Abstract
Light intensities that limit electron flow induce rapid degradation of the photosystem II (PSII) reaction center D1 protein. The mechanism of this phenomenon is not known. We propose that at low excitation rates back electron flow and charge recombination between the QB•− or QA•− semiquinone acceptors and the oxidized S2,3 states of the PSII donor side may cause oxidative damage via generation of active oxygen species. Therefore, damage per photochemical event should increase with decreasing rates of PSII excitation. To test this hypothesis, the effect of the dark interval between single turnover flashes on the inactivation of water oxidation, charge separation and recombination, and the degradation of D1 protein were determined in spinach thylakoids. PSII inactivation per flash increases as the dark interval between the flashes increases, and a plateau is reached at dark intervals, allowing complete charge recombination of the QB•−/S2,3 or QA•−/S2 states (about 200 and 40 s, respectively). At these excitation rates: (i) 0.7% and 0.4% of PSII is inactivated and 0.4% and 0.2% of the D1 protein is degraded per flash, respectively, and (ii) the damage per flash is about 2 orders of magnitude higher than that induced by equal amount of energy delivered by excess continuous light. No PSII damage occurs if flashes are given in anaerobic conditions. These results demonstrate that charge recombination in active PSII is promoted by low rates of excitation and may account for a the high quantum efficiency of the rapid turnover of the D1 protein induced by limiting light.
Following the discovery of the light dependent turnover of reaction center II (RCII) D1 protein (1), the process of excess light-induced photoinactivation and the related degradation of photosystem II (PSII) proteins (photoinhibition) were studied intensively. Significant progress toward understanding the mechanism(s) involved was achieved (reviewed in refs. 2–4) in parallel with the investigation of the protective mechanisms that ensure in vivo maintenance of PSII activity. Photoprotective mechanisms include acclimation to limiting, as well as to high light intensities that exceed the saturation of electron flow (5–8).
Excess light intensities can lead to alteration of the RCII acceptor side activity that blocks electron transfer from QA•− to QB, which is followed by an increase in charge recombination events of the RCII primary radical pair P680•+/Pheo•−. The increased charge recombination promotes formation of 3P680, which may interact with oxygen and generate harmful singlet oxygen (4, 9–12). Due to the short lifetime of the singlet oxygen (1O2), the oxidative damage is confined to PSII and targets the D1 protein for degradation (2). Alternatively, excess light intensity may generate a high ΔpH across the thylakoid membrane. Acidification of the thylakoid lumen may lead to partial release of Ca2+ from the oxygen evolving complex, lower the QA redox potential and down-regulate PSII activity (13, 14). Following inactivation of the oxygen evolving complex, oxidative damage due to the formation of RCII cation radicals YZ•− and P680•+ (9, 15) may trigger the degradation of the D1 protein.
The above mechanism(s) cannot account for PSII damage and the degradation of the RCII–D1 protein when chloroplasts are exposed to nonsaturating light intensities (3). At limiting light intensities, the proton gradient is dissipated via synthesis and utilization of ATP in the process of carbon fixation. Furthermore, it is noteworthy that the quantum yield of photodamage to PSII increases considerably at low light intensity (16).
Electron flow within PSII is gated at the secondary acceptor quinone QB, which is reduced to plastoquinol only following two consecutive photochemical events. The semiquinone QB•− formed after a single charge separation is firmly bound at the QB site and decays slowly to the quinone state by back electron flow to the oxidized S2,3 states of the oxygen evolving complex (2, 4, 17). Based on experimental results obtained using the unicellular green alga Chlamydomonas reinhardtii we have proposed that back electron flow from QB•− to the S2,3 states of the water oxidation complex in the dark interval between two consecutive excitations may occur with high probability at low light intensities (16).
Due to the equilibrium between reduced and oxidized PSII components, the primary radical pair P680•+/Pheo•− may be regenerated in the dark and charge recombination may result in the triplet chlorophyll (3Chl) formation (17, 18). Therefore one would expect that charge recombination and hence damage to PSII will occur with increased efficiency per photon absorbed as the rate of excitation decreases. In this work we present evidence to support this hypothesis.
MATERIALS AND METHODS
Thylakoid Preparation.
Spinach (Spinacia oleracea) plants were grown at 22°C under white fluorescent light with a 10/14-h light/dark regime or in the field during the winter months. Thylakoids were isolated as described (19) and were resuspended in 10 mM phosphate buffer (pH 7.4) containing 5 mM MgCl2, 100 mM sucrose, and 5 mM NaCl.
Light Treatment.
For single turnover light excitation 1 ml of thylakoid suspensions (0.4 mg Chl/ml) were exposed to laser flashes in a 3 ml Pyrex cuvettes (1 cm optical path) with constant stirring by a magnetic bar. The samples were kept under air or nitrogen atmosphere by bubbling oxygen-free N2. Nearly saturating light pulses (532 nm, 300 mJ cm−2/pulse, 9-ns pulse width) were provided by a Quanta Ray (Mountain View, CA) Nd:YAG (yttrium/aluminum garnet) laser DCR-1A. Direct measurements indicate that about 60–70% of the incident light was absorbed by the sample (6 × 1017 absorbed photons per 2.4 × 1017 Chl molecules, equivalent to about 0.6 photons per 10 ps per PSII unit per charge separation time). Lowering the energy output of the laser beam by 40% resulted in a proportional decrease of the flash induced PSII damage, indicating that the light energy used did not exceed that required to saturate the sample (data not shown). A sample holder containing up to eight cuvettes in complete darkness was constructed, and the samples were exposed to laser light pulse regimes by rotating the sample holder. The interval between the pulses for each sample was controlled by a computer which synchronized the laser Q-switch with the rotation of the sample holder. In this way the laser could be operated at 10 Hz, allowing illumination of each sample at different time intervals. The temperature of the sample holder was kept constant at 21°C (±1°C). Control samples were exposed to continuous low intensity fluorescent white light (30 μmol m−2·s−1) or incubated in the dark for a similar time period.
Measurements of PSII Activity.
PSII activity was assayed by 2,6-dichloroindophenol (DCIP) reduction using water as an electron donor (20). Samples were illuminated by saturating red light (Schott cut-off filter above 650 nm) in 1-ml cuvette containing thylakoids equivalent to 4 μg Chl/ml and 1 × 10−5 M DCIP in the thylakoid suspension buffer. Loss of DCIP reduction activity could result from the inactivation of PSII charge separation, the water oxidizing complex and/or inhibition of electron flow from QA•− to QB (10, 12, 14, 21). However charge separation and electron transfer from YZ (the primary electron donor to P680•+), to QA (the primary electron acceptor quinone) as well as charge recombination could persist while the S states cycle or electron transfer from QA•− to the plastoquinol may be impaired. Such residual activity could be detected by thermoluminescence (TL) measurements as the AT band (peak photon emission temperature at about −20°C) (22). TL measurements were carried out as described (22, 23). Samples (20–40 μg Chl) were dark adapted on the TL apparatus stage for 2 min, then excited by continuous illumination while rapidly cooling down (2°C/s) to −40°C. The frozen samples were then heated at 0.6°C/s and the light emitted as a function of temperature was measured by photon counting. For measuring the TL signal originating from QA•−/S2 charge recombination 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; 2 × 10−6 M) was added before the dark adaptation. The signal intensity was calculated as the integrated photon counts under the curve for each TL signal, and the integrated area of a curve produced by a dark adapted control sample was subtracted.
Determination of the Thylakoid D1 Protein Content.
Thylakoid membrane proteins were resolved by SDS/PAGE, and the D1 protein content was measured by immunoblotting using monospecific antibodies and 125I-labeled protein A (Amersham) for detection (16). Autoradiograms, in the linear range of exposure, were scanned and quantified by the National Institutes of Health image program. The amount of D1 protein was normalized to the total amount of protein loaded on the gel as estimated by scanning of Comassie brilliant blue R-250 stained gels of the same samples (16).
RESULTS
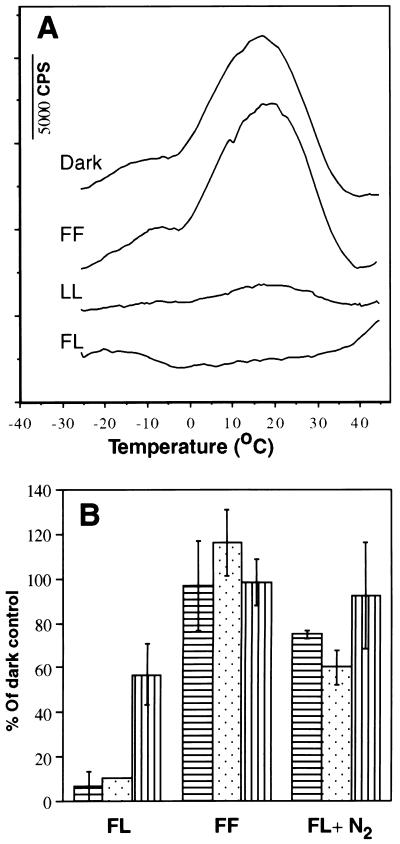
Excitation by single turnover flashes given at a low repetition rate can mimic low levels of continuous illumination if the repetition rate of the light flashes is low enough to prevent rapid double reduction of QB, by consecutive flashes and its protonation to plastoquinol (15). To test the hypothesis that under such conditions, back electron flow from QB•− to S2,3 states may occur via primary pair charge recombination and formation of singlet oxygen (3, 16) spinach thylakoids were exposed under air or anaerobic conditions to 360 flashes with 40-s dark intervals between the flashes. A severe, irreversible loss of the TL signal (Fig. 1A) and DCIP reduction accompanied by D1 protein degradation (Fig. 1B) occurred only in thylakoids exposed to the flashes under aerobic conditions (Fig. 1B). These results indicate that oxygen is involved in the damaging process induced by single turnover flashes, as is the case in continuous high light intensity exposure (19). It is noteworthy that no loss of PSII activity and only a marginal degradation of the D1 protein were observed in thylakoids exposed to 360 flashes delivered under aerobic conditions at 100-ms dark interval between the flashes. At this excitation rate QB is double reduced to plastoquinol (24), lowering significantly the probability for back electron flow and charge recombination (Fig. 1). Thus, the rate of excitation plays a major role in the efficiency of the photodamaging process. Under these conditions photodamage is not directly proportional to the total amount of absorbed energy.
Figure 1.
Effect of the interval between flash excitations and presence of oxygen on the loss of PSII activity and degradation of the D1 protein. Spinach thylakoids were exposed for 4 h to 360 laser flashes at 40-s dark intervals under aerobic (FL) or anaerobic (FL + N2) conditions or to 4 h of continuous low light (LL, 20 μmol m−2·s−1). Alternatively, a total of 360 flashes were given at 100-ms intervals followed by 4 h of further incubation in the dark (FF). PSII activity was measured by TL (A and B, dotted column) and DCIP reduction (B, horizontal line column). D1 protein content was determined by Western blot analysis (B, vertical line column).
To test the hypothesis that charge recombination between the semiquinones QB•− or QA•− and the S2,3 states may damage functional PSII, one could assay the relation between the extent of charge recombination and the damage inflicted by single turnover flashes. To this end it is necessary (i) to determine the lifetime of the QB•− or QA•−/S2,3 states generated by single turnover flashes that decay by charge recombination (25), and (ii) to determine the relationship between the number of flashes delivered at a given rate and the resulting PSII inactivation and degradation of the D1 protein.
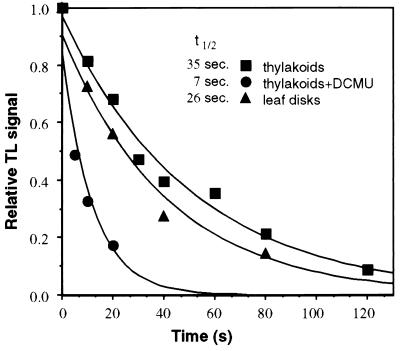
Measurements the decay of the TL signal intensity as a function of the incubation time of the sample in the dark following a single flash excitation, permits to determine the t½ of the QB•−/S2,3 states (25). Following single turnover excitation after dark adaptation most of RCII are in the S2 state (22, 25). Under the conditions used in these experiments we did not resolve the rates of QB•− or QA•− recombination with the S2 or S3 states, and thus the values obtained are the average of the recombination with both S states. The t½ value for the charge recombination of QB•−/S2 states, at room temperature, was found to be ≅35 s (Fig. 2). Similar results were obtained with pea thylakoids (data not shown). This value is comparable to the t½ in vivo as assayed in spinach leaf disks (Fig. 2) as well as to values reported before for spinach thylakoids assayed by both TL and the oxygen flash yield techniques (24, 25).
Figure 2.
Lifetime of QB•− or QA•−/S2,3 charge recombination in spinach thylakoids and leaf disks. Samples were exposed to one saturating light flash at 20°C followed by dark interval periods from 0 to 120 s. At the end of the dark period the samples were rapidly cooled (less than 5 s to reach 5°C) and frozen to −40°C after which the residual TL signal was measured. A total of 40 μg Chl/sample or three pea leaf disks, 0.6 cm diameter, were used per measurement. ▴, Leaf disks; ▪, thylakoids; •, thylakoids with addition of DCMU. ñ
Similar measurements were carried out in the presence of DCMU which binds at the QB site and prevents QA•− oxidation (26). Upon light excitation, only QA•− is formed that can recombine with the S2 states (25). The results of such experiments show that the t½value for QA•−/S2 charge recombination at room temperature is about 5–7 s (Fig. 2). The lifetime of the QA•−/S2 state is shorter because the activation energy for recombination is lower than that required for the recombination of QB•−/S2,3 state (17, 25).
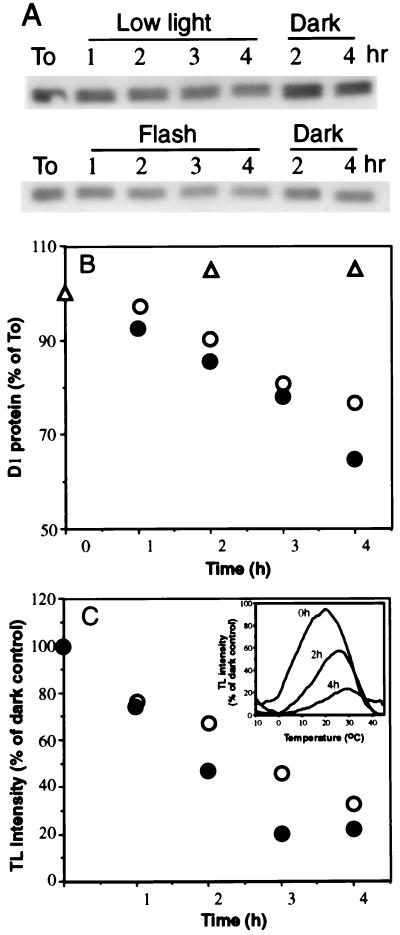
The loss of PSII activity and degradation of the D1 protein were found to be proportional to the number of flashes delivered at a 40-s dark interval over a period of 4 h. The inactivation of PSII and degradation of the D1 protein in such experiments were similar to those obtained in thylakoids exposed to continuous low light intensity (Fig. 3). The TL signal profile did not exhibit an emission band at 7–10°C characteristic of inhibition of electron transfer from QA•− to QB (Fig. 3C Inset) or appearance of the AT band related to loss of electron donation from the water splitting complex (data not shown; see also Fig. 1A). The rate of the D1 protein degradation is slower than that of the PSII photoinactivation. Thus, part of the remaining D1 protein in the photoinactivated PSII may be already “tagged” for degradation as demonstrated before (2, 27, 28). The degradation of the D1 protein is therefore rate limiting for the process of its replacement in vivo with newly synthesized precursor D1 protein (4, 27).
Figure 3.
Kinetics of the PSII inactivation and D1 protein degradation induced by single turnover flashes. Thylakoids were exposed to flashes delivered at 40-s dark interval (•), to continuous white light (○, 30 μmol m−2·s−1) or as a control were kept in the dark for the same period (▵). Lane To, D1 protein level before light exposure. All incubations were at room temperature. Sample where taken at times as indicated and assayed for the D1 protein content (A and B) and TL signal (C). (C Inset) Recorded TL measurements indicating loss of the QB•−/S2,3 emission (22) as a function of increasing exposure time to the flashes. No block in electron flow from QA•− to QB or loss of the oxygen evolving complex activity occurred as evidenced by the absence of the QA•−/S2 band (emission at 7–10°C).
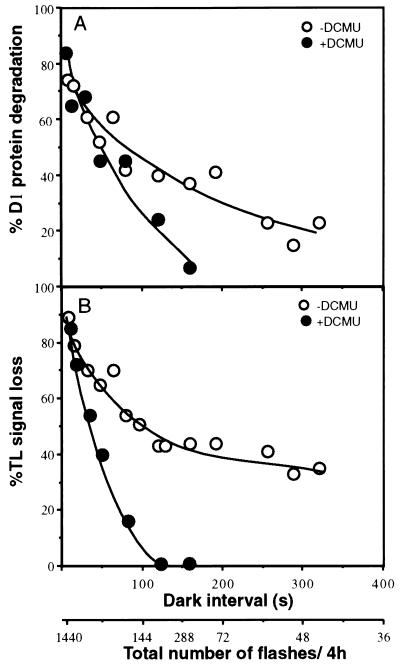
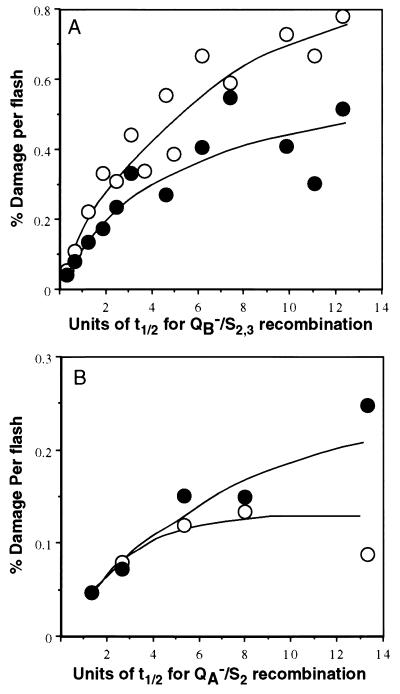
Based on these results we could test the relation between the loss of PSII activity and the degradation of D1 protein relative to the time interval between the flashes. Recombination will occur in a larger fraction of the PSII population present in the QB•−/S2,3 states generated by a single turnover flash if the interval between the flashes increases. Spinach thylakoids were exposed to series of flashes given at 8- to 300-s intervals between the flashes for a period of 4 h, and the degree of PSII damage was subsequently assayed (Fig. 4). For equal exposure times to flash regimes of different repetition rates, the total number of flashes given to a sample increases with the repetition rate. Thus, fewer flashes are delivered as the time interval between the flashes increases, as indicated in the abscissa of Fig. 4. Accordingly, the total loss of PSII activity and degradation of the D1 protein in samples exposed to the various flash regimes for 4 h decreases with the increase in the interval between the flashes. However, based on the proportionality of the damage incurred to the number of flashes (Fig. 3) we have calculated the efficiency of the damage per flash as a function of the interval between the flashes (Fig. 4). The results of this calculation (Fig. 5) show indeed that the efficiency per flash for the loss of PSII activity and D1 protein degradation increases if the interval between the flashes increases. As predicted by the working hypothesis, a clear relation is established between the extent of charge recombination in PSII (expressed as t½ of the TL signal decay) and the damage to PSII. This conclusion is supported by the fact that, qualitatively, a plateau for the damage efficiency per flash is reached when the interval between the flashes reaches an approximate value of about 7–8 t½ units, equivalent with complete charge recombination. The plateau is reached at about the same multiplicity of units irrespective of whether recombination occurred between QB•−/S2,3 or QA•−/S2 states (t½ = 35 s and t½ = 7 s, respectively).
Figure 4.
Relation between the flash induced photoinactivation and degradation of the D1 protein and increasing the dark interval between the flashes. Thylakoids were exposed for 4 h to light flashes in absence or presence of DCMU. The dark intervals between the flashes varied from 8 to 320 s, corresponding to a total of 1800 to 45 flashes delivered per sample, respectively. The samples were analyzed for D1 protein content (A) and TL signal (B). • and ○, Thylakoids with or without addition of DCMU, respectively. The data represent percent of a dark control.
Figure 5.
Increase in the efficiency of damage per flash as a function of the charge recombination extent. Calculated loss of PSII activity/flash (open symbols) and degradation of D1 protein (solid symbols) as a function of the extent of charge recombination during the dark interval expressed as t½ units (Fig. 2) using the data of Fig. 4. Thylakoids were exposed to light flashes in the absence (A) or presence (B) of DCMU, respectively.
DISCUSSION
Mechanism of the Flash-Induced Damage to PSII.
The data presented in this work demonstrate a strong correlation between the extent of back electron flow in PSII from the semiquinone acceptors to the S2,3 oxidation states of the donor side (17) and the single turnover flash-induced damage to PSII. The interpretation of the results is based on the concept that the measured TL signal represents charge recombination via the primary radical pair formed in the dark during this process (22). The formation of the primary radical pair in the dark at least in part of the PSII population in the QB•−/S2,3 or QA•−/S2 states is indicated by the photon emission during de-excitation of singlet P680 generated by charge recombination of 1[P680•+/Pheo•−] (18, 29, 30).
The concept that PSII may be damaged by formation of singlet oxygen via interaction with 3Chl formed during charge recombination is well established in the case of excessive light-induced photoinhibition (9, 11). In the case of limiting light intensity or single turnover flashes one may consider that damage may be due to the formation of the donor side tyrosine radical YZ•− in the process of back electron flow to S2,3. The potential of the YZ•+ radical is sufficient to cause oxidative harm to PSII (9) irrespective of oxygen presence. However the requirement of oxygen demonstrated here points clearly to a mechanism in which 3Chl and 1O2 are involved. Detection of 1O2 was however not attempted in this work. The low concentration of PSII units in our experiments (≅μM) imposed by the limitation of light saturation in a flash, the t½ of the recombination time and the predicted level of 1O2 during the recombination process are below the detection level of presently available methods.
Under anaerobic conditions the photoinactivation of isolated thylakoids is reversible. Recovery of 70–80% of the initial activity requires at least 1 h of dark incubation at room temperature (19). In this work we have tested the degree of photoinactivation immediately following the flash treatment under the nitrogen atmosphere. The photoinactivated samples were kept in ice until the measurement was performed thus avoiding putative reactivation.
It was proposed recently that hydrogen peroxide induces degradation of the D1 protein in the dark (31). Hydrogen peroxide may be generated in PSII as product of incomplete water oxidation (32). The peroxide concentrations required to induce even a limited degradation of the D1 protein were found to be in the mM range (31). Even if hydrogen peroxide is generated in all reaction centers by each flash excitation, its concentration would not exceed a μM level. Production of hydrogen peroxide cannot increase with the dark interval between the flashes. Furthermore, it is highly improbable that the same plateau for hydrogen peroxide induced damage per flash will be obtained for the recombination of QB•−/S2,3 and the QA•−/S2 states.
The results presented here are in agreement with our working hypothesis and indicated that the loss of PSII activity induced by the single turnover flashes (as in the case of low light), occurs in PSII in which both, the acceptor and donor side are functional (3). Loss of PSII activity under these conditions is due to the inactivation of the primary charge separation. This interpretation is in agreement with our preliminary EPR data showing that the PSII signal IIslow, QA•−/Fe2+, the Pheo•− and the multiline EPR signal of the water splitting manganese complex decrease simultaneously during exposure of thylakoids to single turnover flashes or continuous low light (33).
Efficiency of QB•−/S2,3 or QA•−/S2 States Recombination in PSII Photoinactivation and D1 Protein Degradation.
It has been shown previously that the photodamage to PSII is proportional to the amount of absorbed energy above light intensities which saturate electron flow (34). Recently, it was proposed that PSII actually acts as “a photon counter” (35). Furthermore, based on the assumption that photoinhibition is a first-order process, it was proposed that the rate of photodamage/incident light is constant at all light intensities (36). These conclusions are in contradiction to the proposed photoinhibition mechanism ascribing different rates of photoinhibition under various light treatments and electron flow rates (2, 4, 9–12, 37, 38). Moreover a constant rate of photoinactivation does not account for the light-induced activation of the protective mechanism of energy dissipation and down regulation of PSII activity (3, 6, 7).
The results presented here are in disagreement with a constant probability for PSII damage at all light intensities. The efficiency of the single turnover flashes in photoinactivating PSII is considerably higher than that obtained by exposure to excessive continuous light. Based on the data presented here one can calculate that PSII can be completely inactivated by a total of 150 charge separation events and the resulting primary radical pair charge recombinations following decay of the QB•−/S2,3, states. Since, as shown here, the degree of damage increases with the time interval between the charge separation, one can conclude that the recombination events are the reason for the damage incurred to PSII. It is reasonable to assume that not all recombinations and 3Chl formation result in generation of harmful singlet oxygen. Partial protection against this possibility may be ascribed to the presence of the RCII carotens. Oxidation of the reduced semiquinone acceptors in the dark may occur also by alternative routes (14, 39) that may be harmless to PSII. The relative contribution of the alternative decay paths and their significance is not yet known. Quantification of the level of singlet oxygen generated during the charge recombination under the experimental condition used here may provide a final assessment of the damaging efficiency of this process.
On the basis of the total incident energy of the laser flash (150 flashes, total 1.5 × 1020 photons) and the concentration of RCII (about 2.4 × 1014 PSII/sample at 0.4 mg Chl/ml and assuming 250 Chl/PSII) one can calculate a probability of about 4 × 10−5 for the maximal efficiency of the incident photon delivered by the light flashes at 7–8 t½ units of dark interval as compared with a reported constant probability of 7 × 10−8 for inactivation of PSII per incident photon irrespective of the incident light intensity (36). In view of these results it is evident that the process of PSII photoinactivation cannot occur with a constant probability per absorbed photon.
The surprising results obtained in this work can be rationalized by an analysis of the probabilities for 3Chl formation during charge recombination processes between QB•− or QA•− and the S2,3 states (18, 40). Two cases should be considered. (i) Charge recombination occurring within the radical pair, 1[P680•+/Pheo•−] is faster than the inter-system crossing reaction 1[P680•+/Pheo•−] ↔ 3[P680•+/Pheo•−]. In this case efficient deexcitation of 1[P680] by fluorescence is observed. (ii) Charge recombination occurs in the 1[P680•+ Pheo QA/QB•−] state. In this case the process is slower than the inter-system crossing 1[P680•+/Pheo•−] ↔ 3[P680•+/Pheo•−] and the probability for recombination of 3[P680•+/Pheo•−] resulting in 3[P680] formation, and its interaction with oxygen to generate reactive 1O2 is high.
It was reported previously that occupancy of the QB site by DCMU which in light-exposed thylakoids blocks the site in the reduced state and promotes charge recombination between [P680•+/Pheo•−] protects PSII from photodamage (41) and degradation of the D1 protein (27, 42). In the presence of DCMU, in continuous light, the state [P680•+/Pheo QA•−] prevails (10). Due to the effect of the repulsion by the QA•− charge, the recombination time is shorter than the inter-system crossing 1[P680•+/Pheo•−] ↔ 3[P680•+/Pheo•−] (29). In this case the probability for 3Chl formation could be very small (18, 40). In terms of triplet formation efficiency, one may consider the following series: [P680•+ Pheo QA/QB•−] > [P680•+ Pheo/QA•−] > [P680•+/Pheo•−] > [P680•+/Pheo•−/QA•−] (11).
In the case of single turnover flash induced photodamage as reported here, the state [P680•+/Pheo•−/QA•−] is not generated. This is a consequence of the charge recombination occurring between the QA•−/S2 states during the dark interval between the flashes in the presence of DCMU. Thus protection by DCMU against loss of PSII activity and D1 protein degradation is expected to be limited (30–50%) as observed in this work.
One should note that the low light or flash induced damage to PSII in vivo may not activate the protective mechanisms against high light intensity such as the state transition (7), the activation of the xantophyll cycle resulting in thermal dissipation of excess absorbed energy (6, 8), lowering the potential of QA•− (14) and the phosphorylation of the PSII core proteins preventing the D1 protein degradation (4, 43). Thus the damage per light absorbed may be further enhanced in absence of these protective conditions as compared with that induced at light intensities above saturation of electron flow.
The question arises as to what extent the damage induced by back electron flow and charge recombination under low light conditions simulated by single turnover flash excitation is relevant to natural conditions. As an example, light intensities of 1–10 μmol m−2·s−1 prevail in the shadowed lower canopy of plants when exposed in sun light to 1500 μmol m−2·s−1. At these low intensities the rate of PSII excitation in a leaf containing 500 μmol Chl m−2 is compatible with about 2% to 0.2% charge recombinations/charge separation, which according to the data presented here would account for a half lifetime of PSII activity and D1 protein degradation in the range of 2–20 h in accordance with published data (16, 35, 36).
In conclusion, this work demonstrates that back electron flow induced in PSII as a result of low rates of light excitation is highly efficient in generating loss of PSII activity and the related degradation of the D1 protein. The efficiency of this process considerably exceeds that of the photoinhibition induced by excess light and may account for the turnover of the D1 protein observed to occur in vivo at low photon flux densities. Although the quantum yield for 3Chl formation and the related oxidative damage increases with lowering the photon flux density, the same mechanism of back electron flow and charge recombination in active PSII may be responsible for photodamage at all light intensities.
Acknowledgments
This work was supported by a grant from the Israeli Science Foundation awarded to I.O. and partially by a grant from the German Israeli Foundation, awarded to I.O. in cooperation with L. Eichacker and W. Rudiger (Munich), and a Human Frontiers Research Program grant. The support of the Minerva Avron Even-Ari Center for Regulation of Photosynthesis under Environmental Stress Conditions and to H.L. by The Minerva Farkas Center for Light-Induced Processes are acknowledged as well.
Footnotes
Abbreviations: Chl, 3Chl, chlorophyll and triplet chlorophyll respectively; DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea; DCIP, 2,6-dichloroindophenol; 1O2, singlet oxygen; PSII, photosystem II; RCII, reaction center II; P680, Pheo, primary electron donor and acceptor of RCII; QA, QB, the primary and secondary electron acceptor quinones; QA, QB and AT bands, thermoluminescence signals resulting from charge recombination of QA•−, QB•−/S2,3 states of the PSII oxygen evolving complex or with the histidine electron donor to P680•+; TL, thermoluminescence.
References
- 1.Mattoo A K, Pick U, Hoffman F H, Edelman M. Proc Natl Acad Sci USA. 1981;78:1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasil O, Adir N, Ohad I. In: The Photosystems: Structure, Function and Molecular Biology. Barber J, editor. Amsterdam: Elsevier; 1992. pp. 295–348. [Google Scholar]
- 3.Ohad I, Keren N, Zer H, Gong H, Mor T S, Gal A, Tal S, Domovich Y. In: Photoinhibition of Photosynthesis: From the Molecular Mechanisms to the Field. Baker N R, Bowyer J R, editors. Oxford: Bios; 1994. pp. 161–177. [Google Scholar]
- 4.Aro E-M, Virgin I, Andersson B. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 5.Raven J A. In: Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Baker N R, Bowyer J R, editors. Oxford: Bios; 1994. pp. 449–461. [Google Scholar]
- 6.Demmig-Adams B, Adams W W., III Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- 7.Ruban A V, Young A J, Pascal A A, Horton P. Plant Physiol. 1994;104:227–234. doi: 10.1104/pp.104.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koroleva O Y, Brueggemann W, Krause G H. Physiol Plant. 1994;92:577–584. [Google Scholar]
- 9.Hideg E, Spetea C, Vass I. Biochim Biophys Acta. 1994;1186:143–152. [Google Scholar]
- 10.Vass I, Styring S, Hundal T, Koivuniemi A, Aro A-M, Andersson B. Proc Natl Acad Sci USA. 1992;89:1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vass I, Styring S. Biochemistry. 1993;32:3334–3341. doi: 10.1021/bi00064a016. [DOI] [PubMed] [Google Scholar]
- 12.Kirilovsky D, Ducruet J M, Etienne A L. Biochim Biophys Acta. 1990;1020:87–93. [Google Scholar]
- 13.Krieger A, Weis E. Photosynth Res. 1993;37:117–130. doi: 10.1007/BF02187470. [DOI] [PubMed] [Google Scholar]
- 14.Johnson G N, Rutherford A W, Krieger A. Biochim Biophys Acta. 1995;1229:202–207. [Google Scholar]
- 15.Eckert H J, Gieken B, Bernarding J, Napiwotzki A, Eichler H J, Renger G. Photosynth Res. 1991;27:97–108. doi: 10.1007/BF00033249. [DOI] [PubMed] [Google Scholar]
- 16.Keren N, Gong H, Ohad I. J Biol Chem. 1995;270:806–814. doi: 10.1074/jbc.270.2.806. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford A W. Trends Biol Sci. 1989;14:227–232. doi: 10.1016/0968-0004(89)90032-7. [DOI] [PubMed] [Google Scholar]
- 18.Volk M, Gilbert M, Rousseau G, Richter M, Ogrodnik A, Michel-Beyerle M-E. FEBS Lett. 1993;336:357–362. doi: 10.1016/0014-5793(93)80837-k. [DOI] [PubMed] [Google Scholar]
- 19.Hundal T, Aro E M, Carlberg I, Andersson B. FEBS Lett. 1990;267:203–206. doi: 10.1016/0014-5793(90)80925-9. [DOI] [PubMed] [Google Scholar]
- 20.Schuster G, Timberg R, Ohad I. Eur J Biochem. 1988;177:403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohad I, Koike H, Shochat S, Inoue Y. Biochim Biophys Acta. 1988;933:288–298. [Google Scholar]
- 22.Inoue Y. In: Biophysical Techniques in Photosynthesis. Amesz J, Hoff A J, editors. Dordrecht, The Netherlands: Kluwer; 1996. pp. 93–107. [Google Scholar]
- 23.Zer H, Prasil O, Ohad I. J Biol Chem. 1994;269:17670–17676. [PubMed] [Google Scholar]
- 24.Forbush B, Kok B, McGolin M P. Photochem Photobiol. 1971;14:307–321. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 25.Vass I, Horvat G, Demeter S. Biochim Biophys Acta. 1981;634:140–152. doi: 10.1016/0005-2728(81)90134-1. [DOI] [PubMed] [Google Scholar]
- 26.Tietjen K G, Kluth J F, Andree R, Haug M, Lindig M, Muller K H, Wroblowsky H J, Trebst A. Pestic Sci. 1991;31:65–72. [Google Scholar]
- 27.Zer H, Ohad I. Eur J Biochem. 1995;231:448–453. doi: 10.1111/j.1432-1033.1995.tb20718.x. [DOI] [PubMed] [Google Scholar]
- 28.Barber J. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. London: Kluwer; 1995. pp. 159–164. [Google Scholar]
- 29.van Mieghem F, Brettel K, Hillmann B, Kamlowski A, Rutherford A W, Schlodder E. Biochemistry. 1995;34:4798–4813. doi: 10.1021/bi00014a038. [DOI] [PubMed] [Google Scholar]
- 30.Govindjee, van De Ven M, Preston C, Sibert M, Gratton E. Biochim Biophys Acta. 1990;1015:173–179. doi: 10.1016/0005-2728(90)90017-x. [DOI] [PubMed] [Google Scholar]
- 31.Miyao M, Ikeuchi M, Yamamoto N, Ono T. Biochemistry. 1995;34:10019–10026. doi: 10.1021/bi00031a025. [DOI] [PubMed] [Google Scholar]
- 32.Bradley R L, Long K M, Frasch W D. FEBS Lett. 1991;286:209–213. doi: 10.1016/0014-5793(91)80975-9. [DOI] [PubMed] [Google Scholar]
- 33.Keren N, van Kan P J M, Berg A, Gong H, Shochat S, Levanon H, Styring S, Andersson B, Ohad I. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. London: Kluwer; 1995. pp. 299–302. [Google Scholar]
- 34.Jones L W, Kok B. Plant Physiol. 1966;41:1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y I, Anderson J M, Chow W S. Planta. 1996;198:300–309. [Google Scholar]
- 36.Tyystjarvi E, Aro E-M. Proc Natl Acad Sci USA. 1996;93:2213–2218. doi: 10.1073/pnas.93.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setlik I, Suleyman I, Allakhverdiev S I, Nedbal L, Setlikova E, Klimov V V. Photosynth Res. 1990;23:39–48. doi: 10.1007/BF00030061. [DOI] [PubMed] [Google Scholar]
- 38.Ohad I, Adir N, Koike H, Kyle D J, Inoue Y. J Biol Chem. 1990;265:1972–1979. [PubMed] [Google Scholar]
- 39.Barber J, De Las Rivas J. Proc Natl Acad Sci USA. 1993;90:10942–10946. doi: 10.1073/pnas.90.23.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levanon H, Norris J R. In: Light Reaction Path of Photosynthesis. Fong F K, editor. Berlin: Springer; 1982. pp. 152–195. [Google Scholar]
- 41.Jegerschold C, Virgin I, Styring S. Biochemistry. 1990;29:6179–6186. doi: 10.1021/bi00478a010. [DOI] [PubMed] [Google Scholar]
- 42.Jansen A K, Depka B, Trebst B, Edelman M. J Biol Chem. 1993;268:21246–21252. [PubMed] [Google Scholar]
- 43.Dannehl H, Herbik A, Godde D. Physiol Plant. 1995;93:179–186. [Google Scholar]