Abstract
We previously reported that caveolin-1 is a key component in a β1 integrin-dependent mechanotransduction pathway suggesting that caveolae organelles and integrins are functionally linked in their mechanotransduction properties. Here, we exposed BAEC monolayers to shear stress then isolated caveolae vesicles form the plasma membrane. While little β1 integrin was detected in caveolae derived from cells kept in static culture, shear stress induced β1 integrin transposition to the caveolae. To evaluate the significance of shear-induced β1 integrin localization to caveolae, cells were pretreated with cholesterol sequestering compounds or caveolin-1 siRNA to disrupt caveolae structural domains. Cholesterol depletion attenuated integrin-dependent caveolin-1 phosphorylation, Src activation and Csk association with β1 integrin. Reduction of both caveolin-1 protein and membrane cholesterol inhibited downstream shear-induced, integrin–dependent phosphorylation of myosin light chain. Taken together with our previous findings, the data supports the concept that β1 integrin-mediated mechanotransduction is mediated by caveolae domains.
Keywords: caveolae, integrins, mechanotransduction, shear stress, lipid rafts
Introduction
Endothelial signaling responses to shear stress have classically been categorized by their molecular initiation sites. While the list of sites continues to grow, currently known mechanosensitive proteins include ion channels [1], G-protein coupled receptors [2], growth factor receptors [3-5], and the cell adhesion molecule PECAM-1 [6, 7]. Additional domains that are sensitive to mechanical stimulus include focal adhesions [3, 8-10] and caveolae [11-16]. These sites are in turn coupled to an array of secondary signaling molecules that create a robust, temporally and spatially regulated response to mechanical stimulation culminating in extensive phenotypic and morphologic changes. Interestingly, several recent studies suggest a linkage between key elements associated with caveolae and focal adhesions.
Our previous work identified caveolae as important mechanotransduction sites as increasing flow and pressure in situ stimulated protein-tyrosine phosphorylation within caveolae leading to activation of ERK1/2 MAP kinases [15]. Further studies demonstrated that flow stimulated nitric oxide (NO) production from caveolae-associated endothelial nitric oxide synthase (eNOS) [13]. In addition to caveolae, shear stress regulation of protein tyrosine phosphorylation, nitric oxide (NO) production and MAP kinases also appear to require integrin-mediated mechanotransduction processes [17]. More recently, we described a novel mechanotransduction pathway where acute shear stress resulted in integrin-dependent phosphorylation of caveolin-1 on its N-terminal tyrosine 14 residue (pY14) via a Src-family kinase [18]. The functional consequence of caveolin-1 tyrosine phosphorylation was to recruit C-terminal Src like kinase (Csk) to phospho-caveolin-1-containing integrin complexes which, through additional regulation of Src activity, promoted myosin light chain (MLC) phosphorylation, an early indicator for actin stress fiber formation in response to shear stress. While these finding demonstrated that pY14 caveolin-1 is a key component of the integrin mechanotransduction pathway, the role of caveolar structures in this process was not definded. Thus, the purpose of the studies presented here was to evaluate the extent to which caveolae influence integrin mechanotransduction.
Material and Methods
Reagents and Antibodies
Unless otherwise specified, chemical reagents were purchased from Sigma (St. Louis, MO) and Fisher Scientific (Fairlawn, NJ). The following primary antibodies were obtained from commercial sources: Csk, and pY14 caveolin-1 monoclonal antibodies (mAbs), and caveolin-1 polyclonal antibody, (pAb,Transduction Labs); pY14-caveolin-1 pAb, c-Src pAb, Csk pAb (Santa Cruz); β1 integrin pAb, and mAbs (Chemicon); myosin light chain and β-actin mAb (Sigma); pY416 Src pAb (Biosource); Di-phosphorylated MLC antibody was a kind gift from Dr. Peter Vincent (Albany Medical College, Albany, NY). Horse-radish peroxidase conjugated anti-rabbit and anti-mouse secondary antibodies (Amersham).
Cell Culture
Bovine Aortic Endothelial Cells (BAEC) were purchased from Cell Applications, Inc. (San Diego, CA). Cells were cultured in MCDB-131 medium (Sigma) supplemented with 10% Fetal Bovine Serum (Atlanta Biologicals, Atlanta, GA) and 0.05mg/mL gentamycin sulfate (Cambrex Biosciences, Walkersville, MD), and were maintained at 37°C, 97% humidity and 5% carbon dioxide. All experiments were performed using cells below passage 8.
In Vitro Flow Experiments
A parallel plate chamber (Streamer model, Flexcell Corp.) connected to a recirculating flow circuit composed of a variable speed peristaltic pump, a fluid capacitor that damps pulsation, and a reservoir with culture medium was used to imposed laminar shear stress on endothelial cell monolayers, as described in detail in our past work [18]. Prior to placement in the parallel plate chamber, cells were acclimated for 2 hrs in “flow-media” consisting of MCDB-131 containing 0.1% FBS then exposed to acute step change in laminar shear stress applied at a magnitude of 10 dynes/cm2 for either 1, 5, 10 minutes from static conditions. Time-matched, non-sheared, control cultures were incubated in fresh flow media at 37°C.
Membrane raft/caveolae Disassembly
Endothelial cells were incubated in serum free media containing either 10 mM Methyl-β-cyclodextrin (CD) for 30 minutes or 5μg/ml filipin for 5 min at 37°C prior to shear stress, as described in our past work [19-21]. In a separate set of experiments, cholesterol was added back to CD treated cell cultures in order to reconstitute disassembled rafts/caveolae.
Immunoprecipitation
Poly- or monoclonal primary antibody were conjugated to sheep-anti rabbit or sheep-anti mouse-coated paramagnetic beads (Dynal), respectively, as described in our past work [18]. Endothelial cell lysates (250μg) were incubated with the antibody/bead conjugates for 4 hrs at 4°C. The bound fraction was separated from the unbound material, washed three times with lysis buffer, and processed for SDS-PAGE. Lysates were also incubated with beads alone to determine extent of non-specific binding.
Caveolae Immunoaffinity Isolation
Static and shear exposed BAEC's were scraped into ice-cold, detergent-free Tricene buffer (250mM sucrose, 1mM EDTA, 20mM Tricene, pH 7.4) and centrifuged to precipitate nuclear material. The supernatant was mixed with 30% Percoll in Tricene buffer and ultracentrifuged for 25 minutes (Beckman MLS50 rotor, 77,000xg, 4°C). The separated plasma membranes were collected, sonicated (3 × 30 second bursts) and incubated with anti-caveolin-1 (clone 2234, Transduction Laboratories) conjugated goat anti-mouse IgG-coated magnetic beads (Dynal Biotech) for 1 hour at 4°C, as in our past reports [14, 19]. Bound material, representative of caveolae vesicles, was separated magnetically from unbound, non-caveolar membranes.
Caveolin-1 siRNA
BAEC at 85-90% confluence were transfected with 100nM caveolin-1 SMARTpool siRNA or siCONTROL (a non-targeting pool of siRNAs that contains at least four mismatches for all known gene sequences) using DharmaFECT-1 (Dharmacon, Inc., Lafayette, CO) according to the manufacturer's protocol. Cells were used 48 hours post-transfection, a time point which corresponded with a 90% reduction in caveolin-1 protein levels, as described in our past study [19].
Western blotting
Cells were processed in lysis buffer (25 mM HEPES, pH 7.4, 150mM NaCl, 5mM EDTA, 1% TritonX-100, phosphatase and protease inhibitors). Protein content of the various samples were determined by bicinchoninic acid (BCA) analysis. Equivalent amounts of protein from each sample were prepared and separated by SDS-PAGE followed by electrotransfer to nitrocellulose filters (Biorad). Nitrocellulose membranes were incubated with primary antibody, followed by appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham or Pierce Biotechnology). Membranes were exposed to SuperSignal enhanced chemiluminescence substrate and immunoblots scanned, digitized and quantified using Image J software.
Statistical analysis
For each study, data was gathered from at least three independent experiments and pooled according to group. Mean and standard deviation were calculated and differences between groups analyzed with an unpaired two-tailed Student's t test or ANOVA with a post-hoc Tukey test using STATGRAPHICS 4.0 software (Statistical Graphics Corp). Differences between control and experimental groups was deemed significant at p <0.05.
Results
Membrane raft/caveolae structural integrity is required for β1 integrin mediated mechanotransduction responses
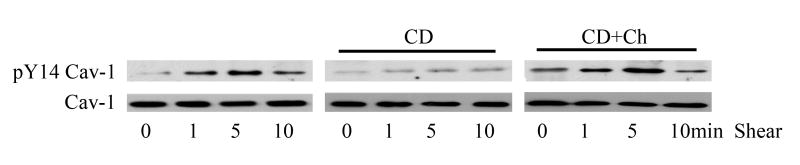
We previously showed that shear-induced caveolin-1 tyrosine phosphorylation is dependent upon β1 integrin and Src activation [18]. To determine whether intact membrane rafts and/or caveolae play a role in this process, BAEC were pretreated with methyl-β-cyclodextrin (CD) to disrupt these cholesterol-rich membrane domains prior to shear stress challenge. Similar to our past findings, we found that in non-treated, control cells, caveolin-1 was rapidly but transiently phosphorylated in response to shear stress (Fig. 1a). In CD treated cells, this mechanotransduction response was essentially blocked (Fig. 1a). Similar results were observed when filipin was used as the cholesterol modifying compound (data not shown). To verify that the observed results were due to manipulation of membrane cholesterol content, cholesterol depleted cells were further incubated with cholesterol loaded CD to replenished plasma membrane cholesterol and reconstitute raft/caveolae domains. Figure 1a shows that shear-induced caveolin-1 tyrosine phosphorylation was recapitulated in cholesterol restored cells.
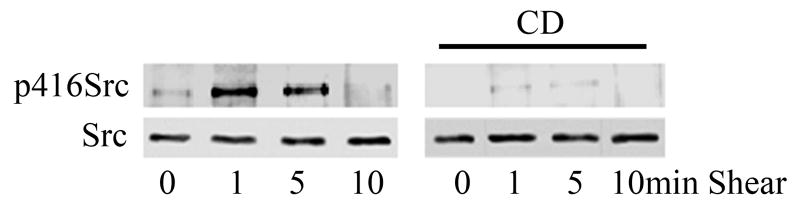
Figure 1. Lipid raft structure facilitates shear-induced phosphorylation of caveolin-1 and Src.
Cell monolayers were pretreated with 10 mM methyl-β-cyclodextrin (CD) for 30 min to disrupt raft/caveolae structure. To validate cholesterol depletion effects, cell membranes were re-loaded with cholesterol by incubation with CD preloaded with cholesterol (CD+Ch). Following shear (10dynes/cm2), cells were lysed, resolved by SDS-PAGE, and Western blotted for pY14 caveolin-1 (A) or Src p416 (B). Total Src and caveolin-1 protein were densitomertrically quantified for normalization.
Since Src has been shown to be a necessary intermediate for several downstream β1 integrin-dependent events, including caveolin-1 tyrosine phosphorylation in response to shear stress [18], we also evaluated Src activity in CD treated BAEC's. Consistent with our past findings [18], shear stress induced a rapid and significant tyrosine 416 auto-phosphorylation of Src, a residue present in the enzymes activation loop whose phosphorylation strongly correlates with enzyme activation. We detected a 2.3 +/− 0.4 fold increase in pY416 signal after only 1 min exposure to shear stress, a time point which slightly preceded peak tyrosine phosphorylation of caveolin-1 at 5min. As in the case of caveolin-1 phosphorylation, CD prevented shear-induced Src activity (Fig. 1b).
Shear-induced formation of β1 integrin / phosphotyrosine caveolin-1 signaling complex requires raft/caveolae domains
We described the formation of a new signaling complex that associated with β1 integrin in response to shear stress [18]. This signaling complex required β1 integrin activation and phosphorylation of caveolin-1. To determine whether rafts/caveolae mediate the formation of this shear-induced signaling complex, co-precipitation experiments were performed following peak phosphorylation of caveolin-1 (5 min). As reported previously [18], shear stress enhanced the association of tyrosine phosphorylated caveolin-1 and C-terminal Src like kinase (Csk), with β1 integrin (Fig 2). Pretreated with CD prevented these recruitment and association events (Fig. 2), which could be restored following cholesterol repletion (data not shown).
Figure 2. Raft/caveolae ablation blocked shear-induced association of Csk with β1 integrin.
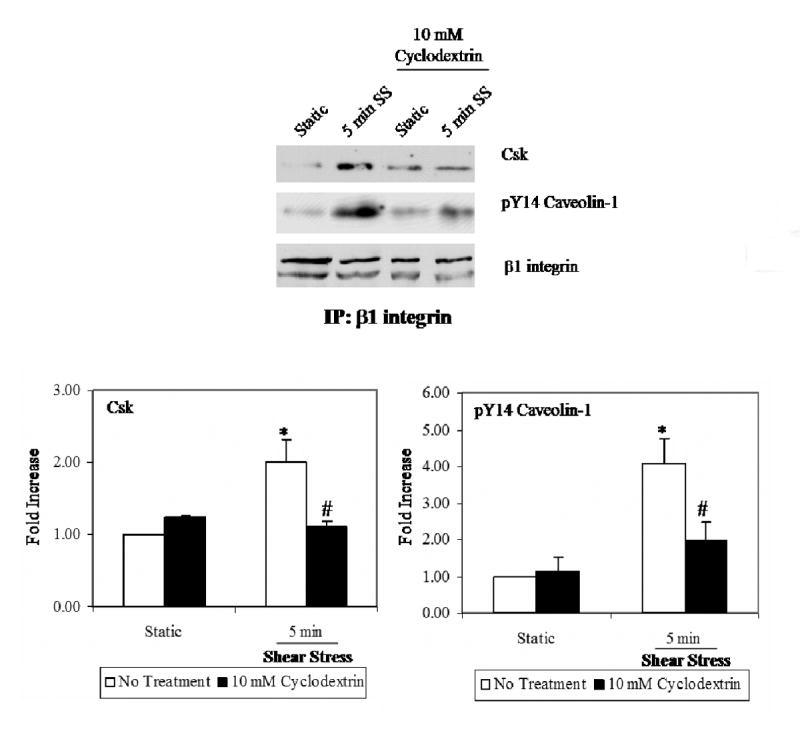
BAEC's were either kept static or sheared for 5 mins. In some experiments, cyclodextrin was added prior to shear treatment. β1 integrin was immuno-precipitated from cell lysates. The results show that shear stimulates increased association of Csk, and pY14-caveolin-1 with β1 integrin (*P.<0.05). Cholesterol removal attenuates the formation of this multi-protein mechanosensory complex (#P<0.05).
Plasma membrane cholesterol and caveolin-1 are required for shear-induced myosin light chain phosphorylation
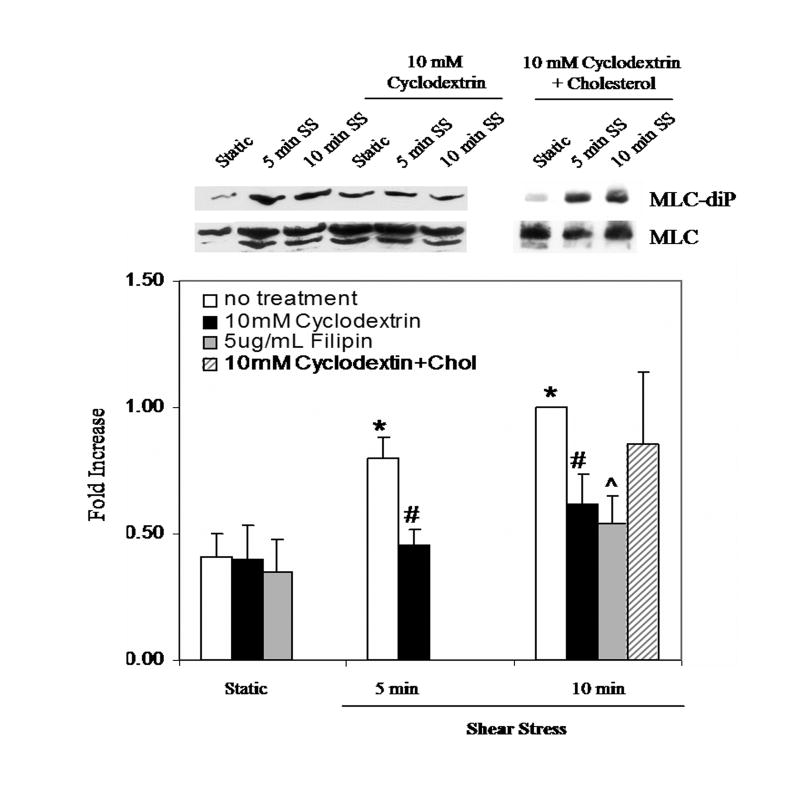
As CD treatment inhibited the increased association of phospho-caveolin-1 and Csk with the β1 integrin following shear stress, we tested the effects of cholesterol removal on myosin light chain (MLC) phosphorylation, a response that we previously established as downstream of β1 integrin activation and caveolin-1 tyrosine phosphorylation following shear stress [18]. As shown in Figure 3a, addition of either CD or filipin effectively blocked the shear-induced increase in MLC di-phosphorylation. The MLC di-phosphorylation response was reconstituted following cholesterol add-back, indicating the inhibitory effect of raft/caveolae dispersal by cholesterol depletion was reversible.
Figure 3. Shear Stress-enhanced Myosin Light Chain phosphorylation is dependent on plasma membrane cholesterol content and caveolin-1.
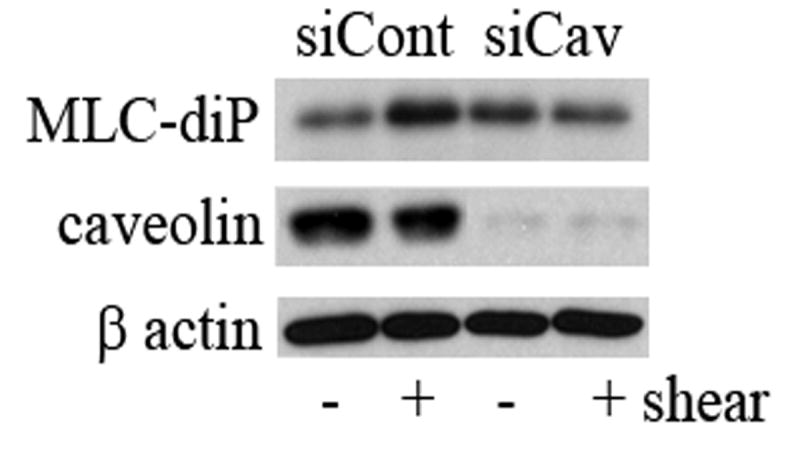
(A) BAEC's were pretreated with either cyclodextrin, filipin, or cyclodextrin followed by cyclodextrin preloaded with cholesterol. Shear stress (10dynes/cm2) for 5 and 10 min was sufficient to stimulate an increase in MLC di-phosphorylation (*P.<0.05). Both cyclodextrin (#P<0.05) and filipin (^P<0.05) significantly decreased shear-induced MLC di-phosphorylation. The effect was reversible by reloading cholesterol into the membranes. (B) Cells were transfected with siCONTROL or cav-1 SMARTpool siRNA. Approximately 85% of cells were successfully transfected as verified by siGLO fluorescent stable siRNA (data not shown). MLC-diphosphorylation in response to shear was attenuated in caveolin-1 depleted cells. β-actin verified equal loading.
To control for generalized effects of CD, BAEC were pretreated with calyculin A, a serine/threonine phosphatase inhibitor in order to prevent de-phosphorylation of MLC via myosin phosphatase. Similar to our previous findings [19], calyculin A applied to CD pretreated cells induced MLC phosphorylation levels without addition of stimuli demonstrating that myosin light chain is capable of being phosphorylated in the presence of CD (data not shown).
To provide a molecular approach for manipulating caveolae/caveolin-1, BAEC were transfected (48 hours) with either caveolin-1 siRNA (cav-1 SMARTpool) or a pool of non-targeting siRNAs (siCONTROL) prior to shear. Similar to our past findings [19], Figure 3b demonstrates a 90% reduction in caveolin-1 protein expression by this method. As in cells pre-treated with cholesterol altering compounds, MLC di-phosphorylation in response shear was significantly attenuated in caveolin-1 depleted cells but not in cells treated with control siRNA's (Fig. 3b) or transfection reagents alone (data not shown).
β1 integrin rapidly translocate to caveolae in response to shear stress
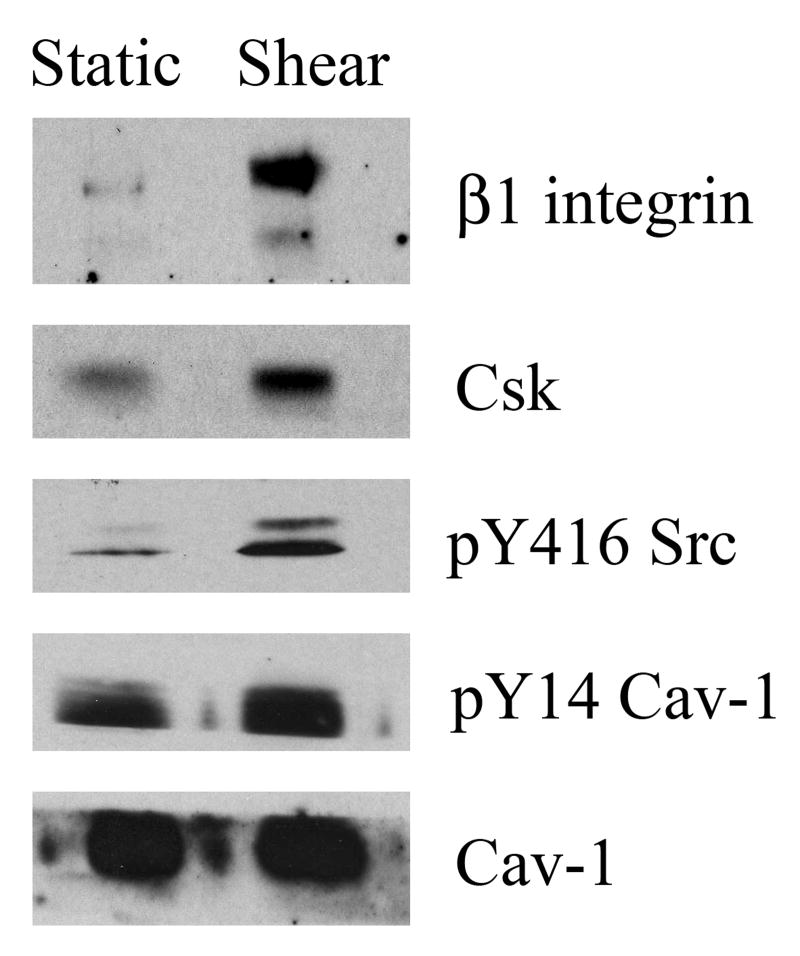
To evaluate whether caveolar microdomains (rather than membrane rafts in general) play a role in the spatial organization of integrin-mediated mechanotransduction events, caveolae were immunoaffinity isolated from static control and sheared BAEC plasma membranes, according to previous studies [14, 19]. We found that very little β1 integrin was present in caveolae derived from cells kept in static culture. However, shear stress induced β1 integrin transposition to the caveolae (3.6 +/− 0.7 fold relative to controls - Fig. 4). Csk was detected in the caveolae from non-sheared control cells and was recruited (2.7 +/− 0.4 fold) to this membrane compartment following acute exposure to shear stress. In addition, we detected a 1.9 +/− 0.3 fold enhancement of caveolin-1 tyrosine phosphorylation within caveolae derived from shear exposed plasma membranes (Fig. 4) while the levels of caveolin-1 associated with caveolae were unchanged by shear stress (Fig. 4).
Figure 4. Shear stress induces β1 integrin translocation into plasmalemmal caveolae.
BAEC were kept static or sheared for 5 min at 10 dynes/cm2. Plasma membranes were isolated by Percoll gradient centrifugation, sonicated and subject to immunoaffinity pull-down of caveolar vesicles. Caveolae bound fractions were resolved by SDS-PAGE and immunoblotted for caveolin-1, pY14 caveolin-1, Csk and β1 integrin.
Discussion
To address the concept that integrins and caveolae, both previously identified as independent mechanotransducing elements, may be functionally linked in their mechanotransduction properties, we used cholesterol modulating agents and caveolin-1 siRNA to disrupt the organization and function of raft/caveolae membrane compartments. We found that cholesterol depletion inhibited shear-induced caveolin-1 tyrosine phosphorylation (Fig. 1a), an observation which paralleled our previous findings showing that β1 integrin blockade inhibited shear-induced caveolin-1 phosphorylation [18]. Similar to caveolin-1 tyrosine phosphorylation, disruption of raft/caveolae structure significantly attenuated shear-activation of Src (Fig. 1b), a proximal event required for caveolin-1 phosphorylation. This finding, considered with the prior studies demonstrating that mechanical activation of Src is mediated by integrins [22], demonstrates that mechanotransduction processes that activate Src, which subsequently phosphorylates caveolin-1, also requires both cholesterol-rich rafts/caveolae domains and integrins.
As described in our past study [18], shear-induced tyrosine phosphorylation of caveolin-1 served a key event for formation of a larger signaling complex that associates with β1 integrin (Fig. 2). The formation of a β1 integrin/phospho-caveolin-1/Csk multi-protein signaling complex served as a modulus to regulate Src activity, myosin light chain phosphorylation and actin stress fibers in response to shear stress. Here, we show that CD prevented the formation of this complex (Fig. 2) and highlights the importance of rafts/caveolae structures in modulating this mechano-sensory signaling array.
When exposed to shear stress, endothelial cells rapidly form actin stress fibers via a signaling process that requires phosphorylation of myosin light chain (MLC). As noted, we showed that shear-induced MLC phosphorylation involves β1 integrin activation, caveolin-1 phosphorylation and Csk recruitment to integrin sites [18]. Our data thus far indicates that proper raft/caveolae cholesterol content is necessary to preserve these β1 integrin-mediated mechanotransduction events. More importantly, both CD and filipin significantly blocked shear-induced MLC phosphorylation (Fig. 3a). As an alternative and independent approach to evaluate caveolae in this process, caveolin-1 protein was depleted via siRNA. We found that BAEC's lacking caveolae/caveolin-1 were insensitive to shear stress effects on the phosphorylation of MLC (Fig. 3b) and confirm that caveolae and caveolin-1 regulate downstream integrin-mediated mechanotransduction events. The experimental findings from studies using cholesterol sequestering compounds are consistent with our recent report showing that filipin pretreatment attenuates thrombin-induced MLC phosphorylation as well as actin stress fiber formation in cultured endothelial cell [19]. However, in those studies, caveolin-1 knockdown did not influence the phosphorylation of MLC by thrombin, as we observed in endothelial cell monolayers exposed to shear stress (Fig. 3b). These findings suggest that membrane rafts may serve as a general signaling compartment that regulate cytoskeletal organization while the participation of caveolae may be more limited to specific stimuli such as fluid mechanical forces.
Since past studies indicate that integrin function may be mediated through their association with rafts compartments, we specifically analyzed caveolae purified by immunoaffinity techniques for the presence of β1 integrin and integrin associated signaling molecules. Here, we provide direct evidence that in response to shear stress, a pool of β1 integrin translocates, presumably from focal adhesions, to caveolae (Fig. 4). In addition, the content of Csk and pY14 caveolin-1 was enhanced in caveolae derived from shear exposed BAEC plasma membranes signifying that key components in the signaling cascade from β1 integrin to MLC are recruited along with β1 integrin to caveolae following shear stress.
It has been suggested that integrin transposition into rafts/caveolae may serve as a mechanism for internalization of these proteins thereby downregulating integrin activity. This process appears to be mediated by phosphorylation of caveolin-1 and its translocation from focal adhesion sites into rafts and/or caveolae compartments purportedly to signal an internalization event [23-25]. While we did not examine β1 integrin trafficking in our studies, under the scenario of β1 integrin internalization by rafts/caveolae, we would speculate that the consequence of caveolae-mediated β1 integrin removal from the plasma membrane may be to regulate integrin-linked effectors of the cytoskeleton such as the small GTPase, RhoA, which is enriched in membrane rafts and caveolae and whose activity and/or downstream effects can be altered by cholesterol depletion [26-28]. As previously suggested [29], RhoA internalization via caveolae could negatively regulate RhoA activity, a concept consitent with the observed temporally activity of RhoA in response to shear stress [10]. At later time points, RhoA may be restored to the plasma membranes since caveolae density is enhanced in endothelial cell cutures exposed to prolonged periods of laminar shear stress [14]. Thus, spatial regulation of RhoA may provide the cell with a means to morphologically adapt to a new flow environment through initial loss of RhoA function thereby decreasing focal adhesion stability followed by activation of newly positioned RhoA to signal for MLC phosporylation resulting in actin stress fiber formation.
In summary, our findings suggest that caveolae and β1 integrins are linked in their functions as mechanotransduction elements within endothelial cells. Future work should determine whether raft/caveolae disruption prohibits shear-induced integrin activation, alters Src targeting to properly phosphorylate caveolin-1 or spatially disturbs integrin relay to small GTPases which subsequently mediates dynamic reorganization of focal adhesion and the cytoskeleton in response to shear stress.
Acknowledgments
We thank Dr. Talia Borbiev (Temple University) for his assistance in the caveolin-1 siRNA experiments. This work was supported by a grant from the NIH (HL66301).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 2.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am J Physiol Cell Physiol. 2002;283:C1540–1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- 4.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 6.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecules? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 8.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 9.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzima E, Angel del Pozo M, Shattil S, Shein S, Schwartz M. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H, Go MY, St-John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol. 2000;278:H1285–1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol. 2003;285:H1720–1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. J Biol Chem. 1998;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in Mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 18.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288:H936–945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 19.Carlile Klusacek M, Rizzo V. Endothelial Cytoskeletal Reorganization In Response To Protease Activated Receptor-1 (Par1) Stimulation Is Mediated By Membrane Rafts But Not Caveolae. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ferraro JT, Daneshmand M, Bizios R, Rizzo V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-induced mechanotransduction pathways in osteoblast cultures. Am J Physiol Cell Physiol. 2004;286:C831–839. doi: 10.1152/ajpcell.00224.2003. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. Faseb J. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253:629–636. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 24.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gingras D, Gauthier F, Lamy S, Desrosiers RR, Beliveau R. Localization of RhoA to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Comm. 1998;247:888–893. doi: 10.1006/bbrc.1998.8885. [DOI] [PubMed] [Google Scholar]
- 27.Michaely PA, Mineo C, Ying YS, Anderson RG. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 29.Sharma DK, Brown JC, Cheng Z, Holicky EL, Marks DL, Pagano RE. The glycosphingolipid, lactosylceramide, regulates beta1-integrin clustering and endocytosis. Cancer Res. 2005;65:8233–8241. doi: 10.1158/0008-5472.CAN-05-0803. [DOI] [PubMed] [Google Scholar]