Abstract
To investigate the roles of K+ channels in the regulation and fine-tuning of cellular excitability, we generated a mutant mouse carrying a disrupted gene for the fast activating, voltage-gated K+ channel Kv3.1. Kv3.1−/− mice are viable and fertile but have significantly reduced body weights compared with their Kv3.1+/− littermates. Wild-type, heterozygous, and homozygous Kv3.1 channel-deficient mice exhibit similar spontaneous locomotor and exploratory activity. In a test for coordinated motor skill, however, homozygous Kv3.1−/− mice perform significantly worse than their heterozygous Kv3.1+/− or wild-type littermates. Both fast and slow skeletal muscles of Kv3.1−/− mice are slower to reach peak force and to relax after contraction, consequently leading to tetanic responses at lower stimulation frequencies. Both mutant muscles generate significantly smaller contractile forces during a single twitch and during tetanic conditions. Although Kv3.1−/− mutants exhibit a normal auditory frequency range, they show significant differences in their acoustic startle responses. Contrary to expectation, homozygous Kv3.1−/− mice do not have increased spontaneous seizure activity.
Potassium (K+) channels are involved in establishing the resting membrane potential, in determining the action potential duration, in modulating release of transmitter, in pacemaker activity, and in rhythmic firing patterns of neurons (1). The Shaker-related multigene family of voltage-gated K+ channels can be divided in at least four subfamilies in mammals: Kv1, Kv2, Kv3, and Kv4. The voltage-gated K+ channel Kv3.1 forms a noninactivating, delayed rectifier involved in the repolarization of the action potential (2, 3). Kv3.1 is expressed in the adult rat brain at high levels in the granule cells of the cerebellum (4, 5), in the reticular nucleus of the thalamus, in a subset of cells in cerebral cortex and hippocampus, and at lower levels in many other brain nuclei, particularly in the brainstem (6, 7). Many Kv3.1 mRNA-expressing cells in hippocampus and cerebral cortex were tentatively identified as GABAergic interneurons, and, recently, it was shown that Kv3.1-expressing hippocampal and striatal neurons contain the calcium-binding protein parvalbumin, a marker for fast-spiking GABAergic inhibitory interneurons (5–9). Outside the nervous system, Kv3.1 mRNA is expressed in skeletal muscle (6) and in murine T lymphocytes where its overexpression may be linked to the development of autoimmunity in mice (10).
In heterologous expression systems, homotetrameric Kv3.1 channels form delayed rectifier-type K+ channels with extremely rapid kinetics of activation and deactivation, a high threshold of activation (approximately −10 mV), and a large unit conductance (25–28 pS) (2, 3, 11). Therefore, a high enough density of Kv3.1 channels may shorten the duration of the action potential of neurons and muscles by rapidly activating Kv3.1 K+ channels during the peak phase of depolarization. These properties of Kv3.1 may be responsible for the fast-spiking activity seen in many GABAergic interneurons.
We report the construction and initial physiological and behavioral characterization of a Kv3.1-deficient mouse. Homozygous Kv3.1−/− mice are viable and fertile. They show normal spontaneous locomotion, unaltered acquisition (learning) and retention (memory) of a conditioned response, and, surprisingly, no spontaneous seizures. Homozygous mutants are, however, smaller in body size and have impaired coordinated motor skills, presumably due to dramatic changes in the contractile properties of their fast and slow skeletal muscles.
MATERIALS AND METHODS
Generation of Kv3.1 Mutant Mice.
Standard procedures were used in processing RNA, DNA, and protein, including Southern, Northern, and Western blot analyses (12, 13). Clones encoding Kv3.1 were isolated from an isogenic genomic library made of embryonic stem (ES) cell DNA. A stretch of 6-kb genomic DNA including the second coding exon was used to assemble the targeting construct. A neomycin-resistance cassette (neo), isolated from pPGKneobpA, was inserted in the S2–S3 linker between the EcoRI and the MscI site (corresponding to codons 273 and 285), replacing 35 bp of genomic DNA in the replacement-targeting vector (see Fig. 1 for details). This construct was used to generate several ES cell clones and germ-line chimeras. The Kv3.1-specific antibody is directed against the C-terminal sequence (residues 567–585) of Kv3.1b; the production and characterization has been described (7).
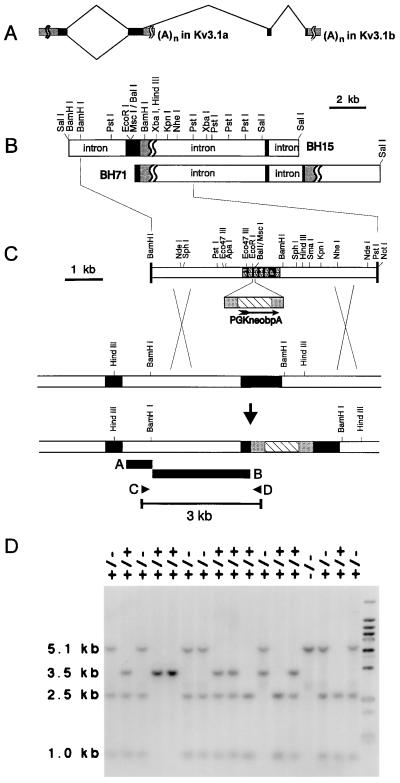
Figure 1.
Homologous recombination by a replacement vector. (A) The exons encoding the two Kv3.1 splice variants Kv3.1a and Kv3.1b are shown at the top. (B) The two genomic DNA clones in EMBL3 used for the construction of the replacement vector are depicted with their relevant restriction sites. (C) The coding region between EcoRI and MscI (35 bp in the S2–S3 linker) was removed and replaced with the neomycin-resistance cassette (PGKneobpA). Homologous recombination between the DNA replacement construct and the targeted locus replaced a functional Kv3.1 allele with the neo cassette-interrupted, nonfunctional Kv3.1 sequence. Homologous recombination was detected by PCR, using primers C and D, or by Southern blot analysis of HindIII-digested genomic DNA hybridized to external probe A or internal probe B. Solid boxes show Kv3.1 coding regions; hatched boxes show the structural gene of the neo cassette; the stippled boxes indicate untranslated regions. (D) Identification of mutant animals. The internal probe B hybridized to a 5.1-kb BamHI fragment derived from the targeted allele and to a 3.5-kb fragment from the 129SvEMS wild-type allele in a Southern blot of genomic tail DNA. The wild-type allele inherited from C57BL/6 contains an additional BamHI site, leading to a 1.0-kb and a 2.5-kb fragment in mice of mixed genetic background.
Behavioral Tests.
Individually housed mice were transferred to the test facility (maintained on a 12-h/12-h light–dark cycle) at least 1 day before testing. Experiments were done between 11 a.m. and 2 p.m.
Open field test.
Male littermates were individually placed in an open field (44 cm × 44 cm Plexiglas cage; opto-varimex and auto-track-system software, Columbus Instruments, Columbus, OH). Spontaneous activity was monitored for 60 min and plotted in 15-min intervals.
Rotarod test.
A mouse was placed on a rod of 3.8 cm diameter (Rotarod test, CR-1 Rotamex System, Columbus Instruments). The rod revolved at 5 rpm and accelerated at 10 rpm/min. The time until the mouse fell off the rotating, accelerating rod was determined (mean ± SE). Immediately after the fall, the mouse received an electrical shock (1 s, 0.2 mA). Each mouse was subjected to five trials per day within a 60-min period.
Active avoidance test.
A mouse was placed in the dark chamber of a light/dark shuttle box (each compartment was 29 × 22 × 13.5 cm) with a small opening to allow transitions between the two compartments. After 10 s, an electrical current (0.2 mA) was applied through a bottom grid for the duration of 20 s. For the first trial on the first day of training, the opening was kept closed until the footshock was initiated. Mice were subjected to 10 trials per day on 5 consecutive days. An avoidance response occurred when a mouse actively avoided the footshock by leaving the dark chamber during the first 10 s.
Startle response.
Mice were tested for their acoustic startle reflex elicited by loud sounds of different intensities (Responder-X Startle Response Monitor and software, Columbus Instruments). A mouse was placed on the force-transducing platform in a small plastic box (12 cm × 8 cm × 6.5 cm) to minimize spontaneous movements. Sounds of defined frequencies (from 3 kHz to 50 kHz) and different relative intensities (500 ms in duration) were applied pseudorandomly every 10 s using a Latin square (four different amplitudes, five times each). The force generated during the first 200 ms after the beginning of the sound was recorded. A startle response occurred when a greater force than ±10 g was detected which equaled four times the standard deviation of the mean force generated by spontaneous movement.
Muscle Contractility Measurements.
Soleus and extensor digitorum longus (EDL) muscles were isolated from mice under anesthesia and suspended vertically between a clamp at the base of a jacketed organ bath containing an oxygenated Krebs solution at 25°C and a Grass Ft.03 force transducer. Muscle length and stimulation voltage were adjusted to yield maximal isometric twitch force. Muscles were stimulated by square pulses of 0.2-ms duration delivered via closely flanking platinum wire electrodes. All stimulations were performed at the optimal length and voltage.
RESULTS
Generation of Kv3.1-Deficient Mice.
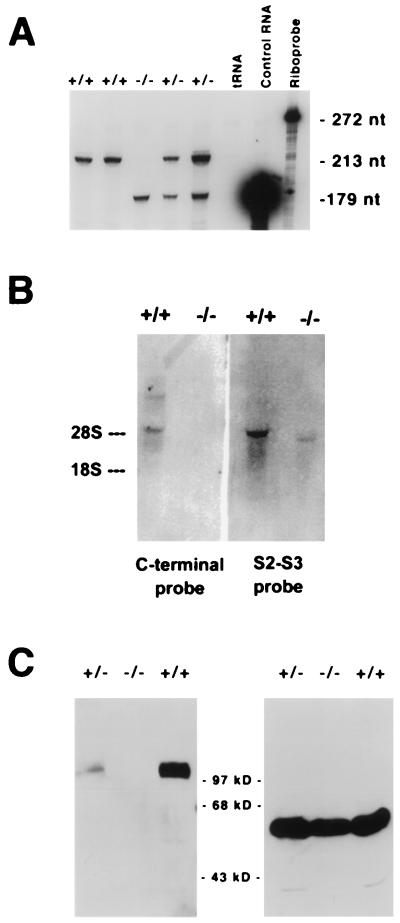
We generated Kv3.1 mutant mice using homologous recombination in ES cells (Fig. 1). To confirm the mutant character, we determined the transcriptional and translational status of the targeted allele. We found that both targeted and nontargeted alleles were transcribed, and, independent of the genotype, transcription levels were approximately equal, suggesting that the insertion of the neo cassette did not affect transcription (Fig. 2A). In contrast to the functional allele, transcription of the targeted allele resulted in a shorter mRNA, probably because transcription was terminated at the new polyadenylation site introduced by the neomycin resistance gene (Fig. 2B). Intact Kv3.1 protein was not found in brain extracts from homozygous Kv3.1−/− mice in contrast to extracts from wild-type mice (Fig. 2C). Compared with wild type, heterozygous Kv3.1+/− mice clearly displayed reduced levels of Kv3.1 protein.
Figure 2.
(A) RNase protection assay. The targeted allele is transcribed at approximately the same level as the functional, nontargeted allele. Ten micrograms of total brain RNA was hybridized to an excess of 32P-labeled probe (272 nt in length) specific for Kv3.1 mRNA. Protected transcripts of the normal allele yielded a fragment of 213 nt after digestion with RNase A and RNase T1; transcripts of the neo cassette-interrupted allele yielded a 179-nt fragment. (B) Northern blot analysis of brain RNA. The targeted allele is transcribed but uses a different polyadenylation site. Two different radioactive probes directed either to the C-terminal end of the coding region of Kv3.1a and -b mRNA or to the S2–S3 region (upstream of the insertion site of the neomycin cassette) were used. The C-terminal probe did not hybridize to the RNA transcribed from the targeted allele; in contrast, the S2-S3 probe identified a Kv3.1-specific RNA that was shorter than the normal Kv3.1b mRNA. The shorter RNA is presumably terminated at the polyadenylation site introduced by the neomycin cassette. (C) Kv3.1 is not expressed in brain of Kv3.1−/− mutants. Western blot analysis performed on brain extract from a Kv3.1+/+ wild type showed a band migrating with an apparent molecular weight of 100 kDa (Left). Extracts from Kv3.1+/− brains showed the same band but at lower intensity. No Kv3.1 protein could be detected in extracts from homozygous Kv3.1−/− null mutant brains. A mAb specific for α2-tubulin was used as a control to monitor comparable amounts of protein on the gel (Right).
Kv3.1-Deficient Mice Are Viable but of Reduced Body Weight.
Heterozygous Kv3.1+/− and homozygous Kv3.1−/− mice were viable and fertile and did not exhibit an obvious mutant phenotype. The ratio of wild-type to heterozygous to homozygous mice was not significantly different from the expected 1:2:1, indicating that Kv3.1 K+ channels are not essential for embryonic development (Table 1). Histological studies did not show an apparent mutant phenotype in gross brain anatomy including the cerebellum.
Table 1.
Genotypes of F2 mice
| Strain | +/+ (%) | +/− (%) | −/− (%) | Total |
|---|---|---|---|---|
| Mixed 129SvEMS × C57BL/6 | 21 (30.9) | 35 (51.5) | 12 (17.6) | 68 |
| Pure 129SvEMS | 6 (18.2) | 22 (66.7) | 5 (15.2) | 33 |
| Overall | 27 (26.7) | 57 (56.4) | 17 (16.8) | 101 |
The ratios of wild-type to heterozygous to homozygous mutants obtained from crosses between F1 animals of 129SvEMS origin or F1 hybrids of mixed background (129SvEMS × C57BL/6) were not significantly different from the expected 1:2:1, indicating that Kv3.1 expression is not required for embryonic development (χ2 = 3.65; P > 0.1).
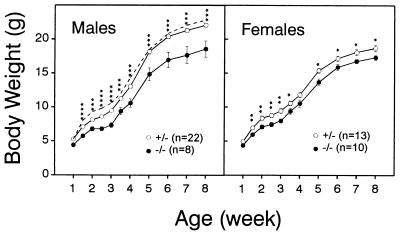
Homozygous Kv3.1−/− mice had significantly lower body weights than their control littermates (Fig. 3). Depending on age, homozygous males (n = 8) were on average 16–22% lighter than their heterozygous siblings (n = 22). The differences were first detected at P10 and increased gradually up to at least 3 months of age (Fig. 3 shows data only up to 8 weeks). In contrast, the growth curves for heterozygous and wild-type males overlapped. A smaller difference in body weight was also evident between heterozygous (n = 13) and homozygous (n = 10) female littermates.
Figure 3.
Growth of Kv3.1+/− and Kv3.1−/− mice. Wild-type and Kv3.1 mutants (129SvEMS) were monitored for their body weights. Male and female homozygous Kv3.1−/− mice had significantly lower weights than their heterozygous littermates (mean ± SE; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001; two-tailed Student’s t test). The dashed line indicates growth of Kv3.1+/+ wild-type males (n = 3; not included in statistical analysis).
Kv3.1−/− Mice Have a Motor-Skill Deficit but Normal Spontaneous Locomotor Activity.
The absence of Kv3.1 channels, which are normally expressed as early as P7 (4, 5) (i.e., before the beginning of synaptogenesis between parallel fibers and Purkinje cells), may change normal excitability properties in the cerebellum and may lead to altered cerebellar physiology. To test for this possibility, we studied mutant mice for the presence of a cerebellar mutant phenotype.
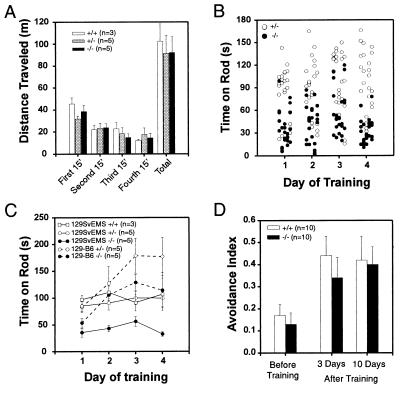
Kv3.1+/+, Kv3.1+/−, and Kv3.1−/− male mice displayed similar spontaneous locomotor activities in an open field test (Fig. 4A). In a test for coordinated motor skills and muscle endurance, however, male homozygous Kv3.1−/− mutants (n = 5) performed significantly worse than their heterozygous Kv3.1+/− littermates (n = 5). Homozygotes were unable to stay for an extended time on a rotating rod that accelerated at 10 rpm/min. Their performance improved neither from one session to the next on the same day (Fig. 4B) nor during a 4-day training period (Fig. 4C). In contrast, heterozygous and wild-type mice could stay on the rod at higher speeds of rotation, even on their first trial. Like homozygous Kv3.1 mutants, heterozygous and wild-type mice of 129SvEMS origin were unable to improve their performance during four days of training (Fig. 4C).
Figure 4.
(A) Spontaneous locomotor activity in the open field. Male littermates (12–14 weeks old) were tested for spontaneous locomotor activity by monitoring their movements in 15-min intervals (mean ± SE). No significant differences among Kv3.1+/+, Kv3.1+/−, and Kv3.1−/− animals were observed. (B) Test for coordinated motor skill. Heterozygous and homozygous male littermates (n = 5 each) were placed on a rotating rod, revolving initially at 5 rpm and accelerating at 10 rpm/min. Performances of individual animals over a 4-day period are plotted. Each column of data represents the five trials of the same animal on that day. The order of animals is the same for each day. Kv3.1+/− mice performed significantly better than their Kv3.1−/− littermates (P < 10−6, ANOVA test). (C) The mean performances for each day are plotted for 4 consecutive days. 129SvEMS mice did not learn during the training period, and the initial differences between Kv3.1+/− (or wild-type) and Kv3.1−/− littermates remained. In contrast, mice of heterogeneous background [F2 offsprings of (C57BL/6 × 129SvEMS)F1 hybrids] improved their performances during the training period; however, the differences between homozygous and heterozygous mutants remained. (D) Active avoidance test. Wild-type and homozygous mutants behaved identically. Before training (i.e., on the first day of the test), Kv3.1+/+ and Kv3.1−/− mice avoided electrical footshocks approximately once out of 10 trials. Three days after the 5-day training period, the performance of wild-type and mutant mice had improved several-fold and remained at that level at least for an additional 7 days.
The Genetic Background Modifies Behavior.
It has been known for some time that different inbred mouse strains exhibit dramatic differences in their behavior (14). More recently, several reports highlighted the problems that may arise when knockout mice of mixed genetic background are studied (15). To reduce physiological and behavioral variability due to gender and genetic differences, most of our studies were done with male littermates on pure 129SvEMS background. When we tested genetically heterogeneous F2 offsprings of (C57BL/6 × 129)F1 hybrids, we obtained strikingly different results. Like mice of pure 129SvEMS origin, heterozygous Kv3.1+/− mutants (n = 5) still outperformed their homozygous Kv3.1−/− littermates (n = 5) on the rotating rod; however, both heterozygous and homozygous mutants of mixed background clearly improved their test scores during training. On the second day of training, homozygous Kv3.1−/− mutants of mixed background performed already better than heterozygous mice of pure 129SvEMS background, and their performance increased again on the third day of training (Fig. 4C).
Kv3.1−/− Mice Show Normal Active Avoidance.
To study acquisition (learning) and retention (memory) of a conditioned response, we subjected wild-type and mutant mice (n = 10 each) to an active avoidance paradigm. Mice were individually placed in a dark chamber from which they could escape through a small opening. After 10 s in the chamber, mice were subjected to a mild continuous footshock. An active avoidance response took place when an individual mouse left the dark chamber within the first 10 s (i.e., before the beginning of the footshock). Each mouse was tested 10 times per day during a 5-day training period (Fig. 4D). On the first day of training, wild-type and homozygous mutant mice avoided the footshock on average only once out of 10 trials. On the eighth day (3 days after the end of training), wild-type and Kv3.1−/− mice had significantly improved their responses and successfully avoided footshocks 3–4 times in 10 trials. On the 15th day (10 days after the end of training), both Kv3.1+/+ and Kv3.1−/− mice still avoided the footshocks ≈4 times out of 10 trials. Both wild-type and homozygous mutant animals improved similarly during the training period, and they retained the active avoidance responses to the same extent for at least 10 days after training.
Kv3.1-Deficient Mice Show Altered Startle Responses.
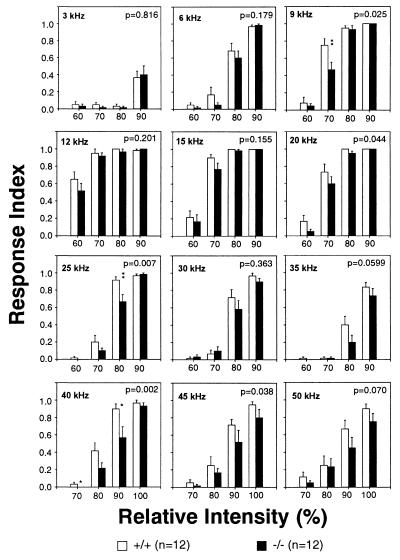
The voltage-gated K+ channel Kv3.1 is expressed in the ventral cochlear nucleus, the superior olive, and the inferior colliculus, several populations of neurons in the auditory pathway involved in signal processing (4–7). We tested mice (n = 12 each) for their startle responses elicited by sounds of different amplitudes and frequencies. Both wild-type and homozygous Kv3.1−/− mice were most sensitive in the frequency range from 9 kHz to 20 kHz with a peak sensitivity around 12 kHz (Fig. 5). At most frequencies the responses of mutants were lower than those of wild-type mice. At 5 of 12 test frequencies we observed significant differences between wild-type and homozygous mutant mice (P < 0.05; two-factor ANOVA test).
Figure 5.
Startle response. Wild-type and homozygous Kv3.1−/− mice were subjected to constant frequency sound (500-ms duration) at four different intensity levels in one session. The intensities are indicated in relative values from 60 to 100, with 100 being the highest possible output of the amplifier-speaker system. The response index is the ratio of the number of startle responses to the total number of trials at that particular intensity level. At five test frequencies a two-factor ANOVA test revealed significant differences (P < 0.05) between wild-type and homozygous mutant animals (the P value is shown in each panel). The asterisks indicate significant differences in multiple comparison tests (Tukey test; ∗, P < 0.05; ∗∗, P < 0.01).
Altered Contractile Properties in Skeletal Muscles of Kv3.1-Deficient Mice.
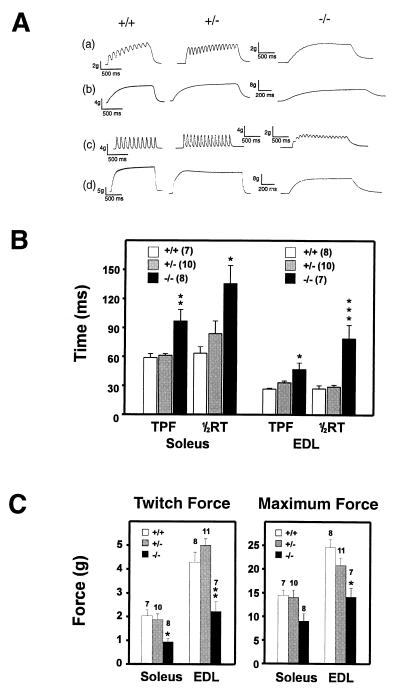
RNase protection experiments showed that, like in the rat, Kv3.1 mRNA was also expressed in mouse skeletal muscle (data not shown), raising the possibility that the inferior performance of Kv3.1−/− mice might reflect a muscle-related phenotype instead of or together with a neuronal one. To determine if the absence of Kv3.1 channels led to changes in muscle contractile properties, we studied in vitro contraction of EDL and soleus muscles. Contractions were induced either by single stimuli or by subtetanic and tetanic stimulations (Fig. 6A). Both EDL and soleus muscles of homozygous mutants showed slower relaxation, and mechanical summation in these muscles occurred at lower frequencies than in those from heterozygous or wild-type mice. For the slow soleus muscle, the TPF and the half time for relaxation was significantly prolonged in Kv3.1−/− muscle compared with Kv3.1+/− or Kv3.1+/+ muscle (Fig. 6B). For the fast EDL muscle, the difference in TPF was not as pronounced as for the slow soleus muscle; however, the half time for muscle twitch relaxation in the Kv3.1−/− mutant was approximately three times that of the heterozygous or wild-type muscle. Hence, the “fast” EDL muscle in homozygous mutants behaved similarly to the slow soleus muscle in heterozygotes or wild type, both in terms of the kinetics of contraction and relaxation. When we determined the forces generated by normal and mutant muscles, the peak forces generated by a single twitch and the maximum forces during a tetanus were significantly reduced in EDL and soleus of homozygotes compared with heterozygotes or wild-type mice (Fig. 6C).
Figure 6.
Contractile properties of fast and slow skeletal muscle in Kv3.1-deficient mice. (A) Soleus (a and b) and EDL muscles (c and d) from Kv3.1+/+, Kv3.1+/−, and Kv3.1−/− mice were subjected to isometric contractions induced by electrical stimulation at either 10 Hz (a and c) or 40 Hz (b and d). Muscle relaxation was slower and mechanical summation occurred at lower frequencies in Kv3.1−/− compared with Kv3.1+/− or Kv3.1+/+ muscles. (B) Time-to-peak-force (TPF) and half-relaxation time (½RT) was measured for single twitches. One-way ANOVA tests followed by Tukey’s multiple comparison tests revealed significant differences for Kv3.1−/− muscles (mean ± SE; n = 7–10 muscles; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001). (C) The forces for a single twitch and for a tetanus were measured for soleus and EDL of wild-type, heterozygous, and homozygous Kv3.1 mice. Both types of skeletal muscles of Kv3.1−/− mutants produced smaller forces than the corresponding muscles from heterozygous or wild-type mice [mean ± SE; n = 7–11 muscles; ∗, P < 0.05; ∗∗, P < 0.01 (Tukey test); P < 0.05 in one-way ANOVA].
Homozygous mutants were smaller than their heterozygous littermates (Fig. 3). To determine whether this size difference reflected a difference in muscle mass, we determined the weights of soleus and EDL muscles. Soleus muscles of wild-type males (n = 7) and heterozygous (n = 4) and homozygous (n = 4) mutants weighed (mean ± SE) 5.84 ± 0.48 mg, 7.88 ± 0.69 mg, and 8.56 ± 1.07 mg, respectively. The apparent increase in muscle weight is not significant because of the small number of measurements. The fast EDL muscles from wild-type males (n = 8) and heterozygous (n = 3) and homozygous (n = 3) mutants weighed 7.44 ± 0.48 mg, 10.8 ± 0.44 mg, and 9.3 ± 0.15 mg, respectively. These results indicated that skeletal muscle mass in mutant mice was not reduced (assuming soleus and EDL are representative). Hence our findings suggest that the lower body weight is not due to reduced skeletal muscle mass.
The Absence of Kv3.1 K+ Channels Does Not Enhance Seizure Activity.
Because activated K+ channels suppress neuronal activity, the absence of a major K+ conductance may lead to spontaneous seizures or increase the frequency of induced seizures. We have not seen an obvious difference in the occurrence of spontaneous seizures between wild type and mutant. So far (after 2 years), we have observed only two mice with spontaneous convulsions, a postpartum mutant female and an injured mutant male. When electrocorticograms of freely moving wild-type mice (n = 3) and homozygous mutants (n = 3) were recorded for 24 h, we neither observed any electrical activity indicative of seizures for wild-type nor for Kv3.1−/− mice (G. Marks, C.S.H., and R.H.J., unpublished observation). Furthermore, intentional vestibular stimulation of wild-type or mutant mice did not induce any observable seizures.
DISCUSSION
Using homologous recombination in ES cells, we have generated a mutant mouse deficient in the gene encoding the subunit of the voltage-gated K+ channel Kv3.1. When expressed as homotetramer, Kv3.1 channels form rapidly activating and deactivating, delayed rectifier-type K+ channel with a high threshold of activation (approximately −10 mV) and a unit conductance (≈25–28 pS) larger than that observed in most other voltage-sensitive K+ channels of the Shaker family (2, 3, 16). Because we did not detect Kv3.1 mRNA during embryonic and early postnatal development in the rat (4) and mouse (data not shown), we expected homozygous Kv3.1-deficient mice to be viable and suitable for the study of physiological and behavioral consequences that result in the absence of a major voltage-gated K+ channel. The targeted Kv3.1 allele is transcribed both in heterozygous and homozygous mutants but the transcript is not translated into full-length Kv3.1 K+ channels (Fig. 2). In Kv3.1+/− mice, the amount of Kv3.1 protein on a Western blot was reduced to a low level compared with that of wild type; in contrast, expression of the Shab-related K+ channel Kv2.1 (17) was not changed (data not shown).
The transcript of the targeted allele could be translated into an N-terminal Kv3.1 fragment (ending passed S2) that would not have been detected. Because the N terminus is involved in subunit interaction (18), the expression of a truncated subunit could interfere with proper channel assembly and generate a dominant negative effect. We currently do not know if a truncated Kv3.1 protein is synthesized. If it were, it would probably have no functional consequences, because we could not detect any phenotypic alterations between Kv3.1+/+ and Kv3.1+/− mice.
Kv3.1−/− Mice Have Reduced Body Weight.
Homozygous Kv3.1−/− mice are viable and fertile (Table 1) indicating that functional Kv3.1 K+ channels are neither essential for embryonic development nor for reproduction. The most apparent difference of Kv3.1−/− mice is their lower body weight. Depending on age, homozygous mutant males are on average up to 22% lighter than their heterozygous and wild-type littermates; in fact, they are similar in size to heterozygous females (Fig. 3). Homozygous mutant females are on average only 5% smaller than their heterozygous littermates. The reasons for the reduced weight and size are currently not clear. Skeletal muscle contributes substantially to total body weight (40% in man); however, the weights of soleus and EDL muscles from wild-type, heterozygous, and homozygous males were not significantly different. It appears, therefore, that the lower weight cannot be attributed to a reduction in muscle tissue. Initial studies suggested no difference in food and water consumption between adult male Kv3.1+/− and Kv3.1−/− mice, making it unlikely that hypophagia in adult animals was the cause of the lower body weight (data not shown).
Behavioral Alterations in Kv3.1-Deficient Mice.
Homozygous and heterozygous littermates and wild-type mice showed no activity differences in the open field. In the rotarod test, however, when mice had to maintain posture control, balance, and endurance under conditions requiring enhanced motor skills, homozygous mutants performed far worse than their heterozygous or wild-type littermates. If one converts “time-on-rod” to the total distance covered, then heterozygous mutants and wild-type mice could run on the rotating rod four to eight times as long as Kv3.1−/− mice. Neither wild-type mice nor heterozygous or homozygous mutants on the 129SvEMS background improved during the 4-day training period. These findings are in stark contrast to the steadily improving performance of mice on a mixed genetic background, and they emphasize the importance of genetically well defined animals when conducting behavioral tests (15).
When mice were conditioned to actively avoid an unpleasant stimulus (electrical footshock), we could not detect any significant differences between wild-type and homozygous animals, indicating that the acquisition of this task (learning) and its retention (memory) was not detectably affected in Kv3.1-deficient mice. It is noteworthy that wild-type and mutant mice on 129SvEMS background avoided footshocks only ≈40% of the time even after extensive training. In contrast, it has been reported that mice on C57BL/6 background achieve an avoidance index of up to 0.8 (19).
Muscular Versus Neuronal Phenotype.
Currently, we cannot decide unequivocally if the impaired performance on the rotarod test is of neuronal (e.g., a form of cerebellar ataxia) or skeletal muscle origin, or both. Our in vitro studies with isolated muscle preparations suggest that a skeletal muscle-related motor deficit is likely. For homozygous Kv3.1 mutants, the TPF and the half time for relaxation are significantly prolonged for EDL and soleus. Moreover, the forces generated by a single muscle twitch and the maximum tetanic forces are significantly reduced in Kv3.1-deficient mice. The fast EDL muscle of homozygous Kv3.1−/− mice shows properties similar to those of wild-type soleus muscle—i.e., Kv3.1-deficient mice no longer contain fast skeletal muscle (if the result for EDL is representative for other fast muscles as well). The TPF is also prolonged, particularly for soleus muscle. In the absence of a K+ channel with a large conductance (25–28 pS), whose major role is to drive the membrane potential of the depolarized sarcolemma rapidly back toward the potassium equilibrium potential (EK), the muscle membrane may not repolarize fast enough for all voltage-gated Na+ channels to recover from inactivation in time to initiate the next action potential.
Kv3.1 K+ channels are expressed in several neuronal populations involved in auditory signal processing, including the ventral cochlear nucleus, the superior olive, and the inferior colliculus (4–7). Recently, it has been shown that a K+ channel with kinetic and pharmacological properties reminiscent of Kv3.1 participates in postsynaptic signal integration in the medial nucleus of the trapezoid body, a brainstem relay nucleus involved in sound source localization (20). We saw significant differences when we compared wild-type and Kv3.1−/− mice in their response to sounds of different amplitudes and frequencies. In the range from 6 kHz to 50 kHz, the normal auditory frequency range of mice (21), we consistently found that wild-type mice startled more easily than their Kv3.1-deficient littermates; however, the frequency of their peak sensitivity (≈12 kHz) was not changed. This indicates that Kv3.1−/− mice can hear when a major K+ conductance is absent in neurons involved in auditory signal processing.
The presence of Kv3.1 channels in the cochlear nucleus, the superior olive, the medial nucleus of the trapezoid body, and the inferior colliculus may endow these neurons with a rapid-firing capability similar to fast-spiking GABAergic interneurons. Although rapidly firing neurons are necessary to maintain phase-locked action potentials, this process is mainly important at low frequencies below the peak sensitivity for the startle response. Apart from altered sensory processing, it is also possible that the execution of the startle response may be affected at the level of the motor system because the contractile properties of skeletal muscles are substantially changed in Kv3.1−/− mice. Moreover, Kv3.1 mRNA is expressed in the dorsal horn (6), a region containing inhibitory interneurons involved in the control of antagonistic muscles. Currently, we cannot decide which of these processes is altered, but the fact that we consistently observed significant differences suggests that one or more of these systems are affected in Kv3.1-deficient mice.
Mutations in Other K+ Channel Genes.
Mice deficient in the Shaker-related voltage-gated K+ channel Kv1.1 show spontaneous, periodic seizures (B. Tempel, personal communication). Contrary to expectation, the absence of Kv3.1 does not lead to enhanced spontaneous seizure activity. It is remarkable that lesions in different K+ channel genes (Kv1.1 and Kv3.1) belonging to the gene family of voltage-gated K+ channels lead to entirely different phenotypes.
How prevalent are ion channel mutations in the human population? During the last few years, three inherited K+ channel disorders have been discovered: two different forms of long QT syndrome and episodic ataxia (22–24). Some human ion channel diseases, including episodic ataxia, hyperkalaemic periodic paralysis, and paramyotonia congenita, display mild, intermittent symptoms that are detectable only under unusual or stressful conditions; otherwise patients appear normal or near normal (25). Other human myopathies linked to abnormal ion channels are life-threatening—e.g., long QT syndrome or other cardiac arrhythmias and myotonia permanens. It is possible that ion-channel “knockout” mice showing subtle physiological or behavioral alterations, like the Kv3.1 mutant, foreshadow pathological conditions already present in some individuals of the human population. Knowledge of the physiology of ion channel-deficient mice may help to develop diagnostic and therapeutic procedures for individuals at risk. Altered or modified K+ channel function has been implicated in disorders as diverse as the epilepsies, cardiac arrhythmias, altered insulin secretion, and T cell mediated immune responses, and it is no surprise that K+ channels have become pharmacological targets of great interest and therapeutic potential.
Acknowledgments
We would like to thank Drs. A. Bradley for the embryonic stem cell line AB1, R. Hammer for an aliquot of AB1 and STO cells, P. Soriano for the neomycin cassette, M. Geppert and K. Kamm for advice, and R. Moreadith and K. Graves for blastocyst injection. We also thank Dr. B. Rudy for anti-Kv3.1 antibodies and Dr. S. Brady for anti-α2-tubulin antibody. This work was supported in part by the Kent Waldrep National Paralysis Foundation and by a grant of the Muscular Dystrophy Association (R.H.J.). All animal protocols were approved by the Institutional Animal Care and Use Committee.
Footnotes
Abbreviations: ES cell, embryonic stem cell; EDL, extensor digitorum longus; TPF, time-to-peak-force.
References
- 1.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 2.Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. FEBS Lett. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- 3.Luneau C J, Williams J B, Marshall J, Levitan E S, Oliva C, Smith J S, Antanavage J, Folander K, Stein R B, Swanson R, Kaczmarek L K, Buhrow S A. Proc Natl Acad Sci USA. 1991;88:3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewe J A, Verma S, Frech G C, Joho R H. J Neurosci. 1992;2:538–548. doi: 10.1523/JNEUROSCI.12-02-00538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perney T M, Marshall J, Martin K A, Hockfield S, Kaczmarek L K. J Neurophysiol. 1992;68:756–7766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- 6.Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiser M, Bueno E, Sekirnjak C, Martone M E, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. J Neurosci. 1995;15:4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz S, Perney T M, Robbins Y Q E, Chesselet M-F. Synapse. 1994;18:55–66. doi: 10.1002/syn.890180108. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Zhang L, Weiser M, Rudy B, McBain C J. J Neurosci. 1996;16:506–518. doi: 10.1523/JNEUROSCI.16-02-00506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grissmer S, Cahalan M D, Chandy K G. J Immunol. 1988;141:1137–1142. [PubMed] [Google Scholar]
- 11.Grissmer S, Nguyen A N, Aiyar J, Hanson D C, Mather R J, Gutman G A, Karmilowicz M J, Auperin D D, Chandy K G. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 12.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Greene/Wiley; 1987. [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 14.McClearn G E. In: The Mouse in Biomedical Research. Foster H L, Small J D, Fox J G, editors. Vol. 4. New York: Academic; 1982. pp. 37–49. [Google Scholar]
- 15.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 16.Stühmer W, Ruppersberg J P, Schröter K H, Sakmann B, Stocker M, Giese K P, Perschke A, Baumann A, Pongs O. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frech G C, VanDongen A M J, Schuster G, Brown A M, Joho R H. Nature (London) 1989;340:642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Yu W, Jan Y N, Jan L Y, Li M. J Biol Chem. 1995;270:24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- 19.Goldowitz D, Koch J. Behav Neural Biol. 1986;46:216–226. doi: 10.1016/s0163-1047(86)90696-5. [DOI] [PubMed] [Google Scholar]
- 20.Brew H M, Forsythe I D. J Neurosci. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay R R. Hearing in Vertebrates: A Psychophysics Databook. Winnetka, IL: Hill-Fay Associates; 1988. [Google Scholar]
- 22.Browne D L, Gancher S T, Nutt J G, Brunt E R P, Smith E A, Kramer P, Litt M. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 23.Curran M E, Splawski I, Timothy K W, Vincent G M, Green E D, Keating M T. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Curran M E, Splawski I, Burn T C, Millholland J M, VanRaay T J, Shen J, Timothy K W, Vincent G M, de Jager T, Schwartz P J, Towbin J A, Moss A J, Atkinson D L, Landes G M, Connors T D, Keating M T. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann-Horn F, Rüdel R. Curr Opin Neurol. 1995;8:402–410. doi: 10.1097/00019052-199510000-00014. [DOI] [PubMed] [Google Scholar]