Abstract
Terminals of a morphological type known as RD (for round vesicles and dense mitochondria, which we define here as the aggregate of types formerly known as RSD and RLD, where “S” is small and “L” is large) constitute at least half of the synaptic inputs to the feline lateral geniculate nucleus, which represents the thalamic relay of retinal input to cortex. It had been thought that the vast majority of these RD terminals were of cortical origin, making the corticogeniculate pathway by far the largest source of input to geniculate relay cells. However, another source of RD terminals recently identified derives from cholinergic cells of the brainstem parabrachial region. (These cells also contain NO.) We used techniques of electron microscopy to determine quantitatively the relative contribution of cortex and brainstem to the population of RD terminals. We identified corticogeniculate terminals by orthograde transport of biocytin injected into the visual cortex and identified brainstem terminals by immunocytochemical labeling for choline acetyltransferase or brain NO synthase (the synthesizing enzymes for acetylcholine and NO, respectively). We estimated the relative numbers of corticogeniculate and brainstem terminals with a two-step algorithm: First, we determined the relative probability of sampling each terminal type in our material, and then we calculated what mixture of identified corticogeniculate and brainstem terminals was needed to recreate the size distribution of the parent RD terminal population. We conclude that brainstem terminals comprise roughly one-half of the RD population. Thus, the cortical input is perhaps half as large and the brainstem input is an order of magnitude larger than had been thought. This further suggests that the brainstem inputs might play a surprisingly complex and subtle role in the control of the geniculocortical relay.
It is often claimed that terminals from visual cortex form the dominant input to the lateral geniculate nucleus, which is the thalamic relay of retinal input to the visual cortex (1–4). This is because corticogeniculate axons end in a characteristic type of synaptic terminal we shall refer to as “RD” (for round vesicles and dense mitochondria), and RD terminals are the majority found in the geniculate neuropil (1, 2, 4, 5). The RD terminal type represents an aggregate of what was previously defined as RSD and RLD terminals (1, 6), where the “S” and “L” refer to small and large, respectively. However, we have shown that, based on size, RSD and RLD terminals form a continuum (6) and that there is thus little justification for separating them, so we prefer to lump them together under the new term “RD.”
Until recently, few candidate sources for RD terminals other than corticogeniculate axons have been identified, and it has been assumed that nearly all of these emanate in the feedback pathway from visual cortex (refs. 1–4 but also see ref. 7). As a result, many functions have been suggested for the corticothalamic pathway in controlling or modifying the thalamic relay (reviewed in ref. 5), and other extrathalamic sources of input have been relegated a less important role. However, we now know that cholinergic terminals from the parabrachial region of the brainstem also display RD morphology, but we have lacked quantitative data permitting us to determine what proportion of the dominant RD terminal population is cortical vs. brainstem in origin. Using material from the cat’s lateral geniculate nucleus, we used an algorithm based on size distributions of RD terminals identified as deriving from cortical neurons or from cholinergic brainstem neurons, and we conclude that the brainstem contribution is much greater than previously thought, providing approximately half of the RD terminals. We suggest that, although the corticothalamic input is large, it is not as dominant as once thought, and inputs from the brainstem are much more important in the geniculate relay than has been appreciated.
Our basic methods have been fully described elsewhere (4, 6, 8, 9). In brief, we deeply anesthetized and killed 11 adult cats and removed the brains. We then cut sagittal sections at a thickness of 50 μm through the A laminae of the lateral geniculate nucleus. For six cats, we studied corticogeniculate terminals identified via orthograde transport of biocytin injected into cortical areas 17, 18, and 19. We deeply anesthetized these cats for cortical injections and maintained anesthesia for the entire 12- to- 36-h survival period needed for transport of the biocytin to the terminals in the lateral geniculate nucleus. In the other five cats, we processed the sections immunocytochemically to reveal synaptic terminals positive for choline acetyltransferase (ChAT) or brain NO synthase (BNOS), the synthesizing enzymes, respectively, for acetylcholine and NO. We have shown that only brainstem parabrachial terminals contain ChAT, that all of these also contain BNOS, and that the vast majority of brainstem terminals are of this variety (7, 9). All geniculate sections were reacted for biocytin or immunocytochemistry. They were then osmicated, embedded in plastic resin, and thin sectioned for electron microscopy. Both laminae A and A1 were sampled, but no attempt was made to distinguish between them. In each section, we located every synaptic contact zone from a terminal and then identified the terminal type; thus, terminals without clear contact zones in the section under study would not enter our sample. For this study, we concentrated on RD terminals, both unlabeled and those labeled with biocytin, ChAT, or BNOS. Other terminals (e.g., from retina) will be described elsewhere. Brainstem terminals were sampled from sections treated for ChAT or BNOS. Likewise, we sampled corticogeniculate terminals from the entire zone of orthograde label seen in the lateral geniculate nucleus. For an unbiased sampling of the parent population of RD terminals, we studied the same material used for labeled corticogeniculate terminals and sampled every RD terminal making a synapse, whether that terminal was labeled with biocytin or not. For a subset of each of these labeled (i.e., with biocytin, ChAT, or BNOS) and unlabeled terminals, we serially reconstructed the entire synaptic zone.
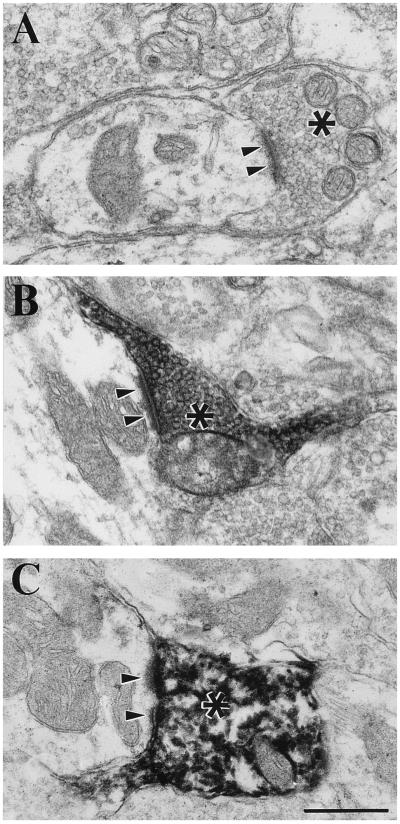
Fig. 1 shows typical examples of the terminal types under study. Our first hints that corticogeniculate terminals were less prominent among RD terminals than we expected were the observations that many unlabeled RD terminals were evident in regions containing labeled corticogeniculate terminals, and surprisingly few unlabeled RD terminals were found among labeled cholinergic terminals. However, we believed that these observations could not be extended to provide quantitative estimates because we could be confident neither that the biocytin injected into cortex labeled all available corticogeniculate terminals (7) nor that the ChAT antibody penetrated sections to label all available cholinergic brainstem terminals.
Figure 1.
Examples of RD terminals (asterisks) and their synaptic contact zones (arrowheads) in the A laminae of the cat lateral geniculate nucleus. (A) Unlabeled RD terminal. (B) Corticogeniculate terminal labeled with biocytin. (C) Brainstem terminal labeled with antibody directed against ChAT. (Bar = 0.5 μm for A–C.)
We thus adopted another strategy. Virtually all cortical and brainstem terminals seem to have RD morphology, so it follows that, if we could determine the relative proportion of corticogeniculate and brainstem terminals present in an unbiased sample of the parent RD population, we could estimate the relative numbers of these labeled terminals. We devised a strategy to do this in a two-step process: (i) We estimated the relative probability of encountering a brainstem (i.e., cholinergic) terminal vs. a cortical one among the parent RD population with our sampling strategy; (ii) we then determined what proportions of labeled brainstem and corticogeniculate terminals were needed to reconstruct the parent population of RD terminals along an arbitrary parameter, which, for the present study, was the cross-sectional area of each terminal. These relative proportions, corrected for sampling biases, then provided an estimate of the actual numbers of these terminal types. The proviso that a small number of RD terminals would be unaccounted for by our approach (i.e., neither labeled from cortex with biocytin nor labeled immunocytochemically with ChAT and/or BNOS) is considered separately below.
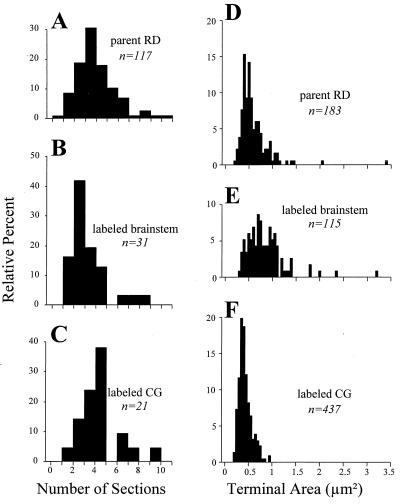
We sampled terminals on the basis of detecting a synaptic zone, so it followed that the relative probability of detecting a terminal was proportional to the probability that a given section would pass through the synaptic contact zone. Because we cut all sections in the same sagittal plane and sampled from similar regions of the lateral geniculate nucleus, any sampling artifacts based on different anisometries in the shape of brainstem vs. corticogeniculate synaptic zones could be ignored. We could thus reduce the relative probability of encountering one of these terminals to the number of sections in a series that contained the synaptic zone. Fig. 2 A–C shows the frequency histograms for the number of serial sections traversed by the synaptic zone for a sample of the parent RD terminals plus labeled brainstem and corticogeniculate terminals. The number of sections representing the synapse are similar for all of these terminal populations (parent RD, 4.5 ± 1.8 sections; corticogeniculate, 4.9 ± 1.8 sections; brainstem, 3.8 ± 1.7 sections). Although the parent RD population did not differ statistically from the other two (P > 0.05 on Mann–Whitney U tests), this number was significantly higher for corticogeniculate than for brainstem terminals (P < 0.01 on a Mann–Whitney U test). We thus conclude that, in the parent RD population, we have oversampled corticogeniculate relative to brainstem terminals by a factor of 4.9/3.8, or 1.3. This analysis also suggests that, if there are substantial numbers of terminals other than corticogeniculate or cholinergic brainstem terminals in the parent RD sample, these do not have synaptic zones that are greatly different in size from the corticogeniculate or brainstem terminals.
Figure 2.
Measurements of synaptic terminals for parent RD terminals (i.e., the larger population from which cholinergic brainstem and corticogeniculate terminals are drawn), brainstem terminals labeled for ChAT or BNOS, and corticogeniculate terminals labeled with biocytin. (A–C) Frequency histograms indicating the extent of the synaptic contact zones. For each of the terminals, the contact zone was serially reconstructed to derive the number of serial sections needed to contain the reconstructed synaptic contact zone. (D–F) Frequency histograms for each terminal type showing their cross-sectional areas. The numbers of terminals in each sample are indicated, and the terminals represented in A–C are a subset of those in D–F.
Fig. 2 D–F shows the overall size distributions of parent RD terminals plus labeled brainstem and corticogeniculate terminals; the latter two populations include those shown in Fig. 2 A–C plus additional terminals that did not have their synaptic zones serially reconstructed. As we have noted (6, 10), brainstem terminals are, on average, significantly larger than corticogeniculate terminals although there is also considerable overlap. This is opposite to the relationship in synaptic zones, which appear to be larger for corticogeniculate terminals. Note, however, that the size range of the parent RD terminals (Fig. 2D) encompasses the full range of corticogeniculate (Fig. 2E) and brainstem terminals (Fig. 2F). If we accept that the sample of parent RD terminals contains a representative sample of corticogeniculate and brainstem terminals, then we should be able to recreate the distribution of Fig. 2D by appropriate combination of the distributions of Fig. 2 E and F. We combined these populations in a stepwise fashion at various intervals from 5% corticogeniculate (and 95% brainstem) terminals to 95% corticogeniculate (and 5% brainstem) terminals and compared these combinations to the distribution of Fig. 2D. To do this, we randomly selected terminals from the corticogeniculate and parabrachial populations in the ratios indicated until 183 were selected to provide the same size population as the target population. The randomized selection process meant that, for each ratio of corticogeniculate and parabrachial terminals, different runs through this algorithm resulted in different individual terminals being selected for each mixture. To minimize the effects of this variation, we ran the algorithm for each mixture three times. Finally, we corrected these for the above mentioned sampling factor as follows. An actual sample from our data consisting of X% corticogeniculate terminals and Y% brainstem terminals was converted to X′% and Y′%, where X′ is 100 × X/(X + 1.3 × Y) and Y′ is 130 × Y/(X + 1.3 × Y). Thus both X% + Y% and X′% + Y′% equal 100%.
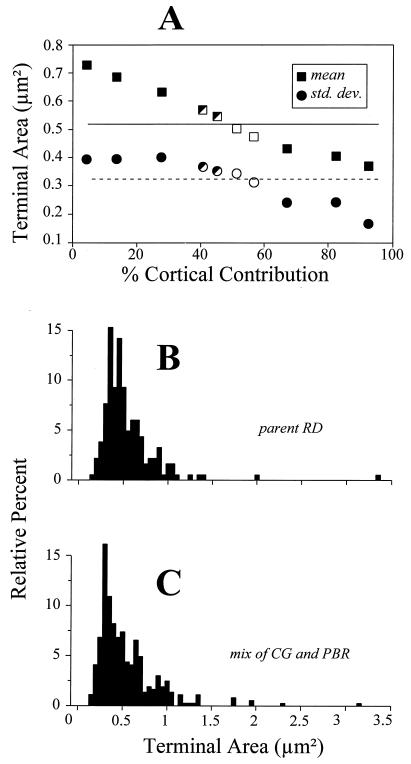
Fig. 3A shows the results of this operation, plotting the population means (squares) and SD (circles) of terminal size for each mixture of corticogeniculate and parabrachial terminals. Each point represents the average value of the three mixtures created for each ratio of terminals. The horizontal lines shown are the mean (solid line) and SD (dashed line) of size for the target population of parent RD terminals, which is redrawn in Fig. 3B. As expected, as the contribution of the smaller corticogeniculate terminals increases, the mean terminal size of the mixed population drops, and the SD shows a similar trend (Fig. 3A). However, note that the resultant populations from every mixture involving <25% or >60% corticogeniculate terminals are statistically different from the target population (filled symbols of Fig. 3A). This is based on Mann–Whitney U tests for means and/or F tests for variance, with P values being <0.05 and usually <0.001. Only at mixtures of corticogeniculate terminals of 53% and 58% (open symbols of Fig. 3A) was every combination statistically indistinguishable from the target population, meaning that every Mann–Whitney U test and F test for all six mixtures had a P value >0.05. Finally, at the mixtures of 40% and 45% corticogeniculate terminals, some of the resultant populations were statistically different from the target, and others were not (half filled symbols of Fig. 3A). As expected, when we averaged all of the six mixtures involving the corticogeniculate contributions of 53% and 58%, the resulting distribution of terminal sizes (Fig. 3C) closely resembled that of the target population (Fig. 3B). Thus, the sizes of unlabeled terminal profiles can best be approximated by mixing corticogeniculate and cholinergic brainstem profiles in a ratio of roughly 1:1 or slightly larger. The parent population of RD terminals represents roughly one-half of terminals found in the geniculate A laminae, so this implies that cholinergic brainstem and corticogeniculate terminals each represent about one-fourth of the terminals there. We emphasize the lack of precision in this calculation, but our goal was merely to obtain a very rough estimate.
Figure 3.
Determination of the mixture of labeled brainstem and corticogeniculate terminals needed to reconstruct the size distribution of parent RD terminals. (A) Means (squares) and SD (circles) of the cross-sectional areas of the terminal populations constructed from various mixtures of corticogeniculate and brainstem terminals (see text for details). Each point represents the average of three independent sampling algorithms whereby corticogeniculate and brainstem terminals were randomly selected for the mixtures. The percentages of corticogeniculate terminals indicated on the abscissa have been corrected for the sampling bias favoring them over brainstem terminals, and thus these values represent X′ as described in the text. The horizontal lines represent the mean (solid line) and SD (dashed line) of the target population of parent RD terminals. Completely filled squares and circles indicate that the mixtures of corticogeniculate and brainstem terminals are significantly different from the parent RD population in terms of cross-sectional area, open symbols indicate that each of the three mixtures is statistically indistinguishable from the parent RD population, and half-filled symbols indicate that some of the mixtures are different from the parent RD population whereas others are not. (B) Terminal size distribution of parent RD population (redrawn from Fig. 2D). (C) Size distribution of combination of all six mixtures of 49% and 53% corticogeniculate terminals, mixtures that were statistically indistinguishable from the parent RD population.
However, one important proviso to this conclusion must be stressed regarding the assumption that essentially all parent RD terminals are either cholinergic from brainstem or corticogeniculate. Another contributor to the parent RD population of Fig. 2D is the population of serotonergic terminals from the dorsal raphé nucleus (11). Both histaminergic terminals from the hypothalamus and noradrenergic terminals from the brainstem exist (12–14), but whether their morphological features are similar to those of RD terminals remains unknown. Terminals from the pretectum have morphology other than RD (9). These other terminal types are thought to be very rare, and thus the above assumption seems justified that only cholinergic brainstem and corticogeniculate terminals need be considered to obtain a very rough estimate of their contribution to the parent RD terminal population. However, even if one or more of these other terminal types prove to contribute substantial numbers to the parent RD population, this would only serve to reduce our overall estimate of the corticogeniculate (and cholinergic) contribution, and they still represent brainstem inputs. Thus, our overall conclusion is unaffected, i.e., corticogeniculate terminals are much less numerous and brainstem terminals are more numerous than previously thought.
We have shown (6) that the synapses from corticogeniculate terminals on relay cells are limited to the peripheral dendrites of these cells whereas cholinergic brainstem terminals contact these cells on proximal dendrites, where retinal inputs also terminate. The peripheral location of corticogeniculate terminals could further attenuate their influence. In contrast, the surprisingly large number of brainstem terminals combined with their proximal location in dendritic arbors amid retinal inputs suggests an anatomical basis for a more powerful regulation of the relay of retinal information through the lateral geniculate nucleus than was imagined previously.
Most considerations of the function of brainstem inputs to thalamus concentrated on effects related to sleep and wakefulness (15–17), and because these effects are global and fairly constant, they do not require a large number of terminals for this operation. More recent data suggest that brainstem inputs, especially the cholinergic ones from the parabrachial region, may play important roles during active vision in terms of controlling the relay of retinal information to cortex (18–20). These possible roles include switching attention between vision and other sensory modalities, controlling the relay during eye movements, and controlling it during different attentional levels. Such roles would suggest a larger anatomical substrate than the previously implied small number of synaptic terminals, and the analysis described in the present account provides this substrate.
Finally, although our observations have been limited to the lateral geniculate nucleus, it is tempting to suggest that a similar pattern exists throughout the thalamus. It is interesting in this context to note that the extent of cholinergic input from the brainstem to different thalamic nuclei varies considerably in the cat (21). For instance, the lateral geniculate nucleus and pulvinar are much more richly innervated by such cholinergic input than are the ventral posterior and medial geniculate nuclei. Our results so far are limited to the cholinergic input to the lateral geniculate nucleus, so it remains unclear what the numerical relationships between corticogeniculate and brainstem inputs are in these other thalamic nuclei.
Acknowledgments
We thank Tony Stretton and Ray Guillery for their help in designing the algorithm used to estimate relative numbers of corticogeniculate and brainstem terminals. This research was funded by U.S. Public Health Service Grant EY03038.
Footnotes
Abbreviations: ChAT, choline acetyltransferase; BNOS, brain NO synthase.
References
- 1.Guillery R W. Z Zellforsch Mikrosk Anat. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- 2.Sherman S M, Koch C. In: The Synaptic Organization of the Brain. 3rd Ed. Shepherd G M, editor. New York: Oxford Univ. Press; 1990. pp. 246–278. [Google Scholar]
- 3.Somogyi J, Hámori J, Silakov V L. Exp Brain Res. 1984;54:485–498. doi: 10.1007/BF00235474. [DOI] [PubMed] [Google Scholar]
- 4.Wilson J R, Friedlander M J, Sherman S M. Proc R Soc London, Ser B. 1984;221:411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 5.Sherman S M, Guillery R W. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 6.Erişir, A., Van Horn, S. C., Bickford, M. E. & Sherman, S. M. (1996) J. Comp. Neurol., in press. [PubMed]
- 7.Weber A J, Kalil R E. J Comp Neurol. 1987;264:171–192. doi: 10.1002/cne.902640204. [DOI] [PubMed] [Google Scholar]
- 8.Bickford M E, Günlük A E, Guido W, Sherman S M. J Comp Neurol. 1993;334:410–430. doi: 10.1002/cne.903340307. [DOI] [PubMed] [Google Scholar]
- 9.Cucchiaro J B, Uhlrich D J, Sherman S M. J Comp Neurol. 1993;334:618–630. doi: 10.1002/cne.903340409. [DOI] [PubMed] [Google Scholar]
- 10.Raczkowski D, Fitzpatrick D. J Comp Neurol. 1989;288:676–690. doi: 10.1002/cne.902880412. [DOI] [PubMed] [Google Scholar]
- 11.de Lima A D, Singer W. J Comp Neurol. 1987;258:339–351. doi: 10.1002/cne.902580303. [DOI] [PubMed] [Google Scholar]
- 12.de Lima A D, Singer W. J Comp Neurol. 1987;259:92–121. doi: 10.1002/cne.902590107. [DOI] [PubMed] [Google Scholar]
- 13.Steriade M, Paré D, Parent A, Smith Y. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 14.Uhlrich D J, Manning K A, Pienkowski T P. Visual Neurosci. 1993;10:225–235. doi: 10.1017/s0952523800003631. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M. Curr Opin Neurobiol. 1993;3:619–625. doi: 10.1016/0959-4388(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 16.Steriade M, Jones E G, Llinás R. Thalamic Oscillations and Signaling. New York: Wiley; 1990. [Google Scholar]
- 17.Steriade M, McCarley R W. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- 18.Hartveit E, Heggelund P. Exp Brain Res. 1995;103:372–384. doi: 10.1007/BF00241496. [DOI] [PubMed] [Google Scholar]
- 19.Lu S-M, Guido W, Sherman S M. Visual Neurosci. 1993;10:631–642. doi: 10.1017/s0952523800005332. [DOI] [PubMed] [Google Scholar]
- 20.Uhlrich D J, Tamamaki N, Murphy P C, Sherman S M. J Neurophysiol. 1995;73:2428–2447. doi: 10.1152/jn.1995.73.6.2428. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick D, Diamond I T, Raczkowski D. J Comp Neurol. 1989;288:647–675. doi: 10.1002/cne.902880411. [DOI] [PubMed] [Google Scholar]