Abstract
Inflammation with expression of interleukin 6 (IL-6) in the brain occurs in many neurodegenerative disorders. To better understand the role of IL-6 in such disorders, we examined performance in a learning task in conjunction with molecular and cellular neuropathology in transgenic mice that express IL-6 chronically from astrocytes in the brain. Transgenic mice exhibited dose- and age-related deficits in avoidance learning that closely corresponded with specific progressive neuropathological changes. These results establish a link between the central nervous system expression of IL-6, inflammatory neurodegeneration, and a learning impairment in transgenic mice. They suggest a critical role for a proinflammatory cytokine in the cognitive deficits and associated neuroinflammatory changes that have been documented in neurodegenerative diseases such as Alzheimer disease and AIDS.
Keywords: dementia, neuropathology, microgliosis, cytokine, calbindin
Interleukin 6 (IL-6) is a plurifunctional cytokine that is involved in the regulation of inflammatory responses (1). Inflammation with concomitant expression of IL-6 in the brain has been reported in many progressive neurodegenerative disorders including Alzheimer disease (2), viral (3) and bacterial (4) meningitis, the AIDS dementia complex (5), and stroke (6). While cognitive impairment is the hallmark of Alzheimer disease and AIDS dementia, the precise relationship between cognitive performance, inflammation in the brain, and neurodegeneration in these disorders remains unknown. The development of transgenic mice (termed GFAP-IL6; GFAP, glial fibrillary acidic protein) that express IL-6 chronically from astrocytes in the central nervous system now provides an opportunity to better explore this problem. Previous work in GFAP-IL6 mice has shown that neuropathological manifestations include neurodegeneration consisting of dendritic vacuolization and reduced branching of dendritic spines in CA1 neurons, loss of parvalbumin-immunoreactive (IR) hippocampal interneurons, as well as atrophy and loss of neurons in the molecular and granular layers of the cerebellum (7–9). Moreover, the chronic expression of IL-6 in these mice induces a progressive and largely localized inflammatory-like response, characterized by astroglial and microglial activation, enhanced-acute phase response and cytokine gene expression and increased blood–brain barrier permeability.
GFAP-IL6 mice used in the present study were derived from a low expressor line (7) shown previously to develop an age-related progression in molecular and cellular central nervous system changes. The level of cerebral IL-6 expression in this transgenic line is comparable to that found in the brains of mice with experimental allergic encephalomyelitis (I.L.C., unpublished observation). The present study was conducted to investigate the influence of chronic expression of IL-6 on the learning of a conditioned avoidance response in a discriminated Y-maze task (10). This task was chosen because it represents a well characterized complex form of learning and has been shown to be sensitive for detection of learning impairments in mice with persistent viral infection (10) and to manipulation of hippocampal function (11). More specifically, this avoidance task measures the ability of the animal to discriminate between right and left and to initiate a response to avoid a negative outcome. In concurrent experiments, specific markers of cellular and molecular pathology were characterized in these mice. To this end we are able to begin to characterize the relationship between behavior and brain pathology in transgenic mice expressing IL-6 in the brain.
MATERIALS AND METHODS
Animals.
A description of the construction of the GFAP-IL6 fusion gene and production of GFAP-IL6 and GFAP-α1-antichymotrypsin (ACT) mice using a C57BL/6 × SJL hybrid strain has been described in detail (7, 12). Mice were housed in groups (2–4 per cage) in temperature- and humidity-controlled rooms with a 12-hr light/12-hr dark cycle (lights on at 10:00). All mice had free access to food and water at all times.
Avoidance Learning Procedure.
Groups of age- and sex-matched nontransgenic or heterozygous and homozygous GFAP-IL6 mice were examined in a longitudinal study at 3, 6, and 12 months of age. The Y-maze apparatus consists of three trough-shaped arms at 45° angles to each other. Each arm is equipped with a sliding door. For the initial day of testing the mice were allowed to freely explore the Y-maze for 8 min. The animals exploratory behavior was observed during this habituation period and the number of arm entries recorded for each animal. On the first trial of the next day, the mice were allowed to enter either arm of the maze. This initial choice was considered the preferred arm and was designated as incorrect on subsequent trials. At the start of each trial, the mouse was placed in the start arm for 15 sec. The door was opened and the mouse allowed 15 sec to move into the correct arm of the maze. If a correct choice was not made in 15 sec or the animal entered the incorrect arm, footshock (0.4 mA) was delivered to all parts of the maze except the correct arm until the animal made a correct choice. Following entry into the correct arm, each mouse was confined to the goal arm for 10 sec. Each trial was separated by a 60-sec inter-trial interval during which the mouse remained in a holding cage. Training continued for 6 days (5 trials per day) with the correct arm remaining the same throughout training. This procedure was repeated when the animals were 6 and 12 months of age. Trials in which the animal entered the correct arm prior to the onset of shock were scored as “correct avoidances.” Entries into the correct arm after the onset of shock were scored as an “escape.” Animals that entered the wrong arm or reentered the start compartment were assigned an “error.” Escape latency was defined as the time, in seconds, from the onset of the footshock until the animal entered the correct arm. To control for possible alterations in somatosensory reactivity to shock, shock sensitivity was assessed in these animals. The method for determining shock sensitivity has been detailed elsewhere (13).
Cellular Pathology Determination.
Animals were perfused with cold saline and the brains removed. Fixed hemibrains were serially sectioned at 40 μm of thickness for subsequent immunocytochemical/computer-aided image analysis. To evaluate the integrity of the synaptic and dendritic system, sections were double- immunolabeled with a combination of mouse monoclonal anti-microtubule-associated protein 2 (MAP2; Boehringer Mannheim) and rabbit polyclonal anti-synaptophysin (SYN; Dako) as described (14, 15). After overnight incubation, sections were incubated with the mixture of fluorescein isothiocyanate-conjugated horse anti-mouse IgG (Vector Laboratories) and Texas Red-conjugated goat anti-rabbit IgG (Vector Laboratories). The double-immunolabeled blind-coded sections were analyzed with the laser scanning confocal microscope (14, 15) followed by quantification of the percent area of neuropil covered by MAP2-IR dendrites and SYN-IR synapses using the image software, as described (14, 15). Further analysis of pattern of selective neuronal damage was performed as described (16) in sections labeled with antibodies against the calcium binding proteins-calbindin and parvalbumin (mouse mAb; Sigma). Anticalbindin or parvalbumin-immunostained cells were counted in ten consecutive fields (0.1 mm; two each) along the side of the gyrus using a ×40 objective and a gridded ×10 eyepiece lens. Additional analysis of the glial response to neurodegeneration was carried out by immunolabeling sections, with rabbit polyclonal anti-GFAP (BioGenex Laboratories, San Ramon, CA) and rat monoclonal anti-F4/80 (Serotec). Immunolabeled sections were reacted with diaminobenzidene and analyzed with the Quantimet 570C (Leica, Deerfield, IL) densitometer, as described (7). All the experiments were performed with sections blind-coded.
Molecular Pathology Determination.
RNA extraction and RNase protection assay were performed as described (8). For quantification, the intensity of each autoradiographic band was measured by densitometry using National Institutes of Health image1.47 software, and the level of expression was expressed in arbitrary units relative to the internal loading control L32.
RESULTS
Avoidance Learning.
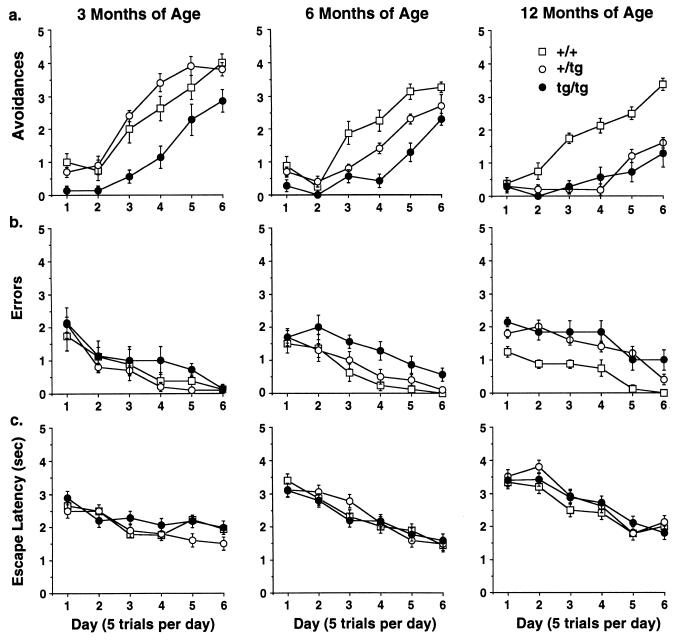
At 3 months of age, mice heterozygous for the IL-6 transgene were able to learn the discriminated avoidance task comparably to that observed in control mice, while homozygous mice were significantly slower to acquire the avoidance response (Fig. 1a). When the same groups of mice were tested at 6 months of age, the heterozygous transgenic animals exhibited a significant deficit in their ability to learn the avoidance response, intermediate to that of the control and homozygous transgenic mice, while the performance in the homozygous transgenic mice worsened. By 12 months of age, the performance of both heterozygous and homozygous transgenic mice had declined further and became indistinguishable. This was confirmed by ANOVA, which revealed a significant group by age interaction [F(4,44) = 6.55, P < 0.0003] and group by age by trial interaction [F(20,220) = 2.70, P = 0.0002]. The analysis of the number of errors followed a similar progression, first affecting homozygous animals and subsequently heterozygous mice {group by age interaction [F(4,44) = 2.96, P = 0.03]}. However, this pattern developed at a later age with no differences in errors observed among the groups at 3 months of age (Fig. 1b). At 6 months of age, homozygous mice exhibited significantly more errors than controls and heterozygous transgenic mice and by 12 months of age both heterozygous and homozygous transgenic mice made more errors than controls.
Figure 1.
Performance of GFAP-IL6 transgenic mice in a discriminated avoidance learning task. (a) Correct avoidances (mean ± SE) on acquisition (3 months of age) and subsequent retests at 6 and 12 months of age for nontransgenic (+/+) (n = 8), heterozygous GFAP-IL6 (+/tg) (n = 10), and homozygous GFAP-IL6 (tg/tg) mice (n = 7). Homozygous mice were significantly different from controls throughout the study and heterozygous mice tested at 3 and months of age. (b) Errors (mean ± SE) on acquisition and subsequent retests for nontransgenic and transgenic mice. (c) Escape latency (mean ± SE) on acquisition and subsequent retests for nontransgenic and transgenic mice.
The discovery of a deficit in the avoidance task indicates a potential alteration in the associative processes necessary for learning this response. Alterations in nonassociative or motivational factors (i.e., performance variables) do not readily account for this differential avoidance performance. Specifically, exploratory behavior during the habituation phase of the Y-maze did not differ among the groups indicating that motor activity was similar across all groups when tested at 3 and 6 months of age (data not shown). In addition, the analysis of shock sensitivity thresholds did not differ among the groups, suggesting that these impairments were not consequent to impaired somatosensory functioning (threshold for all groups was 0.4 mA). Similarly, using a measure of average response latency for the shock escape trials as an index of the motivational properties of the footshock stimulus, we found no statistically significant evidence for any differences among the groups at any age tested (F < 1.0; see Fig. 1c). Taken together, these findings suggest that chronic expression of IL-6 resulted in progressive dose- and age-related deficits in avoidance learning.
To rule out possible nonspecific effects of the GFAP promoter, 12–13-month-old control and GFAP-ACT transgenic mice (12) were tested using the same experimental protocol. GFAP-ACT mice were used as a control because these animals do not exhibit detectable molecular or cellular neuropathological alterations (12). The human ACT is a potent serine protease inhibitor enzyme that protects against injury from serine proteases released by activated macrophages and other cells during the course of an inflammatory response. The absence of inflammation in the GFAP-ACT mice is therefore to be expected and makes this a valid control. There was no significant difference in Y-maze performance between heterozygous transgenic mice and their nontransgenic littermate controls (data not shown). These results provided further support for the conclusion that the observed deficits in avoidance learning in GFAP-IL6 mice were related specifically to the cellular and molecular pathological changes following chronic expression of IL-6 and were not due for example to nonspecific effects associated with the transgene GFAP promoter activity.
Cellular and Molecular Pathology.
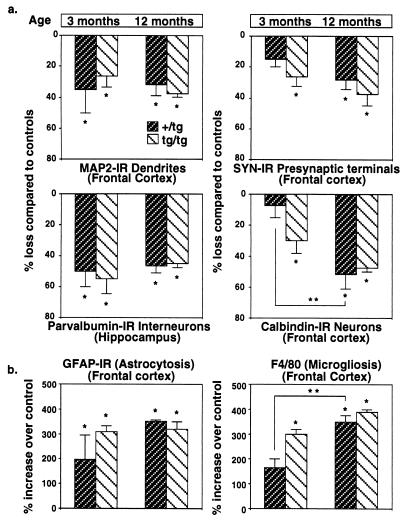
Several markers of neurodegeneration were examined for their correspondence with the learning decline seen in GFAP-IL6 animals. Compared with controls, extensive synaptic damage was observed in 3-month-old homozygous mice and 12-month-old mice of both heterozygous and homozygous transgenic groups [F(4,12) = 15.64, P = 0.0001] as indicated by decreased immunostaining for SYN (Fig. 2a). However, at 3 months of age heterozygous transgenic mice displayed milder synaptic alterations and did not differ from controls. In contrast, pronounced dendritic damage (MAP2) was evident in 3- and 12-month-old GFAP-IL6 mice of both groups when compared with controls [F(4,12) = 8.63, P = 0.002]. Extensive loss of calbindin-IR neurons was observed in 3-month-old homozygous mice and older mice of both transgenic groups when compared with controls [F(4,12) = 15.64, P = 0.0001]. However, calbindin immunostaining was not different in 3-month-old heterozygous transgenic mice compared with controls (Fig. 3). Thus, synaptic damage and loss of calbindin-IR neurons were related to the consequences of chronic IL-6 expression occurring over time in the transgenic mice. Even low doses or short exposure to IL-6 caused significant dendritic damage and loss of parvalbumin-IR neurons. Significant reduction in parvalbumin-IR interneurons is known to occur in the hippocampus of GFAP-IL6 mice (7). Examination of the parvalbumin-containing neurons in the present study confirmed these earlier findings and revealed that the loss of these neurons in the hippocampus was comparable at 3 and 12 months of age in both groups of GFAP-IL6 mice [F(4,12) = 11.12, P = 0.0005]. Taken together, these results indicated that synaptic damage and loss of calbindin-IR neurons, but not dendritic damage and loss of parvalbumin-IR neurons, corresponded most closely with the deficits in avoidance learning evident in these transgenic mice.
Figure 2.
Quantitative analysis of neurodegeneration (a) and gliosis (b) in GFAP-IL6 transgenic mice. A total of 17 mice were analyzed for selected markers utilized to assess the severity of the cellular neuropathology. GFAP-IL6 mice from both age groups were significantly different from nontransgenic controls on measures of MAP2-IR, parvalbumin-IR, and GFAP-IR. A significant loss of SYN-IR was observed in 3-month-old homozygous (tg/tg) transgenic mice and in both groups of 12-month-old transgenic mice when compared with controls, but not in 3-month-old heterozygous (+/tg) transgenic mice. The loss of calbindin-IR neurons and more intense microgliosis observed in 3-month-old heterozygous, and 12-month-old heterozygous and homozygous GFAP-IL6 mice, was significantly different from controls. Three-month-old heterozygous mice did not differ from controls on these measures. All values were expressed as percent increase or loss compared with age matched controls (mean ± SE). ∗, Significantly different from controls (P < 0.01). ∗∗, Twelve-month-old heterozygous mice were significantly different from 3-month-old heterozygous mice (P < 0.01).
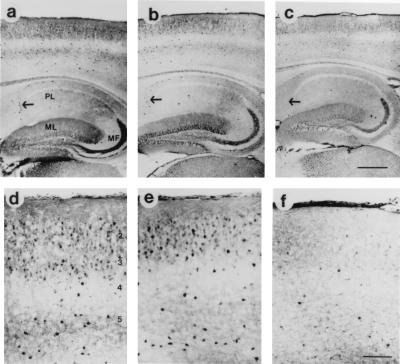
Figure 3.
Alterations in calbindin immunoreactivity in GFAP-IL6 transgenic mice. Low-power view of a 3-month-old (no differences were observed between 3- and 12-month-old nontransgenic mice) nontransgenic mouse (a) and a 3-month-old heterozygous GFAP-IL6 transgenic mouse (b) shows strong immunoreactivity in neocortical areas and in hippocampal interneurons (as indicated by the arrow). The molecular layer (ML) and mossy fiber bundle (MF) also displayed intense calbindin immunoreactivity while the pyramidal cell layer (PL) neuropil was mildly labeled. In contrast, a 12-month-old heterozygous GFAP-IL6 transgenic mouse (c) shows decreased calbindin immunoreactivity in the neocortex and hippocampus. Higher-power view of nontransgenic (d) and a 3-month-old heterozygous GFAP-IL6 transgenic mouse (e) shows strong immunoreactivity associated with neocortical interneurons in layers 2–5. The neuropil in layers 1–3 and layer 5 show a moderate level of immunostaining. In contrast, a 12-month-old heterozygous GFAP-IL6 transgenic mouse (f) shows a widespread decrease in calbindin-IR neurons in all layers of the neocortex. [Bars = 1 mm (c) and 0.1 mm (f).]
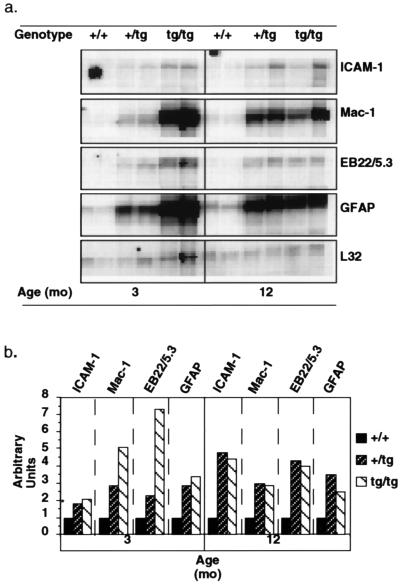
It is unlikely that neuronal injury or death observed in GFAP-IL6 mice is due to direct toxicity mediated by IL-6. First, addition of different concentrations of IL-6 to cultured hippocampal neurons was reported to be associated with a trophic rather than a neurotoxic effect of the cytokine (17, 18). Second, in heterozygous GFAP-IL6 mice expression of transgene-encoded IL-6 was shown previously to be maximal by 3 months of age (8). An alternative possibility is that central nervous system injury in GFAP-IL6 mice is secondary to an inflammatory process induced by IL-6. Consistent with this idea, gliosis and chronic up-regulation of localized acute-phase response gene expression are prominent alterations in the brain of GFAP-IL6 mice (7, 19). In agreement with earlier reports (8), compared with age-matched wild-type controls, 3- and 12-month-old heterozygous and homozygous GFAP-IL6 mice showed significant increases in the expression of the Mac-1 (microglial marker), GFAP (astrocyte marker), EB22/5.3 (acute-phase response gene), and ICAM-1 (inflammatory adhesion molecule) mRNAs (Fig. 4a). Quantification of these responses, however, revealed differences between heterozygous and homozygous mice at 3 months but not 12 months of age, with homozygous mice having markedly higher expression of the Mac-1 and EB22/5.3 genes (Fig. 4b). No significant difference was observed at either age between the heterozygous and homozygous transgenic mice in the level of expression of the GFAP or ICAM-1 genes. The findings with respect to the changes in the microglial cells and astrocytes were further corroborated at the cellular level by quantitative analysis of F4/80 and GFAP immunostained brain sections, respectively (Fig. 2b). Thus, while GFAP staining was increased overall in the transgenic versus control brain, a significant increase in F4/80 staining was observed only in 3-month-old homozygous mice and 12-month-old transgenic mice of both groups when compared with control littermates [F(4,12) = 27.86, P = 0.0001]. At 12 months of age, F4/80 staining was increased comparably in both the heterozygous and homozygous mice. Therefore, these findings indicate that microgliosis and the level of the cerebral acute-phase response, but not astrocytosis nor ICAM-1 gene expression, correspond most closely with the differential avoidance learning and neurodegenerative alterations seen in the heterozygous and homozygous GFAP-IL6 mice.
Figure 4.
Molecular pathological alterations in the brains of GFAP-IL6 transgenic mice. (a) RNase protection assay of poly(A)+-enriched RNA isolated from the brain of nontransgenic (+/+), heterozygous GFAP-IL6 (+/tg), or homozygous (tg/tg) GFAP-IL6 transgenic mice. (b) While cerebral expression of the ICAM-1, Mac-1, EB22/5.3, and GFAP genes was increased in GFAP-IL6 mice, quantitative analysis revealed a marked difference in the levels of expression of the Mac-1 and EB22/5.3 genes between heterozygous and homozygous GFAP-IL6 mice at 3 months but not at 12 months of age. ∗, For ICAM-1, a value of 1 arbitrary unit was assigned to the 3-month-old control group because there was no detectable ICAM-1 signal in these mice.
DISCUSSION
GFAP-IL6 transgenic mice exhibited a progressive age-related decline in avoidance learning performance. These deficits do not appear to be the result of alterations in nonassociative or motivational factors. Cellular and molecular analyses revealed that synaptic damage, loss of calbindin-IR neurons, microglial activation, and the level of cerebral acute-phase response corresponded with the differential avoidance learning observed in heterozygous and homozygous GFAP-IL6 mice. Activation of microglia and up-regulation of cerebral acute-phase response gene expression are implicated in the pathogenesis of human neurodegenerative disorders such as HIV encephalitis and Alzheimer disease (20–22). The contribution of these altered states to the development of neurodegeneration is not known, although it has been speculated that in the case of microglial activation in particular, these cells may elaborate a variety of potentially neurotoxic factors (20–23). Further studies utilizing the GFAP-IL6 transgenic mice may help to resolve these issues.
Several intriguing possibilities for the deficits in avoidance learning observed in the these mice are raised by the present data. First, GFAP-IL6 mice showed a widespread presynaptic loss. Several studies have demonstrated a role for synaptic changes in learning and memory (24, 25). Second, a decrease in calbindin-IR neurons was observed in the frontal cortex and hippocampus. One important function of calbindin is its role as an intraneuronal calcium buffering system, which helps prevent toxic accumulation of cytosolic-free calcium (26–29). Alterations in calcium binding proteins are evident following acute central nervous system insult as well as chronic neurodegenerative disorders, such as Alzheimer disease (27–29). Recent studies have proposed calcium influx as an important mediator in the pathogenesis of HIV-associated neurological damage (30). The decrease in calbindin-IR neurons in HIV encephalitis (31) and in the present study were observed in the frontal cortex. Therefore, cellular alterations observed in the frontal cortex in GFAP-IL6 mice may, in part, underlie their decline in learning performance. Further support for this hypothesis comes from recent observations of learning impairments in mice with reduced calbindin expression (32).
The susceptibility of different neuronal subpopulations to specific neurotoxins may be critically influenced by the expression of endogenous molecules such as calcium binding proteins, which may lead to compromised neural circuits involved in learning. The present study has shown that the severity of the avoidance learning deficits in GFAP-IL6 mice was accompanied by transgene dose-dependent decreases in synaptic complexity and calbindin-IR neurons in the neocortex. Loss of SYN-IR terminals indicates degeneration of association fibers (33), while loss of calbindin immunoreactivity indicates damage to the local circuitry (34). The relationship between the damage to these two distinctive circuits is yet unknown; however, it is possible that abnormal excitatory activity due to loss of local circuit interneurons could lead to excitotoxicity and secondary damage to synapses and dendrites of pyramidal neurons. Neurons expressing high levels of calcium binding proteins in the neocortex and hippocampus correspond to GABAergic interneurons (35). These local circuit interneurons appear to play an important role in determining patterns of neural activation by regulating the rate of firing of excitatory neurons (36). Thus, it is interesting that hippocampal pathophysiology has been previously reported in GFAP-IL6 mice (37). Several studies have demonstrated a functional role for the hippocampus in spatial learning (38–40) and avoidance learning (41). More specifically, Steffensen et al. (37) reported a suppression in theta rhythm in GFAP-IL6 mice and manipulations that effect theta rhythm have been shown to alter the induction of long-term potentiation and performance on tasks of learning and memory (40, 42). Therefore, the alterations observed in cortico-hippocampal function in GFAP-IL6 mice reflect dysfunctional circuitry that likely arises out of altered cellular architecture and these changes could account for the deficits in avoidance learning observed here.
Taken together, these data demonstrate a significant age- and transgene dose-related deficit in avoidance learning in GFAP-IL6 transgenic mice. Of particular interest is the fact that this learning deficit corresponded with specific cellular and molecular changes. Significantly, these studies highlight the utility of this novel transgenic model for integrative analysis to link structural and functional neurological alterations in inflammatory neurodegenerative disease. In particular, these data indicate that loss of synapses and calbindin-containing neurons due to chronic neuroinflammation mediated by activated microglia, may be critical components of human cognitive disorders such as Alzheimer disease and AIDS dementia. Therefore, protection of these neurons from insult and/or reducing microglial activation may be rational therapeutic approaches that could be tested in GFAP-IL6 mice.
Acknowledgments
We thank Floyd E. Bloom and George F. Koob for their helpful comments on this manuscript. This project was supported by grants from the National Institute of Mental Health (MH47680 to I.L.C. and L.H.G. and MH50426 to I.L.C.) and the National Institute of Aging (AG5131 and AG10689 to E.M.). All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals endorsed by the National Institutes of Health. This is manuscript 10162-NP from The Scripps Research Institute.
Footnotes
Abbreviations: IL, interleukin; GFAP, glial fibrillary acidic protein; ACT, α1-antichymotrypsin; MAP, microtubule-associated protein; IR, immunoreactive; SYN, synaptophysin.
References
- 1.Akira S, Hirano T, Taga T, Kishimoto T. FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 2.Bauer J. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 3.Frei K, Leist T P, Meager A, Gallo P, Leppert D, Zinkernagel R M, Fontana A. J Exp Med. 1988;168:449–453. doi: 10.1084/jem.168.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssiau F A, Bukasa K, Sindic C J M, van Dammes J, van Snick J. Clin Exp Immunol. 1988;71:320–323. [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo P, Frei K, Rordorf C, Lazdins J, Tavolto B, Fontana A. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- 6.Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jenson L, Ekholm S, Tarkowski A. Stroke (Dallas) 1995;26:1393–1398. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 7.Campbell I L, Abraham C R, Masliah E, Kemper P, Inglis J D, Oldstone M B A, Mucke L. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang C-S, Stalder A, Samimi A, Campbell I L. Dev Neurosci. 1994;16:212–221. doi: 10.1159/000112109. [DOI] [PubMed] [Google Scholar]
- 9.Brett F M, Mizisin A P, Powell H C, Campbell I L. J Neuropathol Exp Neurol. 1995;54:766–775. doi: 10.1097/00005072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gold L H, Brot M D, Polis I, Schroeder R, Tishon A, de la Torre J C, Oldstone M B A, Koob G F. Behav Neural Biol. 1994;62:100–109. doi: 10.1016/s0163-1047(05)80031-7. [DOI] [PubMed] [Google Scholar]
- 11.Douglas R F. In: The Hippocampus. Isaacson R L, Pibram K H, editors. New York: Plenum; 1975. pp. 327–339. [Google Scholar]
- 12.Mucke L, Masliah E, Campbell I L. Curr Top Microbiol Immunol. 1995;202:187–205. doi: 10.1007/978-3-642-79657-9_13. [DOI] [PubMed] [Google Scholar]
- 13.Schulteis G, Martinez J L., Jr Psychopharmacology. 1990;100:102–109. doi: 10.1007/BF02245798. [DOI] [PubMed] [Google Scholar]
- 14.Masliah E, Achim C L, Ge N, DeTeresa R, Terry R D, Wiley C A. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 15.Mucke L, Abraham C R, Ruppe M D, Rockenstein E M, Toggas S M, Alford M, Masliah E. J Exp Med. 1995;181:1551–1556. doi: 10.1084/jem.181.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masliah E, Ge N, Achim C L, Wiley J. J Neuropathol Exp Neurol. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Satoh T, Nakamura S, Taga T, Matsuda T, Hirano T, Kishimoto T, Kaziro Y. Mol Cell Biol. 1988;8:3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamam T, Miyamoto M, Tsukui H, Nishio C, Hatanaka H. Neurosci Lett. 1989;104:340–344. doi: 10.1016/0304-3940(89)90600-9. [DOI] [PubMed] [Google Scholar]
- 19.Barnum S R, Jones J L, Samimi A, Campbell I L. GLIA. 1996;18:107–117. doi: 10.1002/(SICI)1098-1136(199610)18:2<107::AID-GLIA3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Dickson D W, Mattiace L A, Kure K, Hutchins K, Lyman W D, Brosnan C. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- 21.Kalaria R N. Brain Pathol. 1993;3:333–347. doi: 10.1111/j.1750-3639.1993.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 22.McGeer P L, Kawamata T, Walker D G, Akiyama H, Tooyama I, McGeer E G. GLIA. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 23.Banati R B, Gehrmann J, Schubert P, Kreutzberg G W. GLIA. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 24.Leanza G, Nilsson O G, Wiley R G, Björklund A. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 25.Roch J-M, Masliah E, Roch-Levecq A-C, Sundsmo M P, Otero D A C, Veinbergs I, Saitoh T. Proc Natl Acad Sci USA. 1994;91:7450–7454. doi: 10.1073/pnas.91.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson M P, Rychlik B, Chu C, Christakos S. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 27.Heizmann C W, Braun K. Trends Neurosci. 1992;15:259–264. doi: 10.1016/0166-2236(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland M K, Wong L, Somerville M J, Yoong L K K, Bergeron C, Parmentier M, McLachlan D R. Mol Brain Res. 1993;18:32–42. doi: 10.1016/0169-328x(93)90171-k. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama E, Ohwada J, Iwamoto N, Arai H. Neurosci Lett. 1993;163:223–226. doi: 10.1016/0304-3940(93)90388-2. [DOI] [PubMed] [Google Scholar]
- 30.Dreyer E B, Kaiser P K, Offermann J T, Lipton S A. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 31.Masliah E, Ge N, Achim C L, Wiley C J. J Neuropathol Exp Neurol. 1995;54:350–357. doi: 10.1097/00005072-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Molinari S, Battini R, Ferrari S, Pozzi L, Killcross A S, Robbins T W, Jouvenceau A, Billard J-M, Dutar P, Lamour Y, Baker W A, Cox H, Emson P C. Proc Natl Acad Sci USA. 1996;93:8028–8033. doi: 10.1073/pnas.93.15.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K S, Masliah E, Mallory M, Gage F H. Neuroscience. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- 34.Masliah E, Ge N, Morey M, DeTeresa R, Terry R D, Wiley C A. Lab Invest. 1992;66:285–291. [PubMed] [Google Scholar]
- 35.Celio M R. Science. 1986;231:995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- 36.Conde F, Lund J S, Jacobowitz D M, Baimbridge K G, Lewis D A. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 37.Steffensen S C, Campbell I L, Henriksen S J. Brain Res. 1994;652:149–153. doi: 10.1016/0006-8993(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 38.Lipp H-P, Schwegler H, Crusio W E, Wolfer D P, Leisinger-Trigona M C, Heimrich B, Driscoll P. Experientia. 1989;45:845–859. doi: 10.1007/BF01954059. [DOI] [PubMed] [Google Scholar]
- 39.Grant S G N, O’Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 40.Bach M E, Hawkins R D, Osman M, Kandel E R, Mayford M. Cell. 1995;81:901–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 41.Lipp H-P, Schwegler H, Driscoll P. Science. 1984;225:80–82. doi: 10.1126/science.6729469. [DOI] [PubMed] [Google Scholar]
- 42.Stäubli U, Xu F B. J Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]