Abstract
Many neuromodulators inhibit N-type Ca2+ currents via G protein-coupled pathways in acutely isolated superior cervical ganglion (SCG) neurons. Less is known about which neuromodulators affect release of norepinephrine (NE) at varicosities and terminals of these neurons. To address this question, we used carbon fiber amperometry to measure catecholamine secretion evoked by electrical stimulation at presumed sites of high terminal density in cultures of SCG neurons. The pharmacological properties of action potential-evoked NE release paralleled those of N-type Ca2+ channels: Release was completely blocked by Cd2+ or ω-conotoxin GVIA, reduced 50% by 10 μM NE or 62% by 2 μM UK-14,304, an α2-adrenergic agonist, and reduced 63% by 10 μM oxotremorine M (Oxo-M), a muscarinic agonist. Consistent with action at M2 or M4 receptor subtypes, Oxo-M could be antagonized by 10 μM muscarinic antagonists methoctramine and tropicamide but not by pirenzepine. After overnight incubation with pertussis toxin, inhibition by UK-14,304 and Oxo-M was much reduced. Other neuromodulators known to inhibit Ca2+ channels in these cells, including adenosine, prostaglandin E2, somatostatin, and secretin, also depressed secretion by 34–44%. In cultures treated with ω-conotoxin GVIA, secretion dependent on L-type Ca2+ channels was evoked with long exposure to high K+ Ringer’s solution. This secretion was not sensitive to UK-14,304 or Oxo-M. Evidently, many neuromodulators act on the secretory terminals of SCG neurons, and the depression of NE release at terminals closely parallels the membrane-delimited inhibition of N-type Ca2+ currents in the soma.
Keywords: Ca2+ channel modulation, norepinephrine secretion, adrenergic, muscarinic
Patch-clamp technique studies have shown that N-type Ca2+ channels of the cell soma are modulated via many different G protein-coupled neurotransmitter receptors in superior cervical ganglion (SCG) neurons (1–6; for review, see ref. 7). Many neurotransmitter receptors, including α2-adrenergic, somatostatin, prostaglandin E2 (PGE2), adenosine, M4 muscarinic, pancreatic polypeptide, secretin, vasoactive intestinal peptide, and substance P, inhibit by a fast, membrane-delimited mechanism (7). Angiotensin II receptors and M1 muscarinic receptors inhibit Ca2+ channels via a slow, diffusible cytoplasmic messenger.
Despite intensive work dissecting different modulatory pathways and investigating their underlying mechanisms (7–9), definite physiological roles for Ca2+ channel inhibition in SCG cells have not yet been determined. Two have been proposed (7, 10): (i) In the soma, inhibition of Ca2+ entry could alter cell excitability and action potential firing patterns because the somata possess Ca2+-activated K+ channels and Ca2+-sensitive M-type K+ channels (11–13), and (ii) at sympathetic nerve terminals and varicosities, inhibition of Ca2+ influx could decrease norepinephrine (NE) secretion, as in Dunlap and Fischbach’s (14) general concept of presynaptic inhibition. This hypothesis would require that N-type Ca2+ channels be functionally coupled to many receptors at the distal secretory active zones much as they are at the soma. Studies of radiolabeled NE overflow from sympathetic neurons and of junctional currents in target cells show that NE secretion can be modulated by several neurotransmitters (see ref. 15 for review).
Our study aimed to test the hypothesis that Ca2+ channel modulation is important at secretory active zones. Zhou and Misler (16) recently succeeded in detecting NE secretion from presumed sympathetic terminals in cultured SCG cells using carbon fiber amperometry. The method is stable in time, sensitive enough to detect NE secreted from single synaptic vesicles of SCG cell varicosities, and linear with NE concentration up to 20 μM with the electrodes used in this study. Here we report strong modulation of NE release by several neurotransmitters in a manner that closely parallels Ca2+ channel modulation.
MATERIALS AND METHODS
Cell Culture and Electrochemical Recordings.
Neurons were dissociated from SCG of 2- to 10-day-old Sprague Dawley rats as described (5). Cells were plated at a high density (9 × 105 cells/ml) on poly-l-lysine-coated coverslips and maintained in DMEM (GIBCO)/10% fetal bovine serum (GIBCO)/penicillin (100 units/ml)/streptomycin (100 μg/ml)/nerve growth factor (67 ng/ml, 2.5 S; GIBCO) in 95% CO2/5% O2 at 37°C. Two to 4 days after plating, cells had migrated to form cell clusters connected by axon bundles (16). All measurements shown were performed in this time period and at room temperature.
Electrochemical detection of NE release was as described (16, 17). A carbon fiber (11 μm; Amoco, Tustin, CA) was inserted into a 10-μl polyethylene micropipette tip and insulated with a layer of plastic by melting the pipette tip. The initial sensitivity of the carbon fiber electrodes was ≈1 pA/μM when tested with a known NE concentration. NE secretion was evoked by KCl depolarization or by focal electrical stimulation (Fig. 1A). A multibarreled solution exchange system applied puffs of K+-rich Ringer’s solution or test solutions containing Ca2+ channel modulators or toxins under computer control. For electrical stimulation, we used a small, bipolar electrode (tip size 250 μm) consisting of two platinum wires in each barrel of a pulled θ glass. The stimulation electrode was positioned on an axon bundle ≈200 μm away from the cell clusters studied with the carbon fiber electrode to minimize direct depolarization of the terminals under investigation. Eighteen suprathreshold electric stimuli (20 Hz, 0.07–0.1 mA strength, and 1 ms duration) were applied. Electrochemical currents detected by the carbon fiber electrode at +600 mV were amplified with a List EPC-5 patch-clamp amplifier (List Electronics, Darmstadt, Germany), filtered at 200 or 500 Hz, and stored in an IBM-compatible personal computer. Some recordings shown were digitally filtered at 200 Hz. In recordings acquired with electrical stimulation, artifacts caused by stimulating currents were digitally eliminated by blanking several data points.
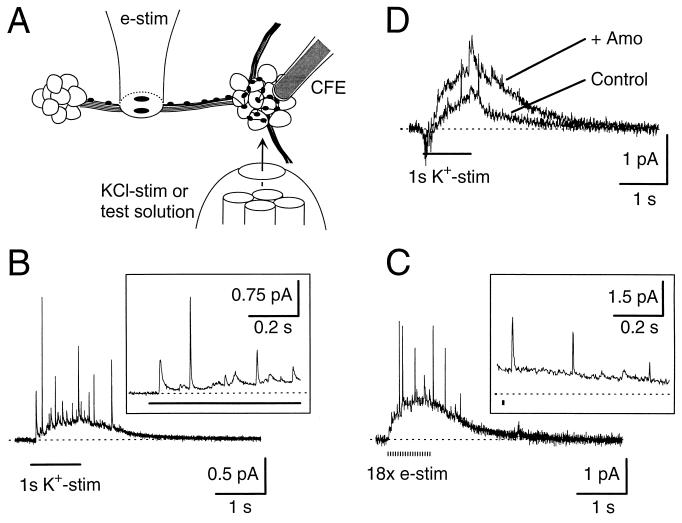
Figure 1.
NE secretion from the varicosities of cultured SCG cells. (A) Schematic illustration of experimental conditions. To detect NE released from varicosities, a carbon fiber electrode (CFE) gently touched a cluster of SCG cells. At rest, the cluster was perfused with Ringer’s solution by a multibarrel solution exchange system. The system was used to puff the K+-rich Ringer’s solution or to apply test solutions. A small bipolar electrode was used to stimulate an axon bundle. Multiple varicosities formed along the one axon are symbolized as small spots. KCl-stim, KCl stimulation; e-stim, electrical stimuli. (Not drawn to scale.) (B) Electrochemical signal evoked by a 1-s, high K+ depolarization (K+-stim) (marked as a line below the trace). Part of recording is shown in the Inset on an expanded time scale illustrating quantal release. Tetrodotoxin (1 μM) was included in all solutions. (C) Electrochemical signal evoked by 18 electrical stimuli (e-stim) (0.1 mA, 1 ms long with a 50-ms interval, marked below the trace) in the absence of TTX. Artifacts resulting from stimulating currents were digitally blanked. (D) NE detected by CFE was increased by NE transporter blocker. Secretion was induced by a 1-s, high K+ depolarization (K+-stim) before (Control) and after (+ Amo) a 1-min incubation with 10 μM amoxapine. A 6-day-old cluster, older than cell clusters, was used in other experiments (see Materials and Methods).
Solutions.
Physiological Ringer’s solution contained: 160 mM NaCl/2.5 mM KCl/2 mM CaCl2/1 mM MgCl2/5 mM Hepes/8 mM glucose, pH adjusted as 7.4 with NaOH. We also added 10 μM amoxapine, an NE uptake blocker, to all bathing solutions if not otherwise indicated. In K+-rich Ringer’s solution used for KCl puffs, 87.5 mM NaCl was replaced by equimolar KCl. The intracellular pipette solution used for whole cell recordings contained: 175 mM KCl/5 mM MgCl2/5 mM Hepes/0.1 mM Na-BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid]/0.3 mM Na2GTP/5 mM K2ATP, pH adjusted as 7.2 with KOH. For solutions containing peptides, 0.01% BSA was added to reduce nonspecific binding. Amoxapine (Research Biochemicals, Natick, MA), PGE2 (Calbiochem), and UK-14,304 (Research Biochemicals) were dissolved in dimethyl sulfoxide, and nifedipine (Sigma) was dissolved in ethanol, as 10- or 20-mM stock solutions. Isoproterenol, methoctramine, oxotremorine M (Oxo-M), pirenzepine, and yohimbine were purchased from Research Biochemicals. Phenylephrine and tropicamide were from Sigma. Pertussis toxin (PTX) was from Calbiochem. Secretin was from Peninsula Laboratories. Somatostatin and ω-conotoxin GVIA were from Peninsula Laboratories and Bachem, respectively.
Analysis.
The amount of NE detected by amperometry was obtained by integrating the electrochemical currents (6.2-s long recordings starting from 250 ms before electrical stimulation) after subtracting any steady current seen before stimulation. This “leak” current was typically 10–20 pA in a fresh probe and declined with time. Relative NE secretion was defined as the ratio of NE secretion in test and control solutions. Bar graphs summarize data from 3–13 experiments. All values in text and figures are given as mean ± SEM.
RESULTS
To measure the NE secretion from sympathetic terminals and varicosities, we used SCG cultures as a model system (16). In these cultures, neurites form many secretory varicosities that envelop cell clusters as judged by abundant immunohistochemical staining for the synaptic vesicle protein SV2 (ref. 16 and unpublished observations). Fig. 1A schematically illustrates the experimental conditions. NE secretion could be evoked by KCl stimulation (Fig. 1B) or by electrical stimulation (Fig. 1C). In either case, the electrochemical signal exhibited two components: There were (i) rapid spikes superimposed on (ii) a several second, slow elevation. The amperometric spikes with rapid onset and exponential decay had current sizes varying from <0.1 to several picoamperes (Insets in Fig. 1 B and C). They have been identified as quantal release of single vesicles of oxidizable neurotransmitters (16, 18). Fast, large spikes presumably arose from quantal release just under the probe whereas slower, smaller spikes arose from slightly further away. The superimposed gradual rise and fall in amperometric current probably reflects a diffuse NE signal summing contributions from many release sites around the cell cluster but distant from the carbon fiber electrode.
Neither current component was detected when the electrode was held at voltages too low to promote catecholamine oxidation (<300 mV). As in a previous report with the same preparation (16), we calculated 104–105 NE molecules per quantum from the integrated charge of a single spike. It is known that secreted NE can be cleared by uptake mechanisms in adrenergic terminals, reducing detection by amperometry or by target cells (19, 20). This also was true in our older cultures despite the relatively low density of cells. In 6-day-old cultures, the overall, integrated NE concentration detected by the carbon fiber electrode was approximately twice as high (231 ± 16%) (n = 3 for 1-s, high K+ depolarization) in our standard solutions containing 10 μM amoxapine, an NE uptake blocker, as without amoxapine (Fig. 1D). Normally we used younger cultures, in which amoxapine’s effect was smaller. Nevertheless, the blocker was used in all solutions to measure unattenuated NE secretion and to avoid studying possible modulation of the uptake mechanism by neuromodulators.
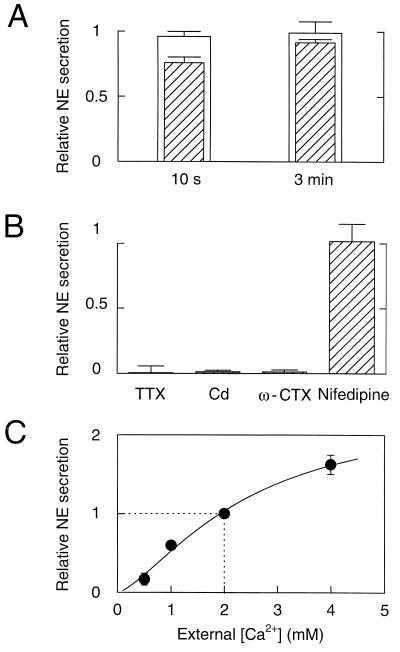
The NE secretion could be evoked repeatedly (>10 times) without significant sign of depletion in most cell clusters provided that the interval between stimuli was sufficiently long. Thus, relative NE secretion evoked by a second electrical stimulation was almost identical to that with the first stimulus after a 10-s interval (Fig. 2A). The recovery after KCl stimulation was slower. In all experiments shown below, we induced secretion every 2 min for electrical stimulation and every 3 min for KCl stimulation. Potassium-induced depolarization of sympathetic terminals may be less influenced by possible modulation of K+ channels and electrical excitability; nevertheless, to examine secretion under more physiological conditions, most experiments were done using electrical stimulation.
Figure 2.
Recovery, pharmacology, and Ca2+ dependence of NE secretion. (A) Recovery of secretion. NE secretion was induced by a 1-s, high K+ deplarization as in Fig. 1B or by electrical stimuli as in Fig. 1C. NE secretion was measured by integrating electrochemical records. Relative NE secretion was calculated as the ratio of NE secretion induced by a second stimulus relative to the first stimulus. Intervals between two stimuli were 10 s or 3 min. White bars denote secretion by electrical stimulation (n = 4), and hatched bars denote secretion by K+ stimulation (n = 4). (B) NE secretion measured in the absence and presence of Na+ and Ca2+ channel blockers. Perfusion of solutions containing blockers started 15 s before electrical stimuli and continued during the recording, except ω-conotoxin GVIA, which was perfused for 1 min. NE secretion relative to controls (see Materials and Methods) was measured in the presence of 300 nM of TTX (0.1 ± 5.2% compared with control value) (n = 6), 100 μM Cd (1.3 ± 1.1%) (n = 10), 1 μM ω-conotoxin GVIA (1.2 ± 1.5%) (n = 5), and 2 μM nifedipine (102 ± 13.5%) (n = 4). (C) Secretion at different external Ca2+ concentrations ([Ca2+]o). NE secretion was induced by electrical stimulation. For [Ca2+]o of 2 mM or lower, MgCl2 was added to make the total divalent concentration 4 mM. NE secretion was normalized to that at 2 mM [Ca2+]o, which was the standard concentration in all other experiments. Data are from 13 cell clusters. The concentration–secretion response was fitted with the equation: relative NE secretion = Max/{1 + (K0.5/[Ca2+]o)a}, where Max =2.41, K0.5 = 2.43 mM Ca2+, and a = 1.43.
Next, we examined the pharmacology of NE secretion from the presumed SCG terminals (Fig. 2B). Preincubation of cell clusters in 300 nM of Tetrodotoxin (TTX) abolished secretion, suggesting that Na+ channel-dependent action potentials are required for secretion evoked by electrical stimulation. A similarly profound block of secretion by 100 μM of Cd2+ indicated that influx of external Ca2+ through Ca2+ channels is required as well. The same extent of inhibition by Cd2+ was observed for secretion induced by KCl stimulation (data not shown). The underlying subtypes of Ca2+ channels were identified using selective blockers. Secretion was nearly abolished by ω-conotoxin GVIA, a selective blocker of N-type Ca2+ channels, demonstrating that N-type channels normally contribute a major portion of the Ca2+ influx at active zones of sympathetic terminals. The actions of ω-conotoxin GVIA were irreversible. In contrast, although the secretion evoked by electrical stimulation was not affected by the L-type Ca2+ channel blocker nifedipine (2 μM), the secretion evoked by a 1-s KCl stimulus could be abolished only by a combination of ω-conotoxin GVIA and nifedipine (data not shown). Apparently, a contribution from the more slowly inactivating L-type channels develops when depolarization is maintained, suggesting that L-type channels are not precisely near the fusion machinery but do lie near enough to deliver Ca2+ during a long depolarization. Alternatively, L-type channels are not activated during short action potentials. In conclusion, the secretion evoked by electrical stimulation is mediated primarily by ω-conotoxin GVIA-sensitive, N-type Ca2+ channels that are activated by TTX-sensitive action potentials.
Next we asked how sensitive the secretion was to small changes of Ca2+ influx. We varied Ca2+ influx by stimulating in solutions containing different external Ca2+ concentrations ([Ca2+]o) and found that secretion could be increased and decreased by this maneuver (Fig. 2C). The fitted Hill equation with a Hill coefficient of 1.43 should be regarded as no more than an empirical description because the experiment involves a slow buildup of residual calcium during a high frequency train of action potentials. If we assume for simplicity that influx is proportional to the external concentration, we would conclude that a 50% decrease of Ca2+ influx relative to control would reduce the integrated secretion by about 40%.
The experiments were initiated to ask if the modulation at distal varicosities and nerve terminals resembles that already studied in cell bodies, so we wanted to ascertain that the NE secretion we were studying does not originate from cell bodies. Therefore, we attempted amperometric recording of NE release from freshly dissociated single cells that lacked visible processes. The cells were whole cell-clamped at −60 mV and depolarized to +10 mV for 2 ms 18 times at 20 Hz, similarly as for electrical stimulation of cultures. The pipette solution contained only 100 μM BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] so that the intracellular Ca2+ concentration could be elevated by Ca2+ influx. Only one quantal event was detected using this standard stimulus protocol in 15 recordings from 9 single cells. However, much longer depolarizations (4 s) did evoke multiple quantal events (2.5 ± 0.5 per recording; 19 recordings) without diffuse signals, indicating that some NE secretion from somata can be induced by unphysiologically prolonged depolarization. In agreement with a previous report on SCG cultures that deletion of cell bodies did not change the secretion of radiolabeled NE from the remaining neurites and varicosities (21), we conclude that electrical stimulation elicits secretion mainly from varicosities and terminals rather than from the cell soma.
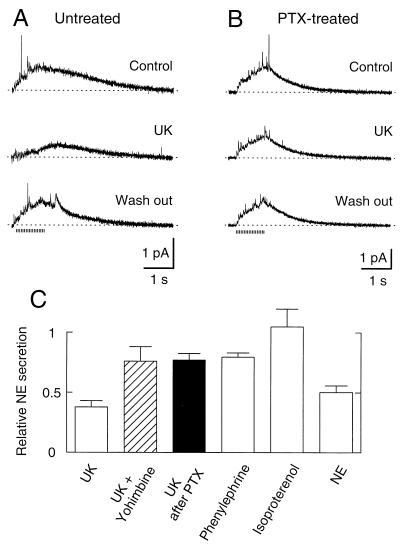
We now turn to modulation of NE secretion by neurotransmitters, emphasizing several parallels with the modulation of Ca2+ channels. Previous studies indicated that α2-adrenergic receptors inhibit Ca2+ channels in the somata of SCG neurons (22–24). We found that 15-s treatment with 2 μM UK-14,304, an α2-adrenergic agonist, also reduced NE secretion in SCG cultures (Fig. 3A). This effect was reversed after a 2-min wash out. The α2-adrenergic antagonist, 10 μM yohimbine, antagonized the UK-14,304 effect (Fig. 3C). The effect of other adrenergic receptor subtypes on secretion was tested (Fig. 3C). Phenylephrine (10 μM), an α1 receptor-preferring agonist, reduced secretion less than UK-14,304. Isoproterenol, a β-adrenergic receptor agonist, had no influence on secretion, suggesting that β-receptors are not involved. The physiological agonist NE (10 μM) reduced secretion by approximately half, comparable to UK-14,304. Because one of the modulatory pathways activated in the soma by adrenergic receptors uses PTX-sensitive G proteins, we studied the action of UK-14,304 on secretion after overnight treatment with PTX. The toxin diminished but did not eliminate the inhibition of secretion by UK-14,304 (Fig. 3 B and C). The reduced UK effect after PTX treatment was not due to experimental variation because cell clusters from parallel dishes without PTX treatment responded normally to UK. The partial action of PTX suggests that the adrenergic effect on secretion has PTX-sensitive and PTX-insensitive components much like the adrenergic modulation of Ca2+ channels (3). In summary, the adrenergic effect is mediated principally by α2-adrenergic receptors acting through at least two classes of G proteins.
Figure 3.
Modulation of NE secretion by adrenergic receptors. (A) Amperometric recordings in Ringer’s (Control), in Ringer’s with 2 μM UK-14,304 (UK), and in Ringer’s solution again (Wash out). The application of UK-14,304 started 15 s before stimuli and continued during the recording. (B) Overnight incubation with 500 ng/ml PTX reduced the inhibition of NE secretion by UK-14,304. PTX was removed before the measurement. (C) Reduction of secretion by agents acting on adrenergic receptor pathways. NE secretion relative to controls was measured with 2 μM UK-14,304 alone (37.7 ± 5.3%) (n = 7), 2 μM UK-14,304 plus 10 μM yohimbine (76.0 ± 12.1%) (n = 3), 2 μM UK-14,304 after PTX treatment (77.0 ± 3.5%) (n = 5), 10 μM phenylephrine (79.6 ± 3.5%) (n = 5), 10 μM isoproterenol (105 ± 14.6%) (n = 5), and 10 μM NE (50.3 ± 5.6%) (n = 3).
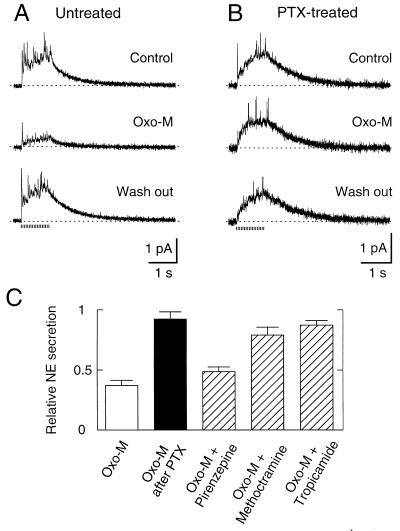
In the soma, muscarinic receptors are known to use two different pathways to inhibit Ca2+ channels. Activation of M1 muscarinic receptors mediates a slow effect through a PTX-insensitive G protein, perhaps Gq/11, and unidentified diffusible second messengers (7, 25). This pathway inhibits not only N-type but also L-type Ca2+ channels and M-type K+ channels. The other modulatory pathway is fast and membrane-delimited and seems to use M4 receptors and PTX-sensitive G proteins. Fig. 4A illustrates the effect of Oxo-M, a muscarinic agonist, on secretion. A 15-s application of 10 μM Oxo-M inhibited secretion by 63% (Fig. 4 A and C). Like the fast, membrane-delimited action of Oxo-M on Ca2+ channels of somata, this action was abolished by overnight treatment with PTX and was relatively insensitive to 10 μM pirenzepine (Fig. 4 B and C). Two other muscarinic antagonists, methoctramine and tropicamide, blocked the Oxo-M effect relatively effectively (Fig. 4C). This profile of antagonist sensitivity would be consistent with actions on presynaptic M2 and/or M4 receptors (26, 27). Evidently, the PTX-insensitive, slow muscarinic pathway is relatively ineffectual at sympathetic secretory active zones. Similar results have been obtained recently with modulation of N- and P-type Ca2+ channels in neostriatal cholinergic interneurons (28).
Figure 4.
Modulation of NE secretion by muscarinic receptors. (A) Amperometric recordings in Ringer’s (Control), in Ringer’s with 10 μM Oxo-M (Oxo-M), and in Ringer’s solution again (Wash out). The application of Oxo-M started 15 s before stimuli and continued during the recording. (B) Overnight incubation with 500 ng/ml PTX eliminates the inhibition of NE secretion by Oxo-M. PTX was absent during the measurement. (C) Reduction of secretion by agents acting on muscarinic receptor pathways. NE secretion relative to controls was measured with Oxo-M (37.0 ± 4.3%) (n = 13), Oxo-M after PTX treatment (92.3 ± 6.0%) (n = 4), Oxo-M plus pirenzepine (48.2 ± 4.3%) (n = 6), Oxo-M plus methoctramine (78.9 ± 6.7%) (n = 7), and Oxo-M plus tropicamide (87.2 ± 3.8%) (n = 5). Concentration of all agents was 10 μM except for PTX.
Care should be taken in interpreting these effects because there could also be K+ channels affected by muscarinic agonists at nerve terminals. In the soma of SCG neurons, M-type K+ channels are inhibited by muscarinic M1 and angiotensin II receptors via the slow, diffusible second messenger pathway (24, 29, 30). Such a reduction of K+ conductance could prolong action potentials, resulting in an increase of NE secretion. However, Oxo-M never increased NE secretion (Fig. 4A), in accordance with our conclusion that the slow muscarinic pathway may be absent from terminals or at least ineffective during short incubations with Oxo-M (Fig. 4 B and C). Furthermore, Oxo-M was as effective as NE in decreasing NE secretion induced by 0.5-s direct depolarization of the terminal membrane with high K+ solutions (data not shown).
An inhibition of “Ca2+ influx-independent secretion” has been described for some neuromodulators in hippocampal synapses (31, 32) and endocrine cells (33). This is usually interpreted as a direct action on the secretory machinery. We tested for a depression of the secretory machinery by looking for modulation of the secretion dependent on L-type Ca2+ channels using 1-s, high K+ stimulation in the presence of 1 μM ω-conotoxin GVIA. We reasoned that L-type channels are not modulated by NE in the soma (29), so any modulation of secretion would result from a direct effect on the secretory machinery. However, there was no effect of 2 μM UK-14,304 (98 ± 10% of control) (n = 4) on NE secretion in these conditions, suggesting that the biochemical steps after Ca2+ influx were not sensitive to the neuromodulator at these active zones. In addition, 10 μM Oxo-M also had no effect on the secretion that depends on L-type channels (103 ± 11% of control) (n = 10), implying no effect of muscarinic receptors on the fusion machinery and an absence of the slow second messenger-mediated pathway for L-type channels at terminals.
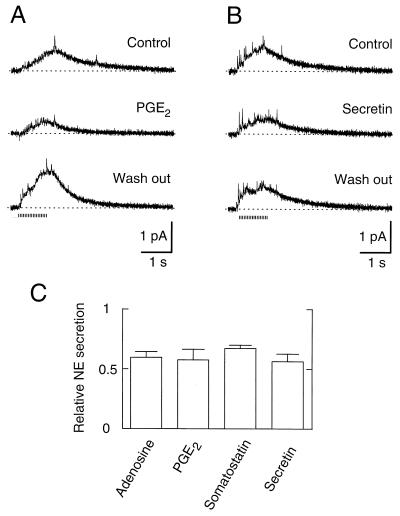
Several neuromodulators in addition to NE and muscarinic agonists can inhibit the N-type Ca2+ channels of SCG cell bodies. Many of them may act as negative regulators of sympathetic action in the periphery, where they are secreted or generated in response to sympathetic activity (NE, PGE2, adenosine; refs. 34 and 35) or released from other nerve cells (somatostatin and secretin). As shown in Fig. 5A, brief preincubation of cell clusters in 1 μM PGE2 resulted in a significant decrease of NE secretion. In some experiments, an additional overrecovery (rebound) of secretion was observed, as shown in the recording after wash out (50% increase in secretion in this experiment). When overrecovery occurred, it developed relatively slowly and persisted for >10 min, decreasing after long wash out. We also found inhibition of secretion in the presence of adenosine (40% at 10 μM) and somatostatin (34% at 250 nM), which inhibit Ca2+ channels in the SCG soma (2, 6). The neuromodulators described above act either solely through a membrane-delimited pathway using a PTX-sensitive Go protein or one additional pathway (7). We also tested a neuromodulator that activates a pathway that does not use Go (36). After a 15-s incubation with 1 μM secretin, which acts through Gs, the NE secretion was 56% of the control value (Fig. 5 B and C), providing additional evidence that the depression of NE release at terminals parallels depression of N-type Ca2+ currents in the soma.
Figure 5.
Modulation of NE secretion by other receptors. (A) Amperometric recordings in Ringer’s (Control), in Ringer’s with 1 μM PGE2 (PGE2), and in Ringer’s solution again (Wash out). The application of PGE2 started 15 s before stimuli and continued during the recording. (B) Recordings as in A except that PGE2 was replaced by 1 μM secretion. (C) Reduction of secretion by neuromodulators. NE secretion relative to controls was measured with 10 μM adenosine (59.6 ± 4.9%) (n = 5), 1 μM PGE2 (57.7 ± 8.8%) (n = 3), 250 nM somatostatin (67.3 ± 5.5%) (n = 4), and 1 μM secretin (56.3 ± 6.4%) (n = 3).
DISCUSSION
A major conclusion of our work is that modulation of catecholamine secretion at sympathetic varicosities and terminals is remarkably similar to the membrane-delimited modulation of N-type Ca2+ channels of the soma. We begin by summarizing the similarities.
In SCG somata, 80–90% of the Ca2+ current is carried by ω-conotoxin GVIA-sensitive N-type Ca2+ channels (37, 38). Likewise, in SCG growth cones, which might be a model for terminals, Ca2+ influx measured by a Ca2+ indicator is greatly attenuated by ω-conotoxin GVIA but much less by 30 μM nifedipine (21). Nevertheless, single channel and whole cell recordings do reveal some L-type channels along with the N-type channels in both the cell body and in the growth cones (29, 37–39). We found that ω-conotoxin GVIA virtually eliminated NE secretion evoked at sympathetic terminals by brief electrical stimuli (Fig. 2B), but a combination of conotoxin and dihydropyridine was needed to block secretion evoked by longer depolarization in high K+ solution. Evidently, N-type channels predominate in secretory active zones of sympathetic neurons and are essential for normal NE secretion. This conclusion fits with findings that ω-conotoxin GVIA abolishes excitatory junction potentials evoked by sympathetic innervation of the guinea pig vas deferens (40).
In somata of SCG neurons, α2-adrenergic, muscarinic, somatostatin, PGE, and secretin receptor agonists depress Ca2+ currents by a fast, membrane-delimited mechanism (7). Except for secretin, each of these actions is at least partially sensitive to PTX. Similarly, we found that the same agonists depress electrically evoked NE secretion from secretory terminals and varicosities, and tests with NE and Oxo-M showed partial or nearly complete PTX sensitivity. We saw no evidence for a slow, second messenger pathway inhibiting secretion at the terminals (Fig. 4). Our observations are in good accord with others in the literature. With whole ganglia, Lipscombe et al. (22) found a reduction of overflow of radiolabeled NE mediated by α2-adrenergic receptors. Measurements of excitatory junction current and electrochemical signals with intact sympathetic nerve terminals and smooth muscle preparation revealed α2-adrenergic receptor-mediated autoinhibition of NE secretion (20, 40).
What is the mechanism of these modulatory actions on secretion? A striking finding in the soma was that N-type Ca2+ channels could be modulated in an apparently identical manner by agonists signaling via at least three different G proteins (7). For example, secretin uses the G protein GS whereas NE uses PTX-sensitive Go or Gi as well as a PTX-insensitive G protein that is not Gs. This convergence from multiple G proteins was then explained by the discovery that the βγ-subunits of the G proteins underlie the fast, membrane-delimited modulation (8, 9). We now find that the same agonists depress the component of NE secretion that depends on N-type channels. The most simplest explanation would be that the G protein βγ-subunits are again responsible and that the inhibition is due to a membrane-delimited reduction of Ca2+ influx through N-type channels of active zones. Our work here on SCG cells shows the appropriate pharmacology, and we found no evidence that inhibition occurs at steps after Ca2+ entry. Measurements with Ca2+-selective dyes indicate that neuropeptide Y inhibits Ca2+ influx through N-type Ca2+ channels in sympathetic terminals innervating cocultured myotubes (41). In addition, like membrane-delimited actions, presynaptic inhibition via γ-aminobutyric acid type B receptors of transmitter release from hippocampal nerve terminals is extremely fast, taking on the order of 200 ms (42).
In concordance with previous work (15), we found that sympathetic terminals and varicosities can be modulated by the same repertoire of neurotransmitters as act at the soma. We have suggested previously that G protein-coupled receptors may be widely distributed over the cell surface because most of them lack efficient localization mechanisms (7). This is largely borne out here, but one exception seems to be that we fail to find clear evidence in active zones for the slow second messenger actions of Oxo-M, which are mediated by M1 receptors in the soma. This conclusion, however, requires further experiments, for example, measurements of Ca2+ influx into active zones before and after activation of the slow pathway. In addition, there may be relative differences because, for example, somatostatin has less effect on NE secretion (34%) than it does on Ca2+ currents of the soma (60%; refs. 2, 5) whereas the figures for other agonists are more similar or in the opposite direction: NE (50 vs. 50%; ref. 3), Oxo-M (63 vs. 80%; ref. 3), and adenosine (40 vs. 30%; ref. 6).
In summary, using amperometric recording, we showed that secretion of NE from sympathetic terminals and varicosities is subject to presynaptic inhibition by a wide range of neurotransmitters. The pharmacological and kinetic similarity of this presynaptic inhibition to fast, membrane-delimited modulation of Ca2+ channels by G protein βγ-subunits in the soma allows us to suggest that presynaptic inhibition involves the same mechanism.
Acknowledgments
We thank Drs. Z. Zhou and S. Misler for valuable training in amperometry and Drs. S. Misler, K. Mackie, J. S. Isaacson, and M. S. Shapiro for reading the manuscript. We thank Dr. J. S. Isaacson for his participation in the early stage of this study. We are grateful to L. Miller and D. Anderson for technical assistance and to E. Martinson for fine mechanical work. This work was supported by National Institutes of Health Grants NS08174 and AR17803.
Footnotes
Abbreviations: SCG, superior cervical ganglion; NE, norepinephrine; Oxo-M, oxotremorine-M; PGE2, prostaglandin E2; TTX, tetrodotoxin; PTX, pertussis toxin; [Ca2+]o, external Ca2+ concentration.
References
- 1.Wanke E, Ferroni A, Malgaroli A, Ambrosini A, Pozzan T, Medolesi J. Proc Natl Acad Sci USA. 1987;84:4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeda S R, Schofield G G. J Physiol (London) 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beech D J, Bernheim L, Hille B. Neuron. 1992;8:97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda S R. J Physiol (London) 1992;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro M S, Hille B. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Ikeda S R. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- 7.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 8.Herlitze S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 10.Miller R J. FASEB J. 1990;4:3291–3299. [PubMed] [Google Scholar]
- 11.Adams P R, Constanti A, Brown D A, Clark R B. Nature (London) 1982;296:746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- 12.Belluzzi O, Sacchi O. J Physiol (London) 1990;422:561–583. doi: 10.1113/jphysiol.1990.sp018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selyanko A A, Brown D A. Neuron. 1996;16:151–162. doi: 10.1016/s0896-6273(00)80032-x. [DOI] [PubMed] [Google Scholar]
- 14.Dunlap K, Fischbach G D. J Physiol (London) 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuder H, Muscholl E. Rev Physiol Biochem Pharmacol. 1995;126:265–412. doi: 10.1007/BFb0049778. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Misler S. Proc Natl Acad Sci USA. 1995;92:6938–6942. doi: 10.1073/pnas.92.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow R H, von Rüden L. In: Single Channel Recordings. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 245–275. [Google Scholar]
- 18.Chow R H, von Rüden L, Neher E. Nature (London) 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 19.Surprenant A, Williams J T. J Physiol (London) 1987;382:87–103. doi: 10.1113/jphysiol.1987.sp016357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Msghina M, Mermet C, Gonon F, Stjärne L. Naunyn-Schmiedebergs Arch Pharmacol. 1992;346:173–186. doi: 10.1007/BF00165299. [DOI] [PubMed] [Google Scholar]
- 21.Przywara D A, Bhave S V, Chowdhury P S, Wakade T D, Wakade A R. Neuroscience. 1993;52:973–986. doi: 10.1016/0306-4522(93)90544-p. [DOI] [PubMed] [Google Scholar]
- 22.Lipscombe D, Kongsamut S, Tsien R W. Nature (London) 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- 23.Schofield G G. Eur J Pharmacol. 1990;180:37–42. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro M S, Wollmuth L P, Hille B. Neuron. 1994;12:1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 25.Bernheim L, Beech D J, Hille B. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 26.Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann M R. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 27.Lazareno S, Birdsall N J M. Br J Pharmacol. 1993;109:1120–1127. doi: 10.1111/j.1476-5381.1993.tb13738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Z, Surmeier D J. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathie A, Bernheim L, Hille B. Neuron. 1992;8:907–914. doi: 10.1016/0896-6273(92)90205-r. [DOI] [PubMed] [Google Scholar]
- 30.Bernheim L, Mathie A, Hille B. Proc Natl Acad Sci USA. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholz K P, Miller R J. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- 32.Capogna M, Gähwiler B H, Thompson S M. J Neurophysiol. 1996;75:2017–2028. doi: 10.1152/jn.1996.75.5.2017. [DOI] [PubMed] [Google Scholar]
- 33.Sher E, Cesare P, Codignola A, Clementi F, Tarroni P, Pollo A, Magnelli V, Carbone E. J Neurosci. 1996;16:3672–3684. doi: 10.1523/JNEUROSCI.16-11-03672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brody M J, Kadowitz P J. Fed Proc. 1974;33:48–60. [PubMed] [Google Scholar]
- 35.Stjärne L. Rev Physiol Biochem Pharmacol. 1989;112:1–137. doi: 10.1007/BFb0027496. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Ikeda S R. Neuron. 1994;13:657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 37.Regan L J, Sah D W Y, Bean B P. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- 38.Mintz I M, Adams M E, Bean B P. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 39.Lipscombe D, Madison D V, Poenie M, Reuter H, Tsien R Y, Tsien R W. Proc Natl Acad Sci USA. 1988;85:2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunnane T C, Searl T J. Adv Second Messenger Phosphoprotein Res. 1994;29:425–459. doi: 10.1016/s1040-7952(06)80029-7. [DOI] [PubMed] [Google Scholar]
- 41.Toth P T, Bindokas V P, Bleakman D, Colmers W F, Miller R J. Nature (London) 1993;364:635–639. doi: 10.1038/364635a0. [DOI] [PubMed] [Google Scholar]
- 42.Pfrieger F W, Gottmann K, Lux H D. Neuron. 1994;12:97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]