Abstract
Morphine produces analgesia at opiate receptors expressed in nociceptive circuits. μ, δ, and κ opiate receptor subtypes are expressed in circuits that can modulate nociception and receive inputs from endogenous opioid neuropeptide ligands. The roles played by each receptor subtype in nociceptive processing in drug-free and morphine-treated states have not been clear, however. We produced homologous, recombinant μ, opiate receptor, heterozygous and homozygous knockout animals that displayed ≈54% and 0% of wild-type levels of μ receptor expression, respectively. These mice expressed κ receptors and δ receptors at near wild-type levels. Untreated knockout mice displayed shorter latencies on tail flick and hot plate tests for spinal and supraspinal nociceptive responses than wild-type mice. These findings support a significant role for endogenous opioid–peptide interactions with μ opiate receptors in normal nociceptive processing. Morphine failed to significantly reduce nociceptive responses in hot plate or tail flick tests of homozygous μ receptor knockout mice, and heterozygote mice displayed right and downward shifts in morphine analgesia dose–effect relationships. These results implicate endogenous opioid–peptide actions at μ opiate receptors in several tests of nociceptive responsiveness and support μ receptor mediation of morphine-induced analgesia in tests of spinal and supraspinal analgesia.
Morphine acts at seven transmembrane domain, G protein-linked receptor products of genes encoding μ, κ, and δ opiate receptor subtypes (1–9). Each of these genes is expressed in neurons in several neuronal circuits implicated in nociception (10–20). μ receptor mediation of much morphine-induced analgesia has been postulated (21, 22). However, studies using compounds with relative preferences for δ and κ receptors have suggested that these other two opiate receptor subtypes also might play significant roles in the analgesic responses induced by morphine-like drugs (22–26). The extent to which each of the three opiate receptor subtype gene products might participate in different features of opiate- or morphine-induced analgesia thus has remained unclear. Elucidation of the selective analgesic contributions of each opiate receptor subtype is of substantial potential importance for developing improved analgesic medications with minimal undesirable effects.
Expression of endogenous opioid–peptide agonists, especially those derived from the preproenkephalin and preprodynorphin genes, in circuits associated with pain perception suggests that opioid–peptide interactions with opiate receptors could be well positioned to modulate nociceptive responses in the absence of exogenously administered opiate drugs (10, 12, 14, 27–32). Studies of pain responses in animals and humans treated with opiate antagonists, however, have documented modifications in nociception in some but not all studies (33, 34). These results also have left uncertainty about the power of endogenous opioid–peptide interactions with opiate receptors in day-to-day nociception modulation.
To test roles for morphine-preferring μ opiate receptors in nociceptive responses in drug-free and opiate-treated animals, we produced homologous, recombinant, μ receptor knockout mice and tested baseline and morphine-altered pain responses in animals with deletion of one or both μ receptor gene copies. Data from these mice provide support for the idea that interactions of endogenous peptides with μ receptors are likely to be involved in nociceptive responses in drug-free animals. They also underscore the large role that the μ opiate receptor subtype plays in mediating the analgesia produced by morphine in model pain test systems.
MATERIALS AND METHODS
Genomic Cloning and Targeting Vector Construction.
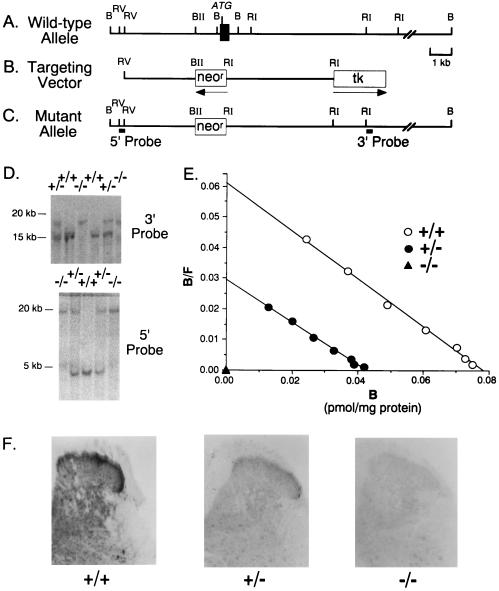
A 595-bp genomic hybridization probe recognizing the 5′ flanking region of the μ receptor’s first exon from the 129/SvEv mouse strain was amplified using 30 cycles of PCR, 1 μg of genomic DNA, and oligonucleotides 5′- ATTGCATATGGTTAGTTGAGTCGGAAGAGTGTTGAGGTAT-3′ and 5′-TGAATGCTTGCTGCGGACTCGGTAGGCTGTA ACTGAGAGC-3′ based on reported sequences (35) and was subcloned into pCRII (Invitrogen) to produce pCRIIm1204-1756. The insert from pCRIIm1204-1756 was radiolabeled by random priming and was used to identify a 16.5-kb μ opiate receptor genomic fragment from a λ-FIX II genomic library prepared from the 129 mouse strain (Stratagene) (Fig. 1A). The 16.5-kb fragment containing 8.3 kb of the 5′ flanking sequence, the 561-bp first exon, and 7.7 kb of first intronic sequences from the mouse μ opiate receptor gene (35) was subcloned into pBluescript (pBS) (Stratagene) to produce pBSμ16.5.
Figure 1.
Construction of μ opiate receptor knockout mice. (A) Representation of 5′ portions of the murine μ opiate receptor gene, with positions of exon I (closed box), start codon (ATG), BamHI (B), EcoRV (RV), BglII (BII), and EcoRI (RI) restriction endonuclease sites noted. (Bar = 1 kb.) (B) Representation of the pμKO2 targeting vector indicating phosphoglycerate kinase neomycin resistance gene (neor) and MC1 thymidine kinase (tk) sequences. The directions of gene transcription are marked by horizontal arrows. Abbreviations and scale as in A. (C) Representation of the predicted mutant allele resulting in the disrupted μ receptor gene. The location of the 5′ and 3′ probes used in the Southern blot analyses are indicated. The putative promoter region and first exon are missing from the mutant allele. Abbreviations and scale as in A and B. (D) Southern blot analysis using a 3′ or 5′ probe to BamHI-digested tail DNA extracted from wild-type (+/+), heterozygote (+/−), and homozygote (−/−) mice. The presence of a single 20-kb fragment indicates a homozygous mutant (−/−) genotype. Wild-type fragments identified by 3′ and 5′ probes are 15 and 5 kb, respectively. (E) Scatchard analyses of saturation radioligand binding data using [3H]DAMGO and membranes prepared from whole brain minus cerebellum specimens from 7-week-old animals. Mean (±SEM; n = 4) values for Bmax were 106 ± 13 and 57 ± 11 fmol/mg protein and undetectable for wild-type (+/+), heterozygote (+/−), and homozygote (−/−) μ receptor knockout mice, respectively. Dissociation constant values (KD) were 0.48 and 0.5 nM and undetectable, respectively. (F) Immunostaining of μ receptor protein in dorsal horn sections through the lumbosacral spinal cord in μ receptor homologous recombinant mice. Darker immunoreactivity is found in superficial laminae of the wild-type (+/+) dorsal horn than in heterozygote (+/−) > homozygote (−/−) mice in two separate experiments. This staining was eliminated by antiserum preabsorption by the peptide to which the antibody was raised but not by preabsorption by an irrelevant peptide (data not shown).
A μ receptor targeting vector was constructed using a 3.2-kb EcoRV–BglII 5′ fragment and a 5.1-kb EcoRI 3′ fragment subcloned into pPGKneo (36) (Fig. 1B). The final construct, designated pmKO2, contained the herpes simplex virus thymidine kinase (tk) gene driven by the MC1 polyoma enhancer at the 3′ end of the EcoRI 3′ fragment. To identify homologous recombinants, 3′ and 5′ hybridization probes were prepared by subcloning 183 bp of the 5′ EcoRV fragment and 420 bp of the 3′ EcoRI fragments from pBSμ16.5 into pBS to produce pBSμ5′183 and pBSμ3′420, respectively (Fig. 1C).
Production of Homologous, Recombinant Embryonic Stem (ES) Cells and Knockout Mice.
Twenty five micrograms of pmKO2 DNA was linearized with BamHI and transfected by electroporation into 107 AB1 ES cells derived from 129/SvEv mice (36) (kindly supplied by Allan Bradley, Baylor College of Medicine, Houston). AB1 ES cells were cultured on mitotically inactive SNL76/7 feeder cells clonally derived from STO cells stably expressing leukemia inhibitory factor and neomycin resistance genes in DMEM containing 15% fetal bovine serum (HyClone) and 0.1 mM β-mercaptoethanol (37). ES cells were selected for homologous recombination by culture in medium containing 500 μg/ml G418 and 2 μM gancyclovir (a generous gift of Syntex, Palo Alto, CA) on days 2–7 after transfection. G418- and gancyclovir-resistant colonies were picked on day 8. BamHI digests of DNA prepared from 800 resistant clones were screened by Southern blot analyses using the insert from pBSμ5′-183 radiolabeled by random priming as hybridization probes. Six positive ES cell lines from the doubly resistant colonies displayed the ≈20-kb BamHI fragment anticipated of homologous recombinants and readily distinguishable from the 5-kb fragment obtained from wild-type DNA.
Chimeric mice were generated by injecting 15–20 homologous, recombinant ES cells into blastocysts harvested from C57BL/6J mice and by implanting the blastocysts into the uteri of pseudopregnant CD-1 mice (Charles River Breeding Laboratories) 2.5 days postcoitus (38). Blastocysts injected with four of the six homologous, recombinant ES cell lines yielded chimeric animals, as assessed by coat color. Southern blot analyses of DNA extracted from tail tip specimens of the offspring of the chimeras revealed that germ line transmission was achieved from two ES cell lines, 1H8 and 5A20 (Fig. 1D). Fifty-four percent of 103 offspring of matings between chimera from the 1H8 cell line and C57BL/6J females revealed disrupted μ receptor alleles, and F2 homozygote, heterozygote, and wild-type offspring of F1 × F1 intercrosses were used for further biochemical and behavioral testing.
Characterization of Opiate Receptor Protein Expression.
μ, δ, and κ receptors were characterized by saturation radioligand binding studies using [3H]DAMGO ([d-Ala2, N-McPhe4, Gly5-ol]enkephalin) [55 Ci/mmol (1 Ci = 37 GBq); DuPont/NEN], DPDPE ([3H][d-Pen2, d-Pen5]enkephalin) (36 Ci/mmol; DuPont/NEN), and [3H]U-69,593 (60 Ci/mmol; Amersham), with membranes prepared from whole brain minus cerebellum specimens of mice killed at ≈7 weeks of age (8, 39, 40). In brief, brains were homogenized in 20 volumes of 50 mM Tris·HCl (pH 7.4) using a Polytron, and the membranes were centrifuged at 40,000 × g for 15 min at 4°C and washed twice with an intervening 25°C, 30-min incubation. Washed membranes containing 500 mg of protein were incubated with [3H]DAMGO (0.156- to 10-nM concentrations), [3H]DPDPE (0.125–8 nM), or [3H]U69,593 (0.125–8 nM) in final volumes of 1 ml for 5 h at 25°C in 50 mM Tris·HCl. A peptidase inhibitor cocktail of 1 mg/ml BSA, 0.1 mg/ml leupeptin, 0.4 mg/ml chymostatin, 0.2 mg/ml bestatin, and 1 mg/ml bacitracin was added to the incubation buffer for μ and δ receptor binding. μ and δ receptor binding buffers each used 50 mM Tris·HCl (pH 7.4), δ receptor binding required 5 mM MgCl2, and Tris·HCl (pH 7.8) was used for κ receptor binding. Reactions were terminated by the addition of 50 mM ice-cold Tris·HCl buffer (pH 7.4), and membrane-associated ligand was estimated after rapid filtration over Whatman GF/B filters using a Brandel (Gaithersburg, MD) apparatus. Nonspecific binding was estimated using 10 mM naloxone for each radioligand. Scatchard analyses were performed using macligand (R. Williams, University of California, Los Angeles).
μ receptor immunohistochemistry was performed as described using a polyclonal antiserum with antibodies recognizing the μ receptor’s C terminus, whose specificities have been documented in Western blotting, immunoprecipitation, and light microscopic immunohistochemical and electron microscope immunohistochemical studies (10, 13, 41, 42).
General Behavioral Characterization.
Rotarod testing. Rotarod testing began when mice were placed on a 3-cm diameter rod rotating at 15 rpm (Ugo Basile, Varesse, Italy), and the number of training trials required for them to stay on the rod for 3 min was recorded. Four hours after this training, mice were placed on the rod as it accelerated from 4 to 40 rpm over 5 min, and the time that they could remain on the accelerating rod was noted.
Screen testing.
Screen testing assessed the ability of mice to hang onto a 4-mm wire mesh screen after it was quickly inverted, with a 2-min cutoff time (43).
Spontaneous locomotor activity.
Spontaneous locomotor activity was assessed as total distance traveled calculated from the number of infrared beam breaks measured over 45 min when mice were placed individually in 46 × 25 × 19-cm clear plastic cages inside Optovarimex activity monitors (Columbus Instruments, Columbus, OH) to which the mice had not been previously exposed under dim light, sound-attenuated conditions. As one measure of “emotionality” (44), the number of fecal boli found after this initial exposure was recorded manually. Habituated locomotor activity was assessed 4 h after the animals’ original exposure to the environment.
Passive avoidance training.
Passive avoidance training began by moving mice from home cages to 40-sec confinement in the brightly lit side of a two-chamber apparatus (45). Access to a dimly lit second chamber was then opened, and the time required for the mouse to enter the second chamber was recorded. Upon entry, the mouse was confined to the second chamber, where it received a 1-sec, 0.3-mAmp, scrambled, unescapable foot shock; it was returned to its home cage 1 min later. Testing for retention was performed 24 h later. Mice were removed from home cages, confined to the brightly lit sides of the apparatus for 40 sec, and allowed access to the second chamber, and the time required for entrance into the dimly lit chamber was recorded, to a maximum of 5 min.
Analgesia Testing.
For hot plate analgesia testing, mice were placed on a 52, 55, or 58°C hot plate, and latency to paw lick was recorded, with a 30-sec cutoff time. For tail flick testing, mice were loosely wrapped in an adsorbent towel, and their tails were immersed ≈2 cm into water heated to 50, 53, or 56°C. The time after immersion at which the tail flick response was noted was recorded. Cutoff time for this test was 15 sec. Morphine effects were assessed in mice injected s.c. at 20-min intervals with saline and then with an ascending cumulative dosing regimen producing total drug doses of 3, 10, 30, and 56 mg/kg morphine sulfate s.c. (46). Hot plate and tail flick responses were tested 20 min after each s.c. injection.
Statistical comparisons were made with ANOVAs followed by Scheffe post hoc analyses for radioligand binding and analgesia test data and χ2 tests comparing genotype distributions to expected values from Mendelizing ratios, as indicated.
RESULTS
Assessing the genotypes of offspring of heterozygote (+/−) × heterozygote (+/−) matings and comparing them with the results of heterozygote × wild-type matings did not provide evidence for any overall significant reduction in viability of animals with the μ receptor gene deletion. Genotypes of 151 progeny of +/− × +/− matings were 18% (−/−), 50% (+/−), and 32% (+/+) genotypes (χ2 = 5.8; P > 0.05; df = 2), and 178 progeny of +/− × +/+ matings were 46.6% (+/−) and 53.4% (+/+) genotypes; values were not different from expected ratios. However, only 33% of the litters from the +/− × +/− matings produced more than seven pups whereas 51% of the litters of the +/− × +/+ matings were of this size.
The ability to produce viable wild-type (+/+), heterozygote (+/−), and homozygote (−/−) knockout mice allowed assessments of the properties of mice of each genotype. Animals of each genotype appear morphologically identical by gross examination, and histologic evaluation of sections from several levels of the brain and spinal cord revealed no obvious differences, including examinations of several areas of high μ receptor expression (Fig. 1; data not shown). Animals of each of three genotypes were indistinguishable from each other in several tests of locomotor skills, learning, and emotionality performed in drug-free conditions. Locomotor activity in a novel environment, habituation to a novel environment, performance on a rotating rod accelerating from 4 to 40 revolutions per minute, and ability to hang suspended on an inverted wire mesh screen were indistinguishable in tests of 7–17 mice of each genotype (data not shown). A test of emotionality (the number of fecal boli emitted under the stress of an open field novelty situation) revealed similar results in animals of each genotype (data not shown). Abilities to acquire and retain a passive avoidance habit tested 24 h after training were similar between animals of each genotype (data not shown). These baseline abilities encouraged us to evaluate the biochemical features and nociceptive responses in these mice.
The knockout animals displayed gene dose-dependent reductions in levels of μ receptor expression. Saturation analyses of [3H]DAMGO binding to brain membrane μ receptors revealed negligible specific binding in homozygotes (−/−). The Bmax value for heterozygote (+/−) mice was 58 ± 11 fmol/mg protein (n = 4), a value that was 54% of the wild-type value (107 ± 14 fmol/mg protein, n = 4; Fig. 1E). These findings were consistent with the results of μ receptor immunostaining. μ receptor immunoreactivity in several brain regions and in the spinal cord dorsal horn was substantially reduced in heterozygotes (+/−) and virtually eliminated in homozygote (−/−) knockout animals (Fig. 1F).
These μ receptor depletions were accompanied by no significant changes in binding to κ or δ opiate receptors. The δ receptor Bmax value identified using [3H]DPDPE displayed no significant differences from wild-type control values (39 ± 2 fmol/mg protein, n = 7); the heterozygotes value was 51 ± 9 fmol/mg protein (n = 7; P = 0.2), and the homozygote value was 49 ± 7 fmol/mg protein (n = 6, P = 0.3). Homozygote (−/−) and heterozygote (+/−) knockout animals displayed κ receptor binding Bmax values for [3H]U69,593 (20 ± 1 and 19 ± 4 fmol/mg protein, n = 4 and 3, respectively) that were again not significantly different from those of wild-type mice (28 ± 5 fmol/mg protein; n = 3; P > 0.1). Receptors from wild-type, +/−, and −/− animals revealed similar affinities for DPDPE (0.45, 0.65, and 0.61 nM, respectively) and for U69,593 (1.1, 0.63, and 0.79 nM, respectively).
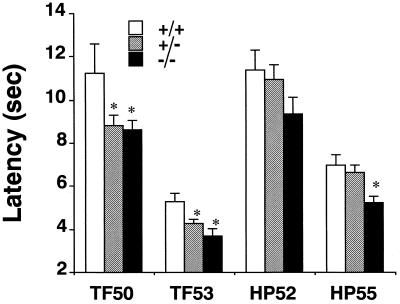
In three different cohorts of knockout mice tested on the tail flick assay under drug-free conditions, knockout mice of both +/− and −/− genotypes displayed shorter tail flick latencies compared with wild-type mice (+/+) when tested at 53°C (P < 0.05; n = 15 for −/−, 24 for +/−, and 14 for +/+; Fig. 2). Stimulus–response testing revealed significant reductions in latencies in tests at both 50 and 53°C (P < 0.05—corrected for repeated measures; n = 11 for −/−, 20 for +/−, and 10 for +/+; Fig. 2) that were not found using a 56°C bath temperature (data not shown). These differences were statistically significant for both heterozygous and homozygous animals.
Figure 2.
Latencies for nociceptive responses in tail flick (TF) and hot plate (HP) tests in unpretreated mice. Mice of +/+ (n = 14), +/− (n = 24), and −/− (n = 15) μ receptor genotypes underwent tail flick testing in 50 or 53°C water and hot plate testing at 52 or 55°C, as indicated. ∗, P < 0.05 compared with wild-type control values.
There was also a more modest reduction in the time to first paw lick on the 55°C hot plate test in three cohorts of tested, drug-free, homozygote mice compared with wild-type mice (+/+), which reached statistical significance (P < 0.05; n = 15 for −/−, n = 24 for +/−, and n = 14 for +/+; Fig. 2). Stimulus–response testing revealed trends toward a similar reduction on a 52°C hot plate that did not reach statistical significance (P = 0.1; n = 11 for −/−, n = 20 for +/−, and n = 10 for +/+; Fig. 2), but little effect on the very short latencies was found at 58°C (data not shown). No effect in heterozygotes, as compared with wild-type mice (+/+), approached statistical significance at any of the three temperatures examined (data not shown).
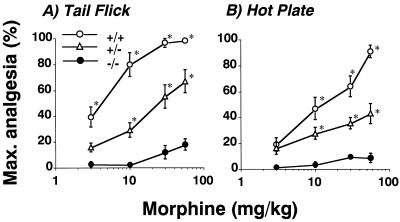
Morphine dose–effect relationships were analyzed in 12 wild-type, 12 +/−, and 10 −/− mice treated with an ascending dose morphine regimen (46). Wild-type mice displayed powerful morphine-induced analgesic effects on both tail flick (Fig. 3A) and hot plate (Fig. 3B) tests (P < 0.001). Heterozygous (+/−) knockout animals revealed right and downward shifts in dose–effect relationships for acutely administered morphine. Homozygous (−/−) knockout animals revealed no statistically significant morphine analgesia on either hot plate or tail flick tests at cumulative doses up to 56 mg/kg. Responses on the tail flick test did display a modest trend toward reduced nociceptive responses at the 56 mg/kg dose (P = 0.12).
Figure 3.
Latencies for nociceptive responses in 53°C tail flick and 55°C hot plate tests in pretreated mice. (A) Dose–response relationships for morphine-induced alterations in latencies on 53°C tail flick testing in mice with wild-type (+/+), heterozygote (+/−), and homozygote (−/−) μ opiate receptor genotypes using a cumulative dose–response paradigm as described. Percentage of maximal analgesia was calculated for each mouse as: 100 × {[(latency to tail flick after morphine) − (latency to tail flick at baseline)]/[(15-sec cutoff time) − (baseline latency)]}. ∗, P < 0.05 compared with preinjection control values for the appropriate genotype. Dose–effect relationships were significant for +/+ and +/− mice but not for −/− mice. Among genotype differences, dose–response relationships also were significant for animals of each genotype [P < 0.001, df(2, 120), F = 66 by repeated measures ANOVA]. (B) Dose–response relationships for morphine-induced alterations in latencies on 55°C hot plate testing in mice with wild-type (+/+), heterozygote (+/−), and homozygote (−/−) μ opiate receptor genotypes using a cumulative dose–response paradigm as described. Percentage of maximal analgesia was calculated for each mouse using a 30-sec cutoff time. ∗, P < 0.05 compared with preinjection control values for the appropriate genotype. Dose–effect relationships were significant for +/+ and +/− mice but not for −/− mice. Among genotype differences, between dose–response relationships also were significant for animals of each genotype [P < 0.001, df(2, 124), F = 27 by repeated measures ANOVA]. Max., maximum.
DISCUSSION
Analyses of animals with μ opiate receptor gene deletions and reduced μ receptor expression provided novel data concerning μ receptor involvement in processing of nociceptive information and in the analgesia exerted by acutely administered morphine. The magnitude of the observed effects might not have been anticipated from previous studies suggesting possibly smaller roles for endogenous opioid systems in modulating nociceptive information and more substantial roles for other opiate receptor subtypes in morphine analgesia. The remarkable failure of other opiate receptor subtypes to show striking adaptations to loss of μ receptors also supports a degree of independence of the regulation of each of these gene products that might not have been anticipated.
The expression of μ opiate receptors in multiple central and peripheral nervous system neuronal circuits has suggested their involvement in a number of different activities of cortical, subcortical, and spinal circuits (10–20). The morphine effects on a broad range of physiological functions (including locomotor activity, neuroendocrine and reproductive systems, respiratory control, pupillomotor systems, and nociceptive functions) support the idea that opiate receptors, including the morphine-preferring μ receptor, could have broad effects in many neural circuits (47). We are currently investigating possible mechanisms that might underlie the modest reduction in the number of −/− animals produced by +/− × +/− matings. However, failure of the animals born with deleted μ receptor expression to display any readily observable alteration in a number of locomotor, autonomic, and other functional tests allowed us to examine nociceptive responses in animals without the kinds of gross defects in other systems that might readily confound measurements of motor responses to nociceptive stimuli.
Absence of substantial changes in brain expression of κ and δ opiate receptors in the μ receptor knockout animals also provided the opportunity to examine nociceptive responses in animals with grossly intact function in other opiate receptor systems. Conceivably, adaptive changes in other systems could contribute to the behavioral differences in nociceptive responses noted here. However, the animals’ normality on a number of functional screening tests and the biochemical observations suggesting that the μ receptor may be independently regulated suggested that partial or total μ receptor absence during development may be compatible with normal or near normal function in a number of brain circuits that express opiate receptors.
Circuits modulating nociception may provide an exception to this general picture. Tail flick testing demonstrates that animals with even 50% reductions from wild-type levels of μ opiate receptor expression display different spinal reflex responses (48) to noxious stimuli than wild-type mice in the absence of exogenous opiate agonists. Conceivably, subtle, adaptive, developmental changes in non-μ systems in these mice could contribute to these results. However, the data are in accord with studies that have used opiate antagonists to implicate endogenous opioid–peptide interactions with opiate receptors in pain modulation although these studies have demonstrated only modest and variable effects (34). Preliminary data examining effects of up to 500 μg/kg s.c. naloxone in wild-type mice of the genotype used here also demonstrate no clear trend toward hyperalgesia (I.S., M.F., and G.R.U., unpublished observation). The data also fit well with the substantial modification of analgesia induced by exogenous opiate drugs in these same mice, as noted below.
The significant alterations in the 50 and 53°C tail flick tests of spinal analgesia in untreated μ receptor +/− and −/− mice contrasted with the fact that hot plate testing of largely supraspinal analgesia (48) yielded significant differences only for −/− mice studied at 55°C. These results fit with data obtained from hot plate testing of transgenic mice that overexpress μ receptors in the catecholaminergic neurons that are thought to make large contributions to these supraspinal analgesic mechanisms (ref. 34; L.L.M, I.S., and G.R.U., unpublished observations). Drug-free testing of these animals revealed only a small trend toward enhanced latency on this test that does not reach significance although these overexpressing mice did show enhanced morphine potency and power in this test by left and upward shifts in morphine dose–effect relationships (L.L.M., I.S., and G.R.U., unpublished observations). Conceivably, action of endogenous opioid peptides at μ receptors may be of more importance for spinal analgesia than for supraspinal analgesia (48), in which neurons using other neurotransmitters and neurotransmitter receptors may play more substantial roles.
Reduced μ receptor expression exerted several distinct influences on responses to acute morphine administration in hot plate and tail flick tests. Elimination of μ receptors in −/− animals virtually abolished morphine’s effects on nociceptive responses in both tests. μ receptor +/− mice displayed right and downward shifts in morphine dose–effect relationships, consistent with lower morphine potencies and efficacies in tests of both spinal and supraspinal analgesia. Comparisons of these morphine effects with those in unpretreated mice suggest that endogenous opioid–peptide/μ receptor interactions might play roles similar to those of morphine/μ receptor interactions in spinal analgesic mechanisms such as those tested by the tail flick procedure. Comparisons of results in the hot plate tests, however, suggest that morphine may recruit supraspinal μ-mediated mechanisms much more powerfully than these mechanisms are engaged by endogenous opioid peptides. These data also fit with the above mentioned enhancement of morphine power and potency in hot plate testing of transgenic mice that overexpress μ receptors in the catecholaminergic neurons that contribute to supraspinal analgesic mechanisms in the absence of significantly different hot plate responses under drug-free conditions (L.L.M., I.S., and G.R.U., unpublished observations).
Taken together, the current results point toward substantial roles for μ receptor expression in virtually all of the morphine-induced analgesia that can be assessed in major supraspinal and major spinal models of nociceptive processes. The greater effect on drug-free nociceptive responses in largely spinal tail flick testing than in largely supraspinal hot plate testing suggests that the impact of opiate peptide occupancy of endogenous μ receptors may be more or less susceptible to adaptive changes in the tail flick than in the hot plate tests. Enhanced understanding of these processes should aid significantly in ongoing efforts to improve therapeutic approaches to pain control so that exogenous pharmacological power is maximized and mechanisms for endogenous pain control are minimally suppressed.
Acknowledgments
We thank Allan Bradley and Yuji Mishina, who generously provided plasmids, AB1 ES cells, and lymphocyte inhibitory factor-secreting STO cells; Yuji Mishina for excellent technical consultations; Lawrence G. Sharp for help with behavioral assessments; Jia-Bei Wang and Akihiro Moriwaki for antiserum production and immunohistochemical characterization; Hsin Fei Lui, Nancy Goodman, and Stephen Kinsey for many technical contributions; Lynda A. Roggio and the Bionetics/Triad animal care staff for careful mouse care and breeding; Angela Flood and Mary Jane Robinson for assistance with the manuscript; and Horace H. Loh for generous prepublication access to murine μ receptor genomic sequence data. We are grateful for the support of the Intramural Research Program, National Institute on Drug Abuse.
Footnotes
Abbreviations: pBS, pBluescript; ES cells, embryonic stem cells; DAMGO, [d-Ala2, N-MePhe4, Gly5-ol]enkephalin; DPDPE, [d-Pen2, d-Pen5]enkephalin.
References
- 1.Evans C J, Keith D J, Morrison H, Magendzo K, Edwards R H. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 2.Kieffer B L, Befort K, Gaveriaux R C, Hirth C G. Proc Natl Acad Sci USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J B, Imai Y, Eppler C M, Gregor P, Spivak C E, Uhl G R. Proc Natl Acad Sci USA. 1993;90:10230–10234. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eppler C M, Hulmes J D, Wang J B, Johnson B, Corbett M, Luthin D R, Uhl G R, Linden J. J Biol Chem. 1993;268:26447–26451. [PubMed] [Google Scholar]
- 5.Chen Y, Mestek A, Liu J, Hurley J A, Yu L. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- 6.Thompson R C, Mansour A, Akil H, Watson S J. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 7.Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, Katsumata S, Yabuuchi K, Satoh M. Neurosci Res. 1994;18:315–322. doi: 10.1016/0168-0102(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda K, Raynor K, Kong H, Breder C D, Takeda J, Reisine T, Bell G I. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minami M, Toya T, Katao Y, Maekawa K, Nakamura S, Onogi T, Kaneko S, Satoh M. FEBS Lett. 1993;329:291–295. doi: 10.1016/0014-5793(93)80240-u. [DOI] [PubMed] [Google Scholar]
- 10.Moriwaki A, Wang J B, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl G R. Neurochem Res. 1996;21:1315–1331. doi: 10.1007/BF02532373. [DOI] [PubMed] [Google Scholar]
- 11.Arvidsson U, Dado R J, Riedl M, Lee J H, Law P Y, Loh H H, Elde R, Wessendorf M W. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee J H, Nakano A H, Lin X, Loh H H, Law P Y, Wessendorf M W, Elde R. Proc Natl Acad Sci USA. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svingos A L, Moriwaki A, Wang J B, Uhl G R, Pickel V M. J Neurosci. 1996;16:4162–4173. doi: 10.1523/JNEUROSCI.16-13-04162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng P Y, Svingos A L, Wang H, Clarke C L, Jenab S, Beczkowska I W, Inturrisi C E, Pickel V M. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman R R, Snyder S H, Kuhar M J, Young W D. Proc Natl Acad Sci USA. 1980;77:6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda C N, Arvidsson U. NeuroReport. 1995;6:1025–1028. doi: 10.1097/00001756-199505090-00019. [DOI] [PubMed] [Google Scholar]
- 17.Mansour A, Khachaturian H, Lewis M E, Akil H, Watson S J. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour A, Fox C A, Thompson R C, Akil H, Watson S J. Brain Res. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 19.Mansour A, Watson S J, Akil H. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- 20.Mansour A, Fox C A, Akil H, Watson S J. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 21.Martin W R, Eades C G, Thompson R E, Huppler R E, Gilbert P E. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 22.Takemori A E, Portoghese P S. Eur J Pharmacol. 1993;242:145–150. doi: 10.1016/0014-2999(93)90074-r. [DOI] [PubMed] [Google Scholar]
- 23.Tung A S, Yaksh T L. Brain Res. 1982;247:75–83. doi: 10.1016/0006-8993(82)91029-0. [DOI] [PubMed] [Google Scholar]
- 24.Uphouse L A, Welch S P, Ward C R, Ellis E F, Embrey J P. Eur J Pharmacol. 1993;242:53–58. doi: 10.1016/0014-2999(93)90009-7. [DOI] [PubMed] [Google Scholar]
- 25.Porreca F, Burks T F. In: Opiates II. Herz A, editor. Berlin: Springer; 1993. pp. 21–52. [Google Scholar]
- 26.Yaksh T L. In: Opiates II. Herz A, editor. Berlin: Springer; 1993. pp. 53–90. [Google Scholar]
- 27.Uhl G R, Goodman R R, Kuhar M J, Childers S R, Snyder S H. Brain Res. 1979;166:75–94. doi: 10.1016/0006-8993(79)90651-6. [DOI] [PubMed] [Google Scholar]
- 28.Nishimori T, Buzzi M G, Chudler E H, Poletti C E, Moskowitz M A, Uhl G R. J Comp Neurol. 1990;302:1002–1018. doi: 10.1002/cne.903020422. [DOI] [PubMed] [Google Scholar]
- 29.Cruz L, Basbaum A I. J Comp Neurol. 1985;240:331–348. doi: 10.1002/cne.902400402. [DOI] [PubMed] [Google Scholar]
- 30.Palkovitz M, Brownstein M J. In: Handbook of Chemical Neuroanatomy. Bjorklund A, Hokfelt T, editors. Amsterdam: Elsevier; 1985. pp. 1–71. [Google Scholar]
- 31.Khachaturian H, Lewis M E, Schäfer MK-H, Watson S J. Trends Neurosci. 1985;8:111–119. [Google Scholar]
- 32.Ruda M A. Science. 1982;215:1523–1525. doi: 10.1126/science.6121374. [DOI] [PubMed] [Google Scholar]
- 33.Levine J D, Gordon N C, Fields H L. Nature (London) 1979;278:740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- 34.Fields H L. In: Opiates II. Herz A, editor. Berlin: Springer; 1993. pp. 3–20. [Google Scholar]
- 35.Min B H, Augustin L B, Felsheim R F, Fuchs J A, Loh H H. Proc Natl Acad Sci USA. 1994;91:9081–9085. doi: 10.1073/pnas.91.19.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 37.Robertson E J. In: Tetracarcinomas and Embryonic Stem Cells. Robertson E J, editor. Oxford: IRL; 1987. pp. 71–112. [Google Scholar]
- 38.Bradley A. In: Tetracarcinomas and Embryonic Stem Cells. Robertson E J, editor. Oxford: IRL; 1987. pp. 113–151. [Google Scholar]
- 39.Rothman R B, Bykov V, Xue B G, Xu H, De C B, Jacobson A E, Rice K C, Kleinman J E, Brady L S. Peptides. 1992;13:977–987. doi: 10.1016/0196-9781(92)90059-c. [DOI] [PubMed] [Google Scholar]
- 40.Vaughn L K, Knapp R J, Toth G, Wan Y P, Hruby V J, Yamamura H I. Life Sci. 1989;45:1001–1008. doi: 10.1016/0024-3205(89)90154-9. [DOI] [PubMed] [Google Scholar]
- 41.Surratt C K, Johnson P S, Moriwaki A, Seidleck B K, Blaschak C J, Wang J B, Uhl G R. J Biol Chem. 1994;269:20548–20553. [PubMed] [Google Scholar]
- 42.Zhang L, Yu Y, Mackin S, Weight F F, Uhl G R, Wang J B. J Biol Chem. 1996;271:11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- 43.Coughenour L L, Mclean J R, Parker R B. Pharmacol Biochem Behav. 1977;6:351–353. doi: 10.1016/0091-3057(77)90036-3. [DOI] [PubMed] [Google Scholar]
- 44.Flint J, Corley R, DeFries J C, Fulker D W, Gray J A, Miller S, Collins A C. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- 45.Sahgal A. In: Behavioral Neuroscience: A Practical Approach. Sahgal A, editor. New York: Oxford Univ. Press; 1993. pp. 49–56. [Google Scholar]
- 46.Elmer G I, Evans J L, Ladenheim B, Epstein C J, Cadet J L. Eur J Pharmacol. 1995;283:227–232. doi: 10.1016/0014-2999(95)00365-r. [DOI] [PubMed] [Google Scholar]
- 47.Reisine T, Pasternak G. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 521–555. [Google Scholar]
- 48.Hull K M, Tolland D E, Maher T J. J Pharmacol Exp Ther. 1994;269:1190–1195. [PubMed] [Google Scholar]