Abstract
Injection of cDNA encoding the neuronal α7 subunit into Xenopus oocytes yields homomeric receptors showing responses to AcCho that have low affinity, fast desensitization, nonlinear current–voltage (I–V) relation, and sensitivity to α-bungarotoxin (α-BTX) and 5-hydroxytryptamine (5HT), both substances acting as antagonists. Mutation of the Leu-247, located in the channel domain, changes 5HT from an antagonist to an agonist, slows the rate of desensitization, renders the I–V relation linear, and increases the affinity for acetylcholine (AcCho). A study was made of receptors expressed after injecting Xenopus oocytes with mixtures of cDNAs encoding the wild-type α7 (WT α7) and the L247T α7 mutated nicotinic AcCho receptors (nAcChoRs). The receptors expressed were again blocked by α-bungarotoxin (100 nM) but exhibited both WT α7 and α7 mutant functional characteristics. Out of eight different types of hybrid receptors identified, most were inhibited by 5HT (1 mM) and showed low sensitivity to AcCho, like the WT α7 receptors, but exhibited a slow rate of desensitization and an I–V relation similar to those of α7 mutant receptors. Together, these findings indicate that the increased nAcChoR affinity and the decreased nAcChoR desensitization after Leu-247 mutation are uncoupled events. We propose that receptor diversity is predicted by permutations of WT α7 and L247T α7 subunits in a pentameric symmetrical model and that even partial replacement of Leu-247 with a polar residue within the leucine ring in the channel domain considerably influences the properties of neuronal α7 nAcChoRs.
Keywords: nicotinic receptors/5-hydroxytryptamine/acetylcholine/Xenopus oocytes
The α-bungarotoxin (α-BTX)-sensitive homomeric α7 neuronal nicotinic acetylcholine (AcCho) receptors (nAcChoRs) expressed in Xenopus oocytes are noncompetitively inhibited by the transmitter serotonin (5-hydroxytryptamine, 5HT) and exhibit fast desensitization, considerable inward rectification of AcCho-evoked current (IAcCho), and relatively low affinity for AcCho (1–3). L247T mutation of the highly conserved leucine residue in the channel domain of the α7 receptor converts 5HT from antagonist to agonist, abolishes the inward rectification, increases the affinity for AcCho, and considerably decreases the rate of nAcChoR desensitization (3–5).
It is believed that, in the closed state, the “leucine ring” mode of association of the nAcChoR α-helices forms a constriction in the channel and that this is disfavored when the transmitter induces receptor conformational changes and creates an open pore (6). However, in the muscle-type nAcChoR, the highly conserved Leu-251 residue, which corresponds to the Leu-247 residue in the homomeric neuronal nAcChoR, has been shown to influence the channel duration but does not serve as a channel gater per se (7, 8). In the homomeric α7 neuronal nAcChoR the leucine ring is said to occlude the channel in the receptor desensitized state (4, 5). The substitution of threonine for leucine enhances the apparent binding affinity for AcCho and decreases the rate of receptor desensitization, suggesting that the Leu-247 converts the desensitized state of the nAcChoR channel into a conducting state with high affinity for AcCho (4, 5, 9).
We coinjected the wild-type (WT) α7 and the L247T α7 mutant cDNAs into Xenopus oocytes and studied the macroscopic current responses to AcCho of the receptors expressed. With a few exceptions, this resulted in the expression of hybrid nAcChoRs with functional profiles that reflect combinations of the profiles of the individual types of homomeric receptors. Our findings suggest that interruptions in the leucine-ring, which occur in heteromeric receptors composed of WT α7 and α7 mutant subunits, considerably influence the receptor functional profile.
MATERIALS AND METHODS
Oocyte Injection.
The cDNAs encoding the chicken neuronal nAcChoR subunits were kindly provided by Marc Ballivet. Full-length cDNAs encoding the WT α7 or L247T α7 neuronal nAcChoR subunits were expressed as described previously (4). Preparation of oocytes and nuclear injection procedures were as detailed elsewhere (10, 11). Stage VI oocytes were injected intranuclearly with cDNA clones using a pressure microinjector (Eppendorf) and a Singer micromanipulator (United Kingdom).
Electrophysiological Recordings.
Membrane currents were recorded 1–4 days after injection, using a voltage-clamp technique with two microelectrodes filled with 3 M KCl (12). The oocytes were placed in a recording chamber (volume, 0.1 ml) perfused continuously with oocyte Ringer (82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1 mM MgCl2/5 mM Hepes/adjusted to pH 7.4 with NaOH) at controlled room temperature (20–21°C) in the presence of atropine (1 μM). Unless otherwise indicated, all of the measurements were performed with the membrane held at −100 mV. To construct dose–response relationships, the oocytes were held at −50 mV and the drugs were applied to the oocyte at 3-min intervals. Current–voltage (I–V) relationships were determined using repetitive exposures to AcCho (0.2 and 100 μM) at various potentials, stepping the holding potential from −50 mV to the required voltage 5 to 10 s before transmitter application. Drugs were dissolved in oocyte Ringer solution and applied by superfusing the oocyte at a flow rate of 12 ml/min. Solution exchange was achieved by using electromagnetic valves (type III; General Valve, Fairfield, NJ). 5HT·HCl was dissolved just before an experiment. Drugs and chemicals were purchased from Sigma.
Experimental Analysis.
The current records were digitized at 50–200 Hz using an analog-to-digital converter (Digidata 1200 Interface, Axon Instruments, Foster City, CA) and stored on a computer for subsequent analysis, using pClamp 6.0.2 routines (Axon Instruments). For more details, see ref. 3.
To determine the half-dissociation constant (EC50) of AcCho, data were fitted, using nonlinear fitting routines (included in sigma plot, Jandel, San Rafael, CA), to the Hill equation:
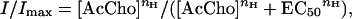 |
1 |
where [AcCho] is the transmitter dose, nH is the Hill coefficient, and Imax is the maximum response. When data were fitted to a sum of two or three Hill equations, considering the hypothesis that multiple nAcChoR populations were expressed (i.e., WT, mutant, and hybrid α7 nAcChoRs), the nH and EC50 values determined for receptor populations of WT α7 and/or L247T α7 nAcChoRs in control experiments were imposed into the equation.
To compare the behavior of different types of nAcCho populations easily, we defined the following parameters. The receptor sensitivity to the transmitter was estimated by calculating the ratio (in percentage) of the current elicited by 0.2 μM AcCho (I0.2) to that elicited by 100 μM (I100). These AcCho concentrations correspond approximately to the EC50 values for L247T α7 (∼0.2 μM; refs. 3–5) and α7 nAcChoRs (∼100 μM; refs. 1 and 3), respectively. The time to half-decay (t½) of IAcCho, defined as the time taken for the current to decay from the peak to half of its value, was used to estimate the rate of receptor desensitization. The t½ of hybrid receptor IAcCho was then compared with t½ of WT α7 IAcCho and was expressed as n-fold increase. The rectification coefficient (nγ), which is the deviation of the I–V curve from linearity, was estimated by the ratio of slope conductances at +20 mV vs. −60 mV, and ranged (in percentage) between 100% (i.e., linear I–V relationship) and 0% (i.e., full rectification).
RESULTS
Properties of Neuronal WT α7 nAcChoR.
Oocytes injected with the WT α7 subunit cDNA responded to AcCho with an inward current (IAcCho) whose peak amplitude depended on transmitter concentration. AcCho at 100 μM (≈EC50; refs. 1–3) elicited an inward current (I100) with a mean peak amplitude of 2.9 μA (n = 11; six donors, range: 125 nA to 9.5 μA), while at 0.2 μM the AcCho current (I0.2) averaged 105 nA (n = 11; six donors; range: 0–400 nA). The ratio [(I0.2/I100) × 100], used as a parameter of receptor sensitivity to AcCho, averaged 2.3 ± 1.1% (range: 0–6%). I100 decayed with t½ = 199 ± 115 ms (mean ± SD; range: 95–590 ms; n = 16; six donors), indicating a fast rate of nAcChoR desensitization. The rate of desensitization was considerably slower at 0.2 μM AcCho (t½ = 2.6 ± 0.8 s; range: 1.6–3.9 s; n = 6; three donors).
In agreement with our previous observations (3), coapplication of 5HT (10–100 μM) with AcCho (10–100 μM) markedly reduced the IAcCho peak amplitude, and I100 was completely suppressed by 5HT at 1 mM concentration (n = 6; three donors).
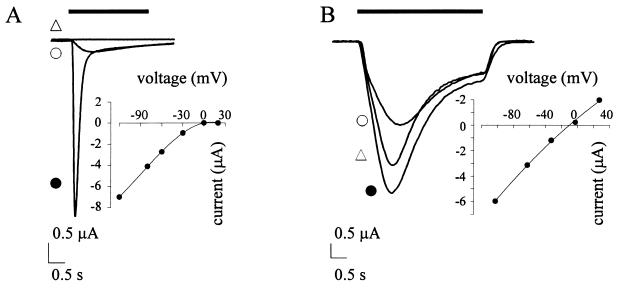
It has already been reported (1, 3) that the IAcCho–voltage relation of WT α7 receptors shows a strong inward rectification at positive potentials. Since the slope conductance at +20 mV was close to zero, the coefficient nγ was very small and ranged between 0 and 8% (5.8 ± 1%; n = 8; four donors), indicating that, under our experimental conditions, homomeric WT α7 receptors again displayed a considerable rectification at positive potentials (Fig. 1A).
Figure 1.
Membrane currents in oocytes expressing homomeric WT α7 (A) and α7 mutant (B) receptors. (A) Superimposed traces and I–V relationship in an oocyte injected with cDNA (2 ng) encoding the α7 nAcChoR. Holding potential −80 mV. Note (i) the inhibition of IAcCho when 5HT (1 mM) was coapplied with AcCho (100 μM); (ii) the marked inward current rectification in the I–V curve; (iii) and the much smaller current elicited by 0.2 μM compared with 100 μM AcCho. (B) Superimposed traces and I–V relationship in another oocyte from the same donor, injected with cDNA (2 ng) encoding for L247T α7 mutant. Note (i) the slower desensitization and (ii) the reduced rectification compared with A. Note also that 5HT is acting as agonist and that the current amplitude at 0.2 μM is ≈50% of that at 100 μM AcCho. AcCho concentration used for I–V relations, 100 μM. The solid line in I–V relations represents a second-order polynomial fit to the data. Bars indicate the time of drug application. Symbols near traces: ▵, 5HT (1 mM) coapplied with AcCho (100 μM) in A or applied alone in (B); •, AcCho at 100 μM; ○, AcCho at 0.2 μM.
Properties of L247T α7 nAcChoR.
It is well established that in oocytes injected with the L247T α7 mutant the IAcCho is maintained for a longer period (3–5) (Fig. 1B). In the present experiments I100 peaked to 4.5 μA (range: 2–9.7 μA; n = 11, six donors) and decayed with t½ = 2.4 ± 1.8 s in four cells and t½ > 10 s in seven other oocytes indicating a relatively slow rate of nAcChoR desensitization. I0.2 recorded in these 11 oocytes peaked to 2 μA (range: 495 nA to 3.7 μA). The ratio [(I0.2/I100) × 100] averaged 47 ± 11% (range: 34–60%), supporting the fact that I0.2 represents ≈50% of the current response at plateau concentrations of AcCho (3–5).
In agreement with data reported previously (3), 5HT applied to oocytes expressing the L247T α7 nAcChoRs gave rise to large inward currents whose amplitude depended on the concentration of 5HT. The maximum 5HT-evoked current (3.5 μA, range: 810 nA to 7.4 μA; n = 8; six donors) was attained at 1 mM.
The amplitude of IAcCho increased linearly at hyperpolarized potentials and showed a very slight rectification at positive potentials. The nγ ranged from 40 to 100% (86 ± 21%; n = 10; four donors), indicating that the I–V relationship in L247T α7 nAcChoR is nearly linear (Fig. 1B).
Properties of nAcChoRs Composed of Both α7 and α7 Mutant Subunits.
In control experiments we found little difference in the peak amplitude of currents elicited by transmitter concentrations corresponding to the EC50s, of α7 mutant- and WT α7-injected oocytes (IAcCho ratio, 0.6–2; 2 ng of injected cDNA). In addition, no significant differences in IAcCho peak amplitude, ranging between 0.8 and 5 μA, were observed when oocytes were injected with increasing amounts of cDNA (1–6 ng) encoding either WT α7 or the α7 mutant (see also ref. 10). These findings suggest that, under our experimental conditions, the receptor expression in WT α7- and α7 mutant-injected oocytes was similar.
Oocytes injected with a mixture of cDNAs encoding the WT α7 and α7 mutant subunits (ratio 1:1) expressed receptors that were activated by AcCho and were blocked by α-BTX (100 nM; n = 6; two donors; e.g. see Fig. 2A) but exhibited a striking functional diversity. This diversity was obviously related to the ratio of cDNAs in the mixture only when this ratio was markedly displaced in favor of either one of the subunits, possibly because of wide fluctuations in the relative expression of WT α7 vs. mutant α7 subunits. For instance, no clear relationship was found between nAcChoRs functional properties and cDNA ratio (1:2; 1:3), in 19 oocytes (four donors). However, in three out of four oocytes (one donor) that were injected with a 1:4 WT α7/L247T α7 ratio, the nAcChoRs showed an α7 mutant-like functional profile, whereas in the remaining cell the nAcChoRs exhibited functional characteristics of both WT α7 and α7 mutant nAcChoRs and in three oocytes (one donor) that were injected with a 6:1 WT α7/L247T α7 cDNA ratio; the nAcChoRs expressed showed a WT α7-like functional profile.
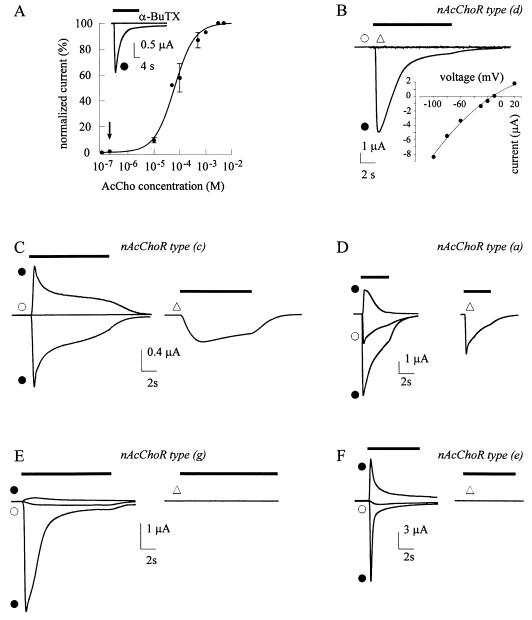
Figure 2.
Membrane currents from oocytes injected with a mixture of cDNAs (ratio 1:1) encoding the WT α7 and L247T α7 subunits. (A) AcCho dose–IAcCho response relationship best fitted to Equation 1. The peak AcCho currents were normalized to that evoked by 5 mM AcCho (IAcCho mean peak amplitude: −3.8 μA). Each point represents the mean ± SD (n = 6; four donors). Data fits to a function consisting of the sum of two or three Eq. 1 were not statistically different from the fit with a single Hill equation and minor number of parameters (F-distribution test; ref. 13). Data derived from six mixture-injected oocytes expressing mainly c, d, e, f, g or h type nAcChoRs (Table 1). Inset shows an example of IAcCho blocked by α-BTX (100 nM) in one of the six oocytes examined. (B) Example of IAcCho elicited by activation of mainly d type receptor population (see Table 1) and corresponding I–V relation. Note the inhibitory action of 5HT, the lack of response to 0.2 μM AcCho, and the slow desensitization. (C) Example of IAcCho evoked by the activation of mainly c type receptor population. Note the lack of response to 0.2 μM AcCho, the outward IAcCho at +20 mV (left), and the inward current elicited by 5HT. (D) Example of currents evoked by activation of a type nAcChoRs. Note a measurable IAcCho at 0.2 μM AcCho and outward IAcCho at +20 mV (left) with I0.2/I100 = 38%. (E) Superimposed currents (left) and lack of 5HT response in an oocyte that expressed a g type nAcChoR population. Note relatively small outward IAcCho at +20 mV. (F) Another oocyte expressing type e nAcChoRs population. Note the fast desensitizing inward IAcCho, outward IAcCho at +20 mV (superimposed) with differences in the desensitization and the lack of response to 5HT (right). Bars, symbols and I–V curve, as Fig. 1. B, D, and E, oocytes from same donor; C and F, from two other donors. Note differences in the current desensitization at positive potentials in C–F.
In most of the oocytes injected with a 1:1 cDNA ratio, the hybrid nAcChoRs displayed a relatively low neurotransmitter sensitivity {[(I0.2/I100) × 100] < 6%; 26 oocytes out of 31 tested, 26/31} and were partially (5/31) or completely inhibited by 1 mM 5HT (13/31), with a clear trend toward the properties of WT α7 nAcChoR (1–3). In the remaining 13 oocytes, 5HT (1 mM) acted as an agonist, eliciting an inward current with quite variable peak amplitude (range: 10 nA to 5.5 μA). On the other hand, in most oocytes the hybrid nAcChoRs desensitized slowly in the continuous presence of the transmitter (24-fold increase of t½; 25/31); and displayed rather linear I–V relationships (nγ = 78 ± 28%; range 23–100%; 23/31), closely resembling the functional profile of the α7 mutant receptors. In about 20% of the oocytes studied (7/31), all of the analyzed functional properties of the nAcChoRs were strongly biased toward those of either WT or mutant nAcChoRs, although they did not show the full behavior of WT or mutant α7 receptors. For instance, in these oocytes, the agonist or antagonist action of 5HT was weaker than in control oocytes (compare Figs. 2D and 1B). Table 1 gives a summary and Fig. 2 gives representative examples of functional profiles of the hybrid nAcChoRs identified.
Table 1.
α7 wild-type-like and α7 mutant-like properties of hybrid AcChoRs expressed in oocytes injected with a mixture of WT α7 and L247T α7 cDNAs
| nAcChoR type | N | 5HT action | Sensitivity | Desensitization | Rectification |
|---|---|---|---|---|---|
| a | 4/2 | M | M (15–38%) | M (27–126%) | M (56–100%) |
| b | 3/1 | WT | WT (0.4–3%) | WT (1.3–2%) | WT (0.8–5%) |
| c | 7/3 | M | WT (0–6%) | M (7–13%) | M (65–100%) |
| d | 8/4 | WT | WT (0–3%) | M (7–27%) | M (33–100%) |
| e | 3/1 | WT | WT (1.8–2%) | WT (1.2–1.3%) | M (23–63%) |
| f | 1/1 | WT | M (30%) | M (20%) | M (25%) |
| g | 3/2 | WT | WT (1.5–4%) | M (7–12%) | WT (5–6%) |
| h | 2/2 | M | WT (3–4%) | M (13–18%) | WT (5–7%) |
N, number of oocytes/number of donors. M, mutant-like properties. WT, wild-type-like properties. Numbers in parentheses, range of variation. For 5HT action M and WT indicate 5HT acting as agonist or antagonist, respectively. For sensitivity M and WT mean [(I0.2/I100) × 100] ≥ 14% or ≤ 6%, respectively; in desensitization, they signify t1/2 n-fold increase ≥4.5% or ≤2.5% (compared with α7 wild type), respectively; and in rectification, nγ ≥ 23 or ≤ 9, respectively. Upper and lower limits for M and WT properties were established by considering mean values (±3σ) of the parameters for the control homomeric nAcChoRs. cDNAs ratio, 1:1.
The ratio I0.2/I100 reflects the receptor apparent affinity well since six oocytes, expressing mainly the c, d, e, f, g, or h types of nAcChoRs, with [(I0.2/I100) × 100] < 6% required a relatively high AcCho concentration for half-maximal response (EC50 = 62 ± 9 μM; nH = 1.12, range: 0.8–1.6 in log–log coordinates at AcCho ≤ 10 μM; four donors; Fig. 2A), while three oocytes (one donor) showing [(I0.2/I100) × 100] > 14% required a lower AcCho concentration for half-maximal response (EC50 = 1.9 ± 1 μM; nH = 1.0; not shown). These dose–response curves were all best fitted by a single Hill equation rather than by an equation composed of a sum of two or three Hill equations, representative of heterogenic nAcChoR populations (14): in this case, WT, L247T, and hybrid α7 nAcChoR. However, 12 oocytes (four donors) injected first with WT α7 cDNA and reinjected 48 h later with α7 mutant cDNA (ratio 1:1), exhibited dose–response curves best fitted by a sum of two Hill equations (i.e. homomeric and hybrid nAcChoRs) when IAcCho was recorded within 24 h, but to a single Hill plot 36 h after the second intranuclear cDNA injection. Similar results were obtained with the same experimental protocol but inverting the order of cDNAs injected. Moreover, failures to respond to 0.2 μM AcCho (≈EC50 of the α7 mutant), incompatible with the expression of a pure α7 mutant receptor population, were observed in seven oocytes (two donors) that exhibited slow desensitization and a rather linear I–V (nγ = 81 ± 25). Together, these data suggest that, with a few exceptions, homomeric WT α7 or L247T α7 nAcChoRs are not expressed in sufficient numbers to be detected by Hill plot analysis.
DISCUSSION
A general view is that the conserved leucines in the M2 domain of ligand-gated channels are involved in receptor–channel gating. However, it is not yet definitely established whether the leucine ring is the determinant of channel gating properties (6–8) or whether its role is restricted to receptor desensitization processes (4, 5). In this study, coinjection of cDNAs encoding neuronal WT α7 and L247T α7 subunits into Xenopus oocytes was used as a tool to investigate the effects of leucine substitution, in at least one of the five α7 subunits, on the properties of the receptors expressed. Our findings clearly show that coexpression of L247T α7 mutant and WT subunits greatly increases the diversity of nAcChoRs. In addition to receptors possessing mainly properties similar to homogenic WT α7 or α7 mutant receptors, six novel types of nAcChoR populations have been identified with singular functional profiles but also sharing some properties. For instance, (i) although with varying potency, 5HT acts as an antagonist, like on WT α7 receptors, on hybrid receptors of types d, f, and g (Table 1), but these receptor populations exhibit very slow desensitization, like α7 mutant receptors. (ii) The c, d, g, and h populations show low transmitter sensitivity, like WT α7 receptors, but desensitize slowly like the mutant. (iii) The c and h populations are activated by 5HT, like the mutant, but resemble the WT α7 in sensitivity. Taken together, these findings show that combinations of α7 subunits with L247T-mutated subunits yield a variety of receptors with functional characteristics that consist of a blend of the properties of WT α7 and L247T mutant homogenic receptors.
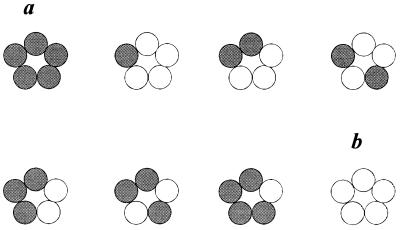
We propose that this nAcChoR diversity may be predicted by the eight possible combinations of α7 WT and L247T α7 mutant subunits in a symmetrical model of the hybrid receptor (Fig. 3). In this model, both the WT α7/L247T α7 subunit ratio and the subunit disposition yield receptor diversity. In other words, not only the number of leucines replaced but also the interruptions in the leucine ring, and their localization, determine the functional properties of hybrid α7 nAcChoRs. This appears to be in contrast with the conclusions drawn for the muscle nAcChoR in which Leu mutations are multiplicative (7) but equivalent (8). In our model instead, not only the number but also the position of Leu mutations would be important in determining receptor profiles such as those identified in Table 1.
Figure 3.
Diagram illustrating the pentameric symmetrical model and predicted hybrid nAcChoR diversity. The model assumes five equivalent subunits and results in eight different combinations, a number equivalent to the identified receptor populations (Table 1). The algorithm calculating the number of possible permutations in a symmetrical model, when the number of receptor subunits n is a prime number, is the following: {[(2n − 2)/n] + 2}. White circles represent WT α7 subunits; gray circles, L247T α7 subunits. a and b hypothetically refer to L247T mutant-like and WT-like nAcChoRs, respectively, identified in Table 1.
Hill plot analyses of AcCho currents (of oocytes injected with the mixtures of cDNAs) indicate that the receptors expressed are not preponderantly homomeric WT α7 and L247T α7 receptors. Furthermore, the variety of functional profiles observed in individual oocytes (Table 1) is a strong indication that hybrid receptors are expressed. Nonetheless, it is surprising that, in spite of the relatively small sample examined so far, the oocytes fall along fairly well defined types. This could happen if populations of receptors are preferred in individual oocytes, perhaps guided by the type of receptor first assembled and inserted in the plasma membrane.
It is thought that the Leu-247 mutation within the channel domain of the α7 subunit renders conductive one of the high affinity desensitized states of the receptor (4, 5). This is inferred from the fact that L247T α7 mutant receptors show a high affinity for AcCho, typical of desensitized nAcChoRs and that those receptors mediate a slowly desensitizing current. In here we show that oocytes injected with a mixture of WT α7 and L247T α7 cDNAs respond to AcCho with a slowly desensitizing current but exhibit a reduced receptor affinity (e.g. Fig. 2 and Table 1). This suggests that increased receptor affinity and reduced receptor desensitization, after incorporation of Leu-247-mutated subunits, are uncoupled events. Thus, the picture that emerges from our findings is that a conserved leucine residue within the channel domain markedly influences the gating properties of the channel but appears not to be linked directly to the mechanism of nAcChoR desensitization.
In the last few years, a considerable number of mutations of single amino acids, some of which may be the structural determinants of neurological disorders, have been found to affect nAcChoR function greatly (15–17). In particular, a large body of evidence demonstrates that mutations of highly conserved leucines in the leucine ring appreciably alter the function of both neuronal α7 and muscle αβγδ nAcChoRs (4–8). Our findings extend the notion that Leu-247 plays an important role in determining the properties of α7 receptors. They also show that in hybrid receptors the L247T mutation appears to be dominant in determining some receptor characteristics, such as desensitization and rectification, while the mode of action of 5HT and sensitivity depend more on the wild-type subunit.
Acknowledgments
We thank Drs. Francesca Grassi, Sergio Fucile, Rogelio Arellano, and Jesus Garcia–Colunga for critical reading of the manuscript and Dr. Laura Maggi for help. This work was supported in part by Ministero Universita’ Ricerca Scientifica Tecnologica (to F.E.), by Consiglio Nazionale delle Ricerche (Progetto Finalizzato Applicazioni Cliniche Ricerca Oncologica (to F.E.), and by the National Institute of Neurological Disorders and Stroke (to R.M.).
Footnotes
Abbreviations: WT α7, wild-type α7 subunit; 5HT, 5-hydroxytryptamine; AcCho, acetylcholine; nAcChoR, nicotinic AcCho receptor; α-BTX, α-bungarotoxin; L247T α7, threonine-for-leucine 247 α7 subunit mutant; IAcCho, current elicited by AcCho; I100, IAcCho elicited by AcCho at 100 μM; I0.2, IAcCho elicited by AcCho at 0.2 μM; EC50, dissociation constant; nH, Hill coefficient; nγ, rectification coefficient.
References
- 1.Couturier S, Bertrand D, Matter J M, Hernandez M C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 2.Schoepfer R, Conroy W G, Whithing P, Gore M, Lindstrom J M. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- 3.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revah F, Bertrand D, Galzi J L, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J P. Nature (London) 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand D, Dellivers-Thiery A, Revah F, Galzi J L, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux J P. Proc Natl Acad Sci USA. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unwin N. Nature (London) 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 7.Filatov G N, White M M. Mol Pharmacol. 1995;48:379–384. [PubMed] [Google Scholar]
- 8.Labarca C, Nowak M W, Zhang H, Tang L, Deshpande P, Lester H A. Nature (London) 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- 9.Scuka M, Mozrzymas J W. Prog Neurobiol. 1992;38:19–33. doi: 10.1016/0301-0082(92)90033-b. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand D, Cooper E, Valera S, Rungger D, Ballivet M. Methods Neurosci. 1991;4:174–193. [Google Scholar]
- 11.Palma E, Bertrand S, Binzoni T, Bertrand D. J Physiol (London) 1996;491:151–161. doi: 10.1113/jphysiol.1996.sp021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miledi R. Proc R Soc London B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 13.Rao C R. Linear Statistical Inference and Its Application. New York: Wiley; 1973. pp. 167–168. [Google Scholar]
- 14.Ramirez-Latorre J, Yu C R, Qu X, Perin F, Karlin A, Role L. Nature (London) 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 15.Beck C, Moulard B, Steinlein O, Guipponi M, Vallee L, Montpied P, Baldy-Moulnier M, Malafosse A. Neurobiol Dis. 1994;1:95–99. doi: 10.1006/nbdi.1994.0012. [DOI] [PubMed] [Google Scholar]
- 16.Steinlein O K, Mulley J C, Propping P, Wallace R H, Phillips H A, Sutherland G R, Scheffer I E, Berkovic S F. Nat Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 17.Ohno K, Hutchinson D O, Milone M, Brengman J M, Bouzat C, Sine S M, Engel A G. Proc Natl Acad Sci USA. 1995;92:758–762. doi: 10.1073/pnas.92.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]