Abstract
Motoneurons in the spinal nucleus of the bulbocavernosus (SNB) express androgen receptors and innervate striated muscles attached to the penis. Previous studies indicated that androgen receptor immunoreactivity in the SNB motoneurons decreases after axotomy and returns to normal only in motoneurons allowed to reinnervate their muscle targets, suggesting that neuron–target interactions play a role in regulating steroid receptor expression in the central nervous system. This study demonstrates that (i) silencing the SNB neuromuscular system with tetrodotoxin did not affect androgen receptor expression in these motoneurons, suggesting that the regulation of androgen receptor is activity-independent; (ii) disruption of axonal transport with vinblastine caused a down-regulation of androgen receptor expression in the SNB motoneurons; and (iii) treatment with brain-derived neurotrophic factor, but not ciliary neurotrophic factor, neurotrophin-4, or glial cell line-derived neurotrophic factor, reversed the axotomy-induced down-regulation of androgen receptor expression. These findings demonstrate neurotrophin regulation of steroid receptor expression in the central nervous system in vivo.
Keywords: spinal nucleus of the bulbocavernosus, ciliary neurotrophic factor, glial cell line-derived neurotrophic factor, neurotrophin-4, vinblastine
Steroid hormones act on the central nervous system (CNS) to organize specific neural circuits during development and to regulate their synaptic organization in adulthood (1, 2). Often the effects of steroid hormones are trophic, in that they promote cell survival; cause somatic, axonal, and dendritic growth; trigger synaptogenesis; and prevent synapse elimination (3). These effects of steroid hormones require binding to ligand-activated receptors that are transcription factors. Protein trophic factors from several different molecular families have similar trophic effects on cell survival and differentiation (4–6). These factors usually act on membrane receptors and involve a cascade of molecular events that includes the activation of different protein kinases (7). The similar effects of steroids and protein trophic factors raises the question of whether the effects of one group are mediated in part by the molecular cascades of the other (8). Recent evidence suggests that steroids and protein trophic factors are colocalized within cells, that steroids regulate expression of trophic factors and/or their receptors (9), and that nerve growth factor can regulate estrogen receptor expression in PC12 cells and explants cultures of neocortex (10, 11). We report that protein trophic factors can regulate expression of steroid receptors in vivo, in an experimentally tractable model system involving a specific population of functionally well defined CNS neurons.
Motoneuronal systems offer significant advantages for studying both the effects of steroids on neurons (1) and the trophic interactions between neuron and target (12). For example, the sexually dimorphic spinal nucleus of the bulbocavernosus (SNB) has proven to be a useful model system for the study of developmental and adult trophic effects of androgens on neurons. SNB motoneurons innervate striated muscles of the penis, the bulbocavernosus (BC), and the levator ani (LA), as well as the external anal sphincter (13, 14). SNB motoneurons and their target BC/LA muscles express androgen receptors (ARs; refs. 13, 15, and 16) and are profoundly influenced by androgen throughout life (1).
Studies on the SNB and other motoneuronal groups indicate that protein trophic factors, synthesized in muscle, Schwann cells, or the motoneurons themselves, regulate neuronal growth and survival during development, regulate neuronal phenotype during adulthood, and influence neuronal response to injury (5, 6, 17, 18). Trophic factors from several classes have potent effects on motoneurons. These include cytokines (8, 19–22), neurotrophins (23–28), and members of the transforming growth factor family (29–32).
When the neuronal cell body is disconnected experimentally from its targets by axotomy, the phenotype of the neuron is dramatically changed, at least partly because target-derived trophic factors can no longer influence the soma (33, 34). For example, axotomy causes down-regulation of proteins related to neurotransmitter synthesis, and up-regulation of trophic factor receptors [e.g., trkB, ciliary neurotrophic factor (CNTF) receptor, and p75 nerve growth factor receptor; refs. 27 and 35–37] and proteins involved in process outgrowth and recovery (e.g., GAP-43 and calcitonin gene-related peptide; refs. 38 and 39). Because some protein trophic factors are regulated by neuronal or muscular activity, blockade of synaptic transmission or neuronal activity leads to changes in expression of trophic factors (40) that result in changes in neuronal phenotype (41, 42).
We recently reported that AR immunoreactivity (ARir) in the SNB motoneurons is significantly reduced after axotomy and that this reduction is fully reversed only in motoneurons allowed to reinnervate the BC/LA muscles (16, 43). This loss of ARir could be explained either by the loss of neuromuscular activity caused by axotomy, or by the loss of protein trophic factors derived from muscle, or both. In particular, we were interested in the idea that motoneuronal steroid receptors might be regulated by target-derived protein trophic factors. In this study, we tested these ideas by blocking neuronal activity with tetrodotoxin (TTX) or by blocking axoplasmic transport with vinblastine (VBL; ref. 44). Moreover, we applied several protein trophic factors to the cut end of the severed motor nerve to determine if specific factors altered the expression of ARir. We chose representatives of three families of factors known to have trophic effects on motoneurons: (i) the cytokine CNTF (20–22, 45), which has potent effects on the developing SNB system (8, 46, 47); (ii) two neurotrophins, brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4; refs. 25–27, 48, and 49); and (iii) the transforming growth factor family member glial cell line-derived neurotrophic factor (GDNF), which is 75 times more effective than the neurotrophins in supporting the survival of purified embryonic rat motoneurons in culture (30–32). The results of these studies indicate that ARir in SNB motoneurons is severely reduced by blockade of axoplasmic transport but not by TTX-induced decrease in neuromuscular activity. Furthermore, BDNF prevents the axotomy-induced reduction of ARir, and GDNF exacerbates the loss of ARir.
MATERIALS AND METHODS
Adult Sprague–Dawley rats (60 days or older) were anesthetized with sodium pentobarbital (55 mg/kg). The motor branch of the pudendal nerve, which contains SNB axons, was exposed as it passes Cowper’s gland just before it enters the BC/LA muscle complex.
Activity Block.
The pudendal nerve was fitted with a cylindrical Silastic cuff (4–5 mm, 1.6 mm i.d., 3.2 mm o.d.) attached via Silastic tubing (0.64 mm i.d., 1.2 mm o.d.) to a subcutaneously implanted mini-osmotic pump (total volume 100 μl, rate 12 μl per day; Alza). In experimental animals (n = 5), the pumps contained 500 mg/ml TTX (Sigma) in Hanks’ balanced salt solution containing 200 units of penicillin and 200 μg of streptomycin. Control animals (n = 5) received pumps filled with vehicle only. Five days later, animals were anesthetized and injected bilaterally with wheat-germ agglutinin conjugated to horseradish peroxidase (WGA-HRP; Sigma) into the BC/LA muscle complex (2.5% in saline, 8 μl per side). The ability of the motoneurons to transport WGA-HRP retrogradely to the cell body allowed an assessment of physical damage caused by the placement of the Silastic cuff. Approximately 20 h after WGA-HRP injections, the activity block was tested in each subject by visual evaluation of muscle response to electrical stimulation of the cuffed pudendal nerve. The animals were then given an overdose of sodium pentobarbital and perfused with heparinized saline followed by 5% phosphate-buffered acrolein. After perfusion, the lower lumbar spinal cord was removed, postfixed for 2 h, immersed in 20% sucrose overnight, and sectioned horizontally (40 μm) on a freezing microtome. Alternate sections were stained immunocytochemically for AR with a 3,3′-diaminobenzidine (DAB) reaction (see below), mounted on subbed slides, and counterstained with cresyl violet. The other sections were reacted with DAB to label the retrogradely transported WGA-HRP, then counterstained immunocytochemically for AR with nickel-intensified DAB (see below), and mounted on subbed slides.
Blockade of Axoplasmic Transport.
The pudendal nerve was exposed unilaterally and wrapped for 15 min with a 2-mg piece of cotton wool soaked in either 50 μl of 300 μM VBL (Sigma) in 0.9% saline (n = 6) or 50 μl of saline alone (n = 6). The cotton-wrapped nerve was isolated from surrounding tissues by a piece of Parafilm. This treatment significantly disrupts axonal flow for at least 4–5 days with little morphological damage to the axon (44, 50). Five days later, animals were anesthetized and injected bilaterally with WGA-HRP into the BC/LA muscle complex to check for the effectiveness of VBL inhibition of retrograde transport. Approximately 20 h after WGA-HRP injections, the animals were anesthetized, and the ability of the nerve to conduct action potentials was assessed by observing the amount of muscle contraction elicited by electrical stimulation of the pudendal nerve. All animals showed a robust response in the muscles after nerve stimulation; therefore, none was excluded from the study. The rats were then perfused, and the lumbar cord was sectioned and processed for WGA-HRP labeling and ARir, as above.
Trophic Factor Treatment.
The pudendal nerve was exposed unilaterally and cut, and the proximal stump was sutured into a Silastic cup (made of medical-grade Silastic adhesive molded into a hollow cylinder 1–2 mm in diameter sealed on one end) containing a 5 × 5 × 3 mm piece of Gelfoam (Upjohn) soaked in approximately 75 μl of one of the following: (i) 0.1 M PBS (n = 5), (ii) BDNF (2.9 mg/ml in PBS, n = 5), (iii) NT-4 (0.58 mg/ml in PBS, n = 5), or (iv) CNTF (3 mg/ml PBS, n = 4; trophic factors provided by Regeneron Pharmaceuticals, Tarrytown, NY). Another group was axotomized, and the cut stumps were left in place without manipulation (n = 5). One week later, the animals were perfused, and the spinal cords were processed as described above. Animals with displaced Silastic cups or infections were excluded from the study.
Three additional groups were added in a follow-up study. The pudendal nerve was severed bilaterally, and the proximal stump was sutured into a Silastic cup containing a piece of Gelfoam soaked in 0.1 M PBS on the control side. On the other side, the proximal stump was left in place without manipulation or sutured into a Silastic cup containing a piece of Gelfoam soaked in either BDNF (2.9 mg/ml PBS, n = 5) or GDNF (1 mg/ml PBS, n = 5; Amgen). Silastic cups were examined 1 week after axotomy, and the animals were killed and perfused as in the previous experiment. After perfusion, the lower lumbar spinal cord was removed and processed as described above.
Histology.
AR immunostaining was carried out according to the protocol of Al-Shamma and Arnold (43). In short, sections were incubated in PG-21 antibody (a gift of G. Prins, University of Illinois, Chicago) for 48 h at 4°C. This antibody was diluted to 0.5–1 μg/ml in 2% normal goat serum, 0.3% Triton X-100 in Tris-buffered saline (TBS). Sections were rinsed and incubated in goat anti-rabbit antibody (for 1 h), then rinsed and incubated in avidin-biotin-peroxidase reagent (1 h; Vector Laboratories Elite ABC kit). After rinsing with TBS, the tissue was incubated in DAB solution (0.05% in TBS) with H2O2 (0.003%) for about 5 min. The latter reaction gives a dark brown nuclear stain. Sections were counterstained with cresyl violet.
Sections from the activity and axoplasmic transport block experiments that were stained for WGA-HRP and counterstained for AR were treated as follows. Sections were incubated in sodium borohydride solution (0.1% in distilled H2O) for 10 min, rinsed three times in TBS, and incubated in DAB solution (0.05% in TBS) with H2O2 (0.003%) for about 15 min. The last step gives a brown reaction product in the cytoplasm of the retrogradely labeled cells. The sections were rinsed and counterstained for AR as above, except that nickel chloride solution (8% in H2O) was added to the final DAB reaction at a concentration of 1:200. The latter reaction gives a dark blue-black nuclear stain that contrasts to the brown cytoplasmic stain. The sections were mounted on subbed slides, dried, rehydrated, washed in distilled water, dehydrated, and cleared in xylenes.
ARir.
ARir was measured in two ways by an observer who was unaware of the treatment condition of the tissue. For the first measure, the density of ARir was judged on a four-point scale: 0 for no detectable labeling, 1 for low, 2 for medium, or 3 for high. Twenty SNB motoneurons with distinct nuclei were sampled from each side of the spinal cord. An equal number of SNB motoneurons were measured on the treated and control side of each section analyzed, and motoneurons were analyzed in alternate sections to avoid measuring a single motoneuron twice. The measure of receptor density was the percentage of motoneurons with medium or high ARir. In the following text, the phrase “change in density of AR” is used as a shorthand for “change in percentage of SNB neurons with medium or high density of AR labeling.” For the second measure, all SNB cells with distinct nuclei containing AR were counted in alternate sections spanning the dorsoventral extent of the SNB.
Data Analysis.
The average cross-sectional area of SNB motoneuronal nuclei on the treated and control sides of the spinal cord was assessed for each animal using a computer with image analysis software (nih image), attached to a light microscope. Twenty AR-immunoreactive nuclei with clear boundaries were sampled from each side of the cord. SNB nuclei that met these criteria were measured in alternate sections sampled from throughout the dorsoventral extent of the SNB. From these measures, the mean nuclear diameter of SNB cells on the treated and control side was calculated for each animal. The raw counts of AR-immunoreactive SNB motoneurons were corrected by the method of Abercrombie (51), normalized with a log transformation, and subjected to an ANOVA.
For all of the experiments, a two-way ANOVA was run on each of the two measures of ARir. Each of these two ANOVAs had one within-group factor (side of cord; treated vs. intact control) and one between-group factor (type of treatment), and was followed by protected planned comparisons where appropriate (two-tailed Bonferroni t test). For all tests, P values of <0.05 were considered statistically significant. Data are presented as the mean ± SEM.
RESULTS
Activity Block.
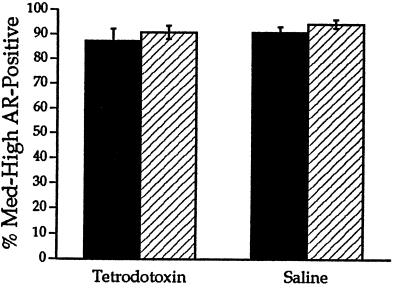
After 1 week of treatment with TTX, stimulation of the treated pudendal nerve in each rat failed to elicit any response in the target musculature, in contrast to a robust response in control rats. In addition, WGA-HRP labeling did not differ between the treated and control rats. These findings indicate that the activity block was complete and did not affect retrograde transport in treated motoneurons. There were no significant differences between treated and control rats in the density of ARir or number of AR-immunoreactive motoneurons (ANOVA, P > 0.05; Figs. 1 and 2), indicating that TTX did not reduce the level of ARir.
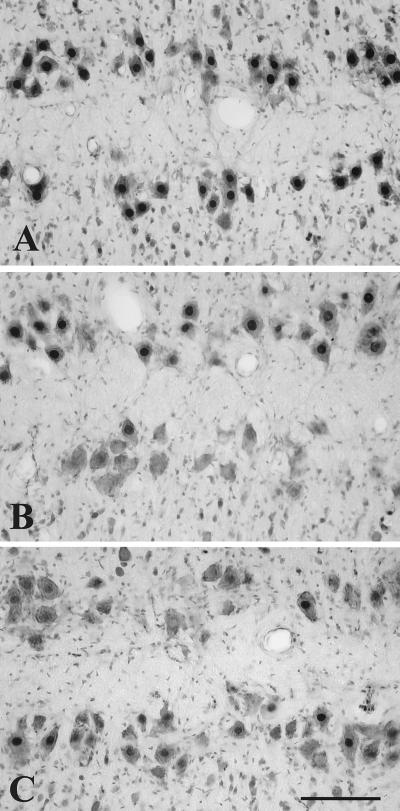
Figure 1.
Composite photomicrograph showing ARir (dark nuclear stain) in SNB motoneurons in two horizontal rows, counterstained with cresyl violet (gray cytoplasm). (A) TTX treatment did not affect ARir on the treated side (bottom row) compared with the control side. (B) In contrast, VBL treatment caused a significant decrease in ARir on the treated side (bottom row) compared with the control side. Note the absence of dense nuclear labeling in motoneurons on the treated side of the cord. (C) Compared with PBS (top row), treatment of axotomized motoneurons with BDNF (bottom row) significantly attenuated the decrease in ARir after axotomy. Note the paucity of dense nuclear labeling on the PBS-treated side compared with the BDNF-treated side. (Bar = 100 μm.)
Figure 2.
TTX treatment of SNB motoneurons did not affect the density of ARir on the treated side (filled bar) compared with the contralateral control side (hatched bar) and the saline-treated control group.
Blockade of Axoplasmic Transport.
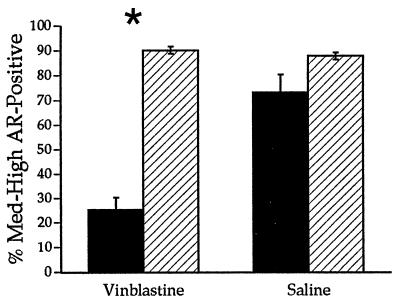
One week after treatment with VBL, WGA-HRP labeling was absent on the treated side compared with complete labeling on the control side, indicating that VBL successfully blocked retrograde transport. The group treated with saline showed no noticeable differences in the number of WGA-HRP-labeled motoneurons between the treated and control sides. In contrast, electrical stimulation of VBL-treated SNB axons elicited a robust response in the target musculature, demonstrating that neuronal activity was not disrupted. There was a significant effect of side on density of ARir and a significant interaction between side and treatment group (ANOVA, P < 0.0001 and 0.0002, respectively). These effects reflect the decrease in the density of ARir on the treated side of the VBL group to 28% of the contralateral control side, compared with no significant difference in ARir between sides of the saline-treated control group (Figs. 1 and 3). Similarly, there was a significant effect of side on the total number of AR-immunoreactive motoneurons (P < 0.05). Further, post hoc analysis revealed a significant decline in the number of AR-immunoreactive motoneurons on the treated side of the VBL group to 49% of the control side (56.3 ± 11.5 vs. 116 ± 6.4, respectively).
Figure 3.
VBL treatment caused a significant drop in the density of ARir on the treated side (filled bar) compared with the intact contralateral control side (hatched bar) and the saline-treated control group. ∗, P < 0.0001.
Trophic Factor Treatment.
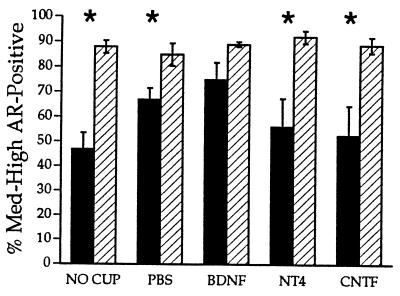
There was a significant effect of side on both the density and number of AR-immunoreactive motoneurons (P < 0.0003 and 0.0001, respectively), and a significant interaction between side and treatment on the number of AR-immunoreactive motoneurons (P < 0.02). Post hoc analysis indicated that axotomized motoneurons receiving no treatment showed the most dramatic drop in the density measure and number of AR-immunoreactive motoneurons to 53% and 76% of the contralateral control side, respectively (P < 0.002 and 0.004, respectively; Fig. 4; ARir number 87 ± 4.2 vs. 113 ± 3.8). Treatment with CNTF or NT-4 did not restore the density (59% and 61% of control; P < 0.02 and 0.006, respectively; Fig. 4) or number (76% and 88% of control, respectively; CNTF: P < 0.04, 85 ± 10.7 vs. 111 ± 9.5; NT-4: P < 0.05, 97 ± 6.9 vs. 110 ± 8) of AR-immunoreactive motoneurons. Treatment with PBS caused a restoration in the number of AR-immunoreactive motoneurons to control levels (94% of control; P > 0.05, 101 ± 5 PBS vs. 108 ± 6 control), although the density measure remained significantly reduced from control levels (79% of control; P < 0.05; Fig. 4). In contrast to all other treatments, BDNF restored both the density and number of AR-immunoreactive motoneurons to control levels (84% and 107% of control, respectively; P > 0.05; ARir number 110 ± 9.1 BDNF vs. 104 ± 5.4 control).
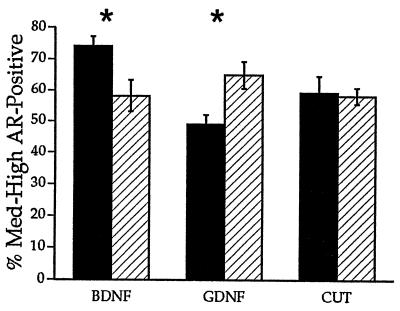
Figure 4.
After unilateral axotomy, treatment with BDNF, but not with PBS, CNTF, or NT-4, or no treatment, prevented the axotomy-induced decrease in ARir. ∗, P < 0.05; filled bar, treatment; hatched bar, control.
The previous experiment suggested that BDNF partially prevented axotomy-induced loss of ARir in SNB motoneurons, but left open the possibility that PBS alone might also have a similar effect. We re-investigated the effects of BDNF and PBS using a within-animal design that allows direct comparison of the two. Moreover, we used the same design to compare the effects of PBS and no treatment, and PBS and GDNF. The pudendal nerve was cut bilaterally in all rats, and groups of rats received (i) PBS applied to the cut nerve on one side and BDNF on the other side, (ii) PBS on one side and GDNF on the other, or (iii) PBS on one side and no treatment on the other.
There was a significant interaction between side and treatment group in both the density and number of AR-immunoreactive motoneurons (P < 0.002 and 0.007, respectively). Post hoc analysis confirmed the restorative effects of BDNF. Both the density measure and the number of AR-immunoreactive motoneurons were higher on the BDNF-treated side compared with the PBS control side (127.14%, P < 0.03, and 113.4%, P < 0.04, respectively; ARir number 110.3 ± 6.7 vs. 97.2 ± 4.3). In contrast, there were no significant differences in the density measure and number of AR-immunoreactive motoneurons between the untreated and PBS-treated control sides (101.7% and 90.5%, respectively; P > 0.05; ARir number 67 ± 5 untreated vs. 74.2 ± 3.1 PBS). Surprisingly, GDNF caused a significant decrease in both the density measure and the number of AR-immunoreactive motoneurons on the treated side compared with the PBS-treated control side (75.4%, P < 0.01, and 77.9%, P < 0.03, respectively; Figs. 1 and 5; ARir number 67.3 ± 7.1 vs. 86.2 ± 5).
Figure 5.
After bilateral axotomy, the density of ARir was higher on the BDNF-treated side compared with the PBS-treated control side (P < 0.03). Filled bar, treatment; hatched bar, PBS. There was no significant difference in the density of ARir between the untreated side and the PBS-treated control side. GDNF caused a significant drop in the density of ARir on the treated side compared with the PBS-treated control side. ∗, Significant difference (P < 0.05).
DISCUSSION
This study strongly supports the idea that protein neurotrophic factors, specifically BDNF, can regulate the expression of neuronal ARs, which are ligand-activated nuclear transcription factors in the nuclear hormone receptor superfamily (52). The evidence supporting this idea is as follows. (i) Severing the axonal connection between SNB motoneurons and their target muscles causes a steep decline in ARir, which is fully reestablished only after the motoneurons regrow to the muscles (16, 43). (ii) Blockade of axoplasmic transport with VBL mimicked the effect of axotomy, reducing the number of AR-immunoreactive SNB motoneurons to 49% of control and the incidence of well labeled motoneurons to 29% of control. Thus, axoplasmic transport is required for normal expression of AR. In contrast, inhibiting neuronal and muscular activity with TTX had no effect. (iii) Application of the neurotrophin BDNF, but not NT-4, CNTF, or GDNF, prevented the loss of motoneuronal ARir caused by axotomy.
Treatment with VBL confirmed our initial studies showing the importance of target-derived factors on the expression of AR in SNB motoneurons (16, 43). VBL disrupts axonal transport without grossly affecting synaptic transmission, and it causes extensive changes in the molecular profile of the treated motoneurons. For example, application of VBL to the sciatic nerve causes an increase in motoneuronal expression of α calcitonin gene-related peptide (50). VBL blocks axonal flow most effectively about 5 days after treatment (44). The similar decrease in ARir after axotomy and VBL treatment suggests that muscle-derived factors regulate levels of ARs in SNB motoneurons. Alternatively, VBL may have influenced Schwann cell expression of trophic factors.
Previous studies have shown that disruption of neuronal activity with TTX or botulinum toxin causes axotomy-like changes in the silenced motoneurons, which can be reversed by electrical stimulation of the muscle and hence are attributed to changes in muscle properties (41, 42, 53). Accordingly, it was important to determine if the loss of ARir after axotomy could be attributed to loss of neuronal or muscular activity. We found no effect of TTX on expression of ARir. The absence of a TTX effect indicates that AR expression is not influenced by synaptic transmission, by neuronal or muscular activity, or by trophic factors that are regulated by such activity (40).
The effects of axotomy and VBL pointed to a role for muscle-derived trophic factors in regulation of AR. Therefore, we compared the effects of BDNF, NT-4, CNTF, and PBS, each applied to the cut end of axotomized SNB motoneurons. Only BDNF restored ARir in axotomized SNB motoneurons to intact contralateral control levels. However, PBS treatment appeared more effective than NT-4 or CNTF, and closer to BDNF in its effects on ARir. To compare the effect of PBS and trophic factors in the same animal, we sectioned pudendal nerves bilaterally and applied PBS to one proximal nerve, and BDNF or GDNF to the other. A third group received PBS on one side and no treatment on the other. This experiment clearly confirmed that BDNF was significantly better than PBS at preventing the effects of axotomy on ARir, and indeed, that PBS was not different from no treatment. In contrast to BDNF, GDNF caused a significant decrease in ARir compared with PBS.
Because only one dose was used for each trophic factor, we cannot conclude that these results prove that NT-4 or CNTF are biologically ineffective in regulating ARir. Moreover, because diffusion of the factors was uncontrolled, the different trophic factors may have been available for different periods of time, depending on their rates of diffusion and catabolism. The doses were selected based on the solubility of each trophic factor, and in some cases, on doses that have been found effective in previous studies (31, 49). Although further studies are required to rule out effects of NT-4 and CNTF, the present results have clearly shown that at these doses, BDNF increases expression of ARir, and GDNF decreases ARir.
Each of the trophic factors that we used acts on different receptors, except for BDNF and NT-4, which both act via the trkB receptor (54, 55). trkB is expressed in SNB and other motoneurons (27). In facial and sciatic motoneurons, trkB mRNA is up-regulated by axotomy, suggesting a role for BDNF and/or NT-4 in survival and regeneration of injured motoneurons (35, 56). However, the regulation of mRNA levels of NT-4 and BDNF differ in adult neuromuscular systems. For example, after axotomy or neuromuscular blockade with TTX or α-bungarotoxin, NT-4 mRNA is rapidly down-regulated in adult skeletal muscle (40, 57). This down-regulation suggests that NT-4 is likely involved in the maintenance of the neuromuscular system rather than as a mediator of regrowth after injury. Because NT-4 treatment did not affect AR expression in the SNB motoneurons, this factor is probably not involved in the regulation of AR. These results are consistent with the results of the first experiment employing TTX to block synaptic transmission. The failure of TTX to alter AR levels in the SNB suggests that these receptors do not depend on factors regulated by muscular activity, including NT-4. Viewed from a different perspective, because the BC/LA muscle targets of the SNB are fast twitch muscles, our results are compatible with the finding of Funakoshi et al. (40) that NT-4 is expressed primarily in slow twitch muscle fibers.
Unlike NT-4, BDNF mRNA levels are up-regulated after peripheral nerve lesion in the Schwann cells surrounding the distal portion of the nerve and in the denervated muscle (27, 57, 58). Further, BDNF is retrogradely transported from muscle to α motoneurons, as demonstrated by radiolabeled BDNF injections into limb muscles (27). The up-regulation of BDNF and trkB expression in injured neuromuscular systems (27, 56) suggest that this neurotrophic factor is involved in regeneration and recovery after injury. In the present experiments, BDNF treatment of axotomized SNB motoneurons, with a dose known to reverse the effects of axotomy in adult facial motoneurons (49), prevented loss of ARir after 1 week. This result suggests that BDNF acts directly on the SNB motoneurons to regulate AR expression. The differing effects of BDNF and NT-4 on the same motoneurons raise the possibility of differential processing of these two neurotrophins at the trkB receptor. This differential processing of the neurotrophin signal might be the result of different binding determinants between the two ligands (59) or differences in dimerization between different forms of the full-length and truncated trkB receptor (60), or both. Although a direct BDNF action on motoneurons is reasonable, given the available data, it is also possible that BDNF acted on other cells (e.g., Schwann cells) to alter ARir in SNB motoneurons indirectly.
The inhibitory effect of GDNF on ARir was surprising. The same dose of GDNF has previously been found to reverse axotomy-induced changes in adult motoneurons (31). Further work is needed to determine whether the inhibitory effect of GDNF is dose-related and whether other lines of evidence (e.g., expression of GDNF and its receptor in the SNB system) support a role for GDNF in regulation of AR in vivo.
Previous reports have implicated glucocorticoid (61) and estrogen interactions with neurotrophins in the CNS (9, 62). Stress induces changes in BDNF and neurotrophin-3 mRNA levels in the hippocampus, presumably in response to changes in levels of glucocorticoids (63, 64). Estrogens and neurotrophins are implicated in reciprocal interactions in the developing CNS (9, 65). Estrogen receptors colocalize with the low affinity p75 and with trkA and trkB mRNA in specific forebrain cells (11, 66, 67). NGF treatment, of rat PC12 cells or neocortical explants in vitro, causes an up-regulation of estrogen binding (10, 11). In PC12 cells, dorsal root ganglion cells, and basal forebrain, estrogen regulates trkA receptor (10, 68, 69). Similarly, estrogen regulates BDNF mRNA in hippocampus (70). Differential estrogenic regulation of these neurotrophin receptors is thought to modulate the sensitivity of the CNS to neurotrophins during development and in adulthood, and neurotrophins such as NGF may underlie the developmental regulation of estrogen receptors in the cortex.
Our results extend previous work and demonstrate neurotrophin regulation of steroid receptors in vivo, in an experimentally tractable neuromuscular system (compare with ref. 71). These results raise numerous questions about the functional role of protein trophic factors in regulation of nuclear receptors, and about the reciprocal relation between steroids and trophic factors in the intact CNS. Further work is needed to determine the molecular mechanisms by which protein trophic factors regulate levels of steroid receptors, whether these or other trophic factors are required for expression of steroid receptors, whether there is redundancy in the trophic factors that modify expression of steroid receptors, and what role trophic factors play in determining the temporal and spacial distribution of steroid receptor expression in the CNS.
Acknowledgments
We thank Dominique Toran-Allerand and Jane Lubischer for comments on an earlier draft. The PG-21 antibody was a kind gift of Dr. Gail Prins. BDNF, CNTF, and NT-4 were provided under a research agreement with Regeneron Pharmaceuticals. GDNF was provided under a research agreement with Amgen. This work was supported by National Institutes of Health Grants HD10839 and HD15021.
Footnotes
Abbreviations: CNS, central nervous system; SNB, spinal nucleus of the bulbocavernosus; BC, bulbocavernosus; LA, levator ani; AR, androgen receptor; CNTF, ciliary neurotrophic factor; BDNF, brain-derived neurotrophic factor; NT-4, neurotrophin-4; GDNF, glial cell line-derived neurotrophic factor; ARir, AR immunoreactivity; TTX, tetrodotoxin; VBL, vinblastine; WGA-HRP, wheat-germ agglutinin conjugated to horseradish peroxidase; DAB, 3,3′-diaminobenzidine; TBS, Tris-buffered saline.
References
- 1.Arnold A P, Jordan C L. In: Frontiers of Neuroendocrinology. Martini L, Ganong W F, editors. Vol. 10. New York: Raven; 1988. pp. 185–214. [Google Scholar]
- 2.Arnold A P. In: Hormones, Brain and Behavior in Vertebrates. Balthazart J, editor. Basel: Karger; 1990. pp. 82–91. [Google Scholar]
- 3.Jones K J. Ann NY Acad Sci. 1994;743:141–164. doi: 10.1111/j.1749-6632.1994.tb55791.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies A M. Nature (London) 1994;368:193–194. doi: 10.1038/368193a0. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R M. Neurobiol Aging. 1994;15:249–251. doi: 10.1016/0197-4580(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 6.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 7.Heumann R. Curr Opin Neurobiol. 1994;4:668–679. doi: 10.1016/0959-4388(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 8.Forger N G, Roberts S L, Wong V, Breedlove S M. J Neurosci. 1993;13:4720–4726. doi: 10.1523/JNEUROSCI.13-11-04720.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toran-Allerand C D. Dev Neurosci. 1996;18:36–48. doi: 10.1159/000111393. [DOI] [PubMed] [Google Scholar]
- 10.Sohrabji F, Greene L A, Miranda R C, Toran-Allerand C D. J Neurobiol. 1994;25:974–988. doi: 10.1002/neu.480250807. [DOI] [PubMed] [Google Scholar]
- 11.Miranda R C, Sohrabji F, Singh M, Toran-Allerand C D. J Neurobiol. 1996;31:77–87. doi: 10.1002/(SICI)1097-4695(199609)31:1<77::AID-NEU7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Grinnell A D. Physiol Rev. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- 13.Breedlove S M, Arnold A P. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 14.McKenna K E, Nadelhaft I. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay R R, Dube J Y, Ho-Kim M A, Lesage R. Steroids. 1977;29:185–195. doi: 10.1016/0039-128x(77)90038-1. [DOI] [PubMed] [Google Scholar]
- 16.Lubischer J L, Arnold A P. Brain Res. 1995;694:61–68. doi: 10.1016/0006-8993(95)00766-j. [DOI] [PubMed] [Google Scholar]
- 17.Barde Y-A. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 18.Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 19.Barbin G, Manthorpe M, Varon S. J Neurochem. 1984;43:1468–1478. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 20.Sendtner M, Kreutzberg G W, Thoenen H. Nature (London) 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 21.Sendtner M, Holtmann M B, Kolbeck R, Thoenen H, Barde Y-A. Nature (London) 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 22.Sahenk Z, Seharaseyon J, Mendell J R. Brain Res. 1994;655:246–250. doi: 10.1016/0006-8993(94)91621-7. [DOI] [PubMed] [Google Scholar]
- 23.Ip N Y, Ibanez C F, Nye S H, McClain J, Jones P F, Gies D R, Belluscio L, Le B M, Espinosa R, Squinto S P, Persson H, Yancopoulos G D. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Levi-Montalcini R. Nature (London) 1989;237:1154–1162. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 25.Yan Q, Elliott J L, Snider W D. Nature (London) 1992;360:753–759. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 26.Henderson C E, Camu W, Mettling C, Gouin A, Pouisen K, Karihaloo M, Rullamas J, Evans T, McMahon S B, Armanini M P, Berkemeier L, Phillips H S, Rosenthal A. Nature (London) 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 27.Koliatsos V E, Clatterbuck R E, Winslow J W, Cayouette M H, Price D L. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 28.Davies A M, Horton A, Burton L E, Schmelzer C, Vandlen R, Rosenthal A. J Neurosci. 1993;13:4961–4967. doi: 10.1523/JNEUROSCI.13-11-04961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L-F H, Doherty D H, Lile J D, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 30.Henderson C E, Phillips H S, Pollock R A, Davies A M, Lemeulle C, Armanini M, Simpson L C, Moffet B, Vandlen R A, Koliatsos V E, Rosenthal A. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Matheson C, Lopez O T. Nature (London) 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Wu E, Lin L-F H, Lei M, Oppenheim R W, Houenou L J. Proc Natl Acad Sci USA. 1995;92:9771–9775. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grafstein B. Exp Neurol. 1975;48:32–51. doi: 10.1016/0014-4886(75)90170-3. [DOI] [PubMed] [Google Scholar]
- 34.Kuno M. Neurosci Res. 1990;9:155–172. doi: 10.1016/0168-0102(90)90001-u. [DOI] [PubMed] [Google Scholar]
- 35.Piehl F, Frisen J, Risling M, Hokfelt T, Cullheim S. Neuroreport. 1994;5:697–700. doi: 10.1097/00001756-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong D M, Brady R, Hersh L B, Hayes R C, Wiley R G. J Comp Neurol. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- 37.Mata M, Jin C-F, Fink D J. Brain Res. 1993;610:162–165. doi: 10.1016/0006-8993(93)91231-g. [DOI] [PubMed] [Google Scholar]
- 38.Saika T, Senba E, Noguchi K, Sato M, Yoshida S, Kubo T, Matsunaga T, Tohyama M. Mol Brain Res. 1991;9:157–160. doi: 10.1016/0169-328x(91)90142-k. [DOI] [PubMed] [Google Scholar]
- 39.Tetzlaff W, Alexander S W, Miller F D, Bisby M A. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez C F. Science. 1995;268:1495–1503. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 41.Czeh G, Gallego R, Kuno M, Kudo M. J Physiol (London) 1978;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinter M J, Vanden Noven S, Muccio D, Wallace N. J Neurosci. 1991;11:657–666. doi: 10.1523/JNEUROSCI.11-03-00657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Shamma H A, Arnold A P. J Neurobiol. 1995;28:341–353. doi: 10.1002/neu.480280307. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald M, Woolf C J, Gibson S J, Mallaburn P S. J Neurosci. 1984;4:430–441. doi: 10.1523/JNEUROSCI.04-02-00430.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsumoto H, Ikeda K, Holmlund T, Greene T, Cedarbaum J M, Wong V, Lindsay R M. Ann Neurol. 1994;36:142–148. doi: 10.1002/ana.410360205. [DOI] [PubMed] [Google Scholar]
- 46.Forger N, Wong V, Breedlove S. J Neurobiol. 1995;28:354–362. doi: 10.1002/neu.480280308. [DOI] [PubMed] [Google Scholar]
- 47.Jordan C L. Dev Neurosci. 1996;18:185–198. doi: 10.1159/000111407. [DOI] [PubMed] [Google Scholar]
- 48.Friedman B, Kleinfeld D, Ip N Y, Verge V M K, Moulton R, Boland P, Zlotchenko E, Lindsay R M, Liu L. J Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan Q, Matheson C, Lopez O T, Miller J A. J Neurosci. 1994;14:5281–5291. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh K, Noguchi K, Senba E. Brain Res. 1992;599:153–157. doi: 10.1016/0006-8993(92)90864-6. [DOI] [PubMed] [Google Scholar]
- 51.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 52.Lopes da Silva S, Burbach J P. Trends Neurosci. 1995;18:542–548. doi: 10.1016/0166-2236(95)98376-a. [DOI] [PubMed] [Google Scholar]
- 53.Gallego R, Kuno M, Nunez R, Snider W D. J Physiol (London) 1979;291:179–189. doi: 10.1113/jphysiol.1979.sp012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soppet D, Escandon E, Maragos J, Middlemas D, Reid S, Blair J, Burton L, Kaplan D, Hunter T, Nikolics K, Prada L. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 55.Squinto S P, Stitt T N, Aldrich T H, Davis S, Bianco S M, Radziejewski C, Glass D J, Masiakowski P, Furth M E, Valenzuela D M, DiStefano P S, Yancopoulos G D. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi N R, Bedard A M, Hincke M T, Tetzlaff W. Eur J Neurosci. 1996;8:1018–1029. doi: 10.1111/j.1460-9568.1996.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 57.Griesbeck O, Parsadanian A S, Sendtner M, Thoenen H. J Neurosci Res. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- 58.Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge V M K, Persson H. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibanez C F. J Neurobiol. 1994;25:1349–1361. doi: 10.1002/neu.480251104. [DOI] [PubMed] [Google Scholar]
- 60.Eide F F, Vining E R, Eide B L, Zang K, Wang X Y, Reichardt L F. J Neurosci. 1996;15:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindholm D, Castren M, Hengerer B, Leingartner A, Castren E, Thoenen H. Ann NY Acad Sci. 1994;746:195–202. doi: 10.1111/j.1749-6632.1994.tb39234.x. [DOI] [PubMed] [Google Scholar]
- 62.Gibbs R B. Ann NY Acad Sci. 1994;743:163–199. doi: 10.1111/j.1749-6632.1994.tb55792.x. [DOI] [PubMed] [Google Scholar]
- 63.Smith M A, Makino S, Kvetnansky R, Post R M. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chao H M, McEwen B S. Mol Brain Res. 1994;26:271–276. doi: 10.1016/0169-328x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 65.Miranda R C, Sohrabji F, Toran-Allerand C D. Horm Behav. 1994;28:367–375. doi: 10.1006/hbeh.1994.1033. [DOI] [PubMed] [Google Scholar]
- 66.Toran-Allerand C D, Miranda R C, Bentham W D L, Sohrabji F, Brown T J, Hochberg R B, MacLusky N J. Proc Natl Acad Sci USA. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miranda R C, Sohrabji F, Toran-Allerand C D. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sohrabji F, Miranda R C, Toran-Allerand C D. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMillan P J, Singer C A, Dorsa D M. J Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh M, Meyer E M, Simpkins J W. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 71.Perez J, Kelley D B. J Neurosci. 1996;16:6625–6633. doi: 10.1523/JNEUROSCI.16-21-06625.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]