Abstract
The transmembrane (TM) domains of viral fusion proteins are required for fusion, but their precise role is unknown. G protein, the fusion protein of vesicular stomatitis virus, was previously shown to lose syncytia-forming ability if six residues (GLIIGL) were deleted from its TM domain. The 20-residue TM domain of wild-type (TM20) G protein was thus changed into a TM domain of 14 residues (TM14). To assess possible sequence specificity for this loss of function, the two Gly residues in TM20 were replaced with either Ala or Leu. Both mutations resulted in complete loss of fusion activity, as measured by fusion-dependent reporter gene transfer. Single substitutions decreased activity by about half. TM14 was weakly active (15%) but reintroduction of a Gly residue into TM14 by a single Ile → Gly substitution increased activity to 80%. All mutants retained normal hemifusion activity, i.e., lipid mixing between the outer leaflets of the reacting membranes. Thus, at least one TM Gly residue is required for a late step in fusion mediated by G protein. Gly residues were significantly (2.6-fold; P = 0.004) more abundant in the TM domains of viral fusion proteins than in those of nonfusion proteins and were distributed differently within the TM domain. Thus, Gly residues in the TM domain of other viral fusion proteins may also prove to be important for fusion activity.
Keywords: virus envelope, α-helix, glycine hinge, anchor sequence
All enveloped viruses initiate infection by membrane fusion between the virus and its target cell, a process mediated by a viral fusion protein. The transmembrane (TM) domain is essential in this reaction, as has been suggested by several recent studies (1–8). A construct of the influenza virus fusion protein hemagglutinin (HA) was prepared in which the entire external domain of HA was attached to a glycerylphosphatidylinositol (GPI) moiety that anchored the protein only into the external leaflet of the membrane bilayer. This protein lost the ability to mediate complete fusion, i.e., aqueous content mixing, but retained the ability to promote hemifusion, i.e., mixing of lipids of the two external leaflets (1, 2). Membranes that underwent GPI-HA-mediated hemifusion were able to complete the fusion reaction upon addition of agents that destabilized the inner, but not the outer, membrane leaflet (3). Observations with fusion proteins of Moloney murine leukemia virus (4), HIV-1 (5, 6), and vesicular stomatitis virus (VSV; refs. 7 and 8) showed that removal or certain alterations of their TM domains prevented cell syncytia formation, indicative of a loss of fusion activity. The requirement for a TM domain in viral fusion thus appears to be general, although the mechanism of its action remains unknown.
Studies of the influenza GPI-HA mutant protein also suggested that hemifusion is an essential early step in membrane fusion. The product of hemifusion is a highly localized “hemifusion diaphragm” or “transmonolayer contact region,” in which the cytoplasmic leaflets of the two reacting membranes form a single bilayer separating the two aqueous compartments. This structure is inherently unstable (9), rearranging to form the lower free energy “fusion pore” that then enlarges to complete the fusion process (3, 10, 11). The TM domain appears to play a role in fusion pore formation and/or enlargement, because it is not involved in the formation of the hemifusion diaphragm.
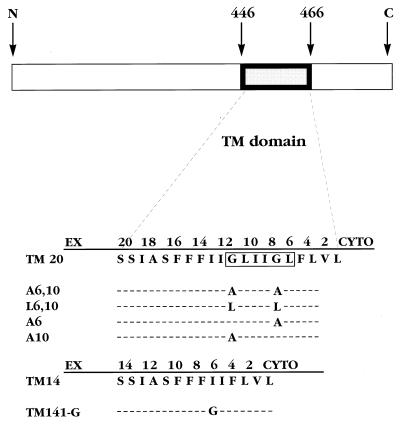
VSV fusion resembles influenza fusion in being mediated solely by a trimeric viral protein, named G. G protein possesses a single TM domain of 20 amino acids and is hereafter referred to as TM20 (Fig. 1). Infection of insect cells with recombinant baculoviruses expressing TM20 resulted in extensive syncytia formation upon exposure to low pH (7). In contrast, a mutant G protein lacking six residues from the TM domain (and, therefore, named TM14; lacking GLIIGL, residues 5–10 in Fig. 1) produced no syncytia despite being expressed, assembled, and transported to the surface normally (7). TM14 was also deficient in syncytia forming activity when expressed in HeLa cells. However, a slightly longer construct, TM16 (lacking LIIG, residues 6–9 in Fig. 1) possessed full syncytia forming activity in HeLa cells (M. Whitt, personal communication). In another study, a mutant GPI-linked G protein was also incapable of inducing syncytia formation (8). These observations showed that the TM domain of VSV G protein is required for fusion but that the shortened TM domain in TM14 is unable to function in this regard.
Figure 1.
Amino acid sequences of the TM domains of VSV G protein (Indiana serotype) and the mutants used in this study. The wild-type (TM20) sequence is shown, numbered from the cytoplasmic end (cyto), with the residues that were deleted to form TM14 (12) boxed.
We now report evidence that a Gly residue within the TM domain of VSV G protein TM20 is required for fusion and that TM14, which lacks a Gly residue, regains nearly wild-type activity when Gly is introduced into its TM domain. A pronounced difference in the distribution of certain amino acid residues between the TM domains of fusion and nonfusion proteins was also found, suggesting that specific TM sequence motifs may be generally required in viral fusion reactions.
MATERIALS AND METHODS
Plasmids and Mutant Construction.
Genes encoding TM20 and TM14 (originally from Jack Rose; ref. 12) were excised from a baculovirus vector (7) with BamHI and ligated into pGEM-3zf. Site-specific mutagenesis (13) was carried out by using single-strand binding protein (Pharmacia) added to the polymerase–ligase reaction mixture. Primer sequences for the five new mutants shown in Fig. 1 are available upon request. Mutants were identified initially by digestion at restriction sites engineered into each primer and confirmed by DNA sequencing.
Reporter Gene Activation Assay for G Protein-Mediated Fusion.
Confluent monolayers of HeLa cells (35-mm2 plates) were transfected (14) with the desired pGEM plasmids, or with pGEM alone, and then infected with vaccinia virus VTF7–3 encoding T7 polymerase at a multiplicity of infection of 0.25 (American Type Culture Collection; ref. 15) and incubated overnight. Other confluent HeLa monolayers were infected with control vaccinia virus WR (American Type Culture Collection) or with vaccinia VCB21R, encoding β-galactosidase behind a T7 promoter (a gift from E. Berger; ref. 16) at a multiplicity of infection of 5. These cells were trypsinized, incubated overnight at 37°C (17), and resuspended in fresh medium at 5 × 105 cells per ml. One hundred microliters of VCB21R- or WR-infected cells was added to each dish containing the transfected VTF7–3-infected cells. After 1 h at 37°C, the medium was carefully removed and 400 μl of low pH buffer (10 mM Mes in PBS at pH 5.2) was added (18) for 5 min, followed by 1 ml of DMEM to restore neutrality. Fusion was allowed to proceed for 1 h, and the cells were fixed with 20 μl of 20% formaldehyde/2% glutaraldehyde in PBS (5 min, 4°C). The fixative was removed and 1 ml of staining solution (5 mM potassium ferricyanide/2 mM magnesium chloride/1 mg of 5-bromo-4-chloro-3-indolyl β-d-galactoside; Boehringer Mannheim) was added. After overnight incubation at 37°C, the number of blue-staining foci were counted under a microscope.
Fusion and Hemifusion (Lipid Mixing) Assays Using High Five Target Cells.
High Five cells (Invitrogen) were loaded with octadecylrhodamine (R18) or lucifer yellow by incubation with dye (0.1 and 0.5 mg/ml, respectively) for 3 h at room temperature. Cells were pelleted and washed with PBS until the supernatant became colorless. Confluent HeLa cells grown on coverslips were infected with vaccinia virus VTF7–3 (multiplicity of infection of 1) and 30 min later were transfected with the appropriate plasmid (14). After 3 h the dye-loaded High Five cells were added and allowed to settle onto the HeLa cells for 30 min. The supernatant was carefully removed by pipette, and low pH buffer was added for 3 min, followed by neutralization with DMEM as above. After incubation for 45 min at 37°C, the coverslips were washed once with PBS and viewed immediately. The highly fluorescent, round, dye-loaded High Five target cells are readily distinguished from the underlying HeLa cells.
RESULTS
TM14 was previously found to be inactive in syncytia formation, whereas TM16 was fully active (see Introduction). This raised the question whether TM14 was inactive simply because the TM domain was too short or because an essential sequence element had been removed. Comparison of the sequence removed from TM14 (GLIIGL; residues 5–10 in Fig. 1) with that removed from TM16 (LIIG; residues 6–9 in Fig. 1) showed that both Gly residues were removed from TM14 but that one of these was retained in TM16. To test the importance of Gly residues in the G protein TM domain, plasmids were prepared encoding G proteins with substitutions of either one or both Gly residues (Fig. 1). These were expressed in HeLa cells, and fusion activity was determined from the fusion-dependent transfer of a soluble cytoplasmic protein (T7 polymerase) between cells (17). As shown in Fig. 2, replacement of both TM Gly residues by either Leu or Ala resulted in essentially complete inactivation of fusion activity. Replacement of either Gly residue singly with Ala led to a reduction of activity, to about half of the wild-type level (Fig. 2). The amount of cell surface expression of the mutant proteins, as determined by flow cytometry, was equivalent (1.0–1.3 times that of TM20; data not shown), so that decreased surface density could not account for the loss of fusion activity. Thus, the two Gly residues act equivalently; one of the two must be present for fusion activity, and the second augments the activity.
Figure 2.
Fusion activity of VSV G protein and of the mutants shown in Fig. 1, as determined by the reporter gene activation assay. Results are the mean and SD (error bars) of at least three experiments, each normalized to results with TM20, because absolute numbers varied. In each experiment the number of foci in at least two plates transfected with each construct were counted. Transfection efficiencies were uniformly 45–55%, in all experiments and for each construct. TM20 plates generally had 100–200 foci per plate. Background foci produced by pGEM-transfected cells lacking any G insert (usually 10–20% of TM20 levels) were subtracted from all values.
TM14, which was inactive in syncytia formation (7), was also tested for fusion activity by using the reporter gene transfer assay. A low but significant amount of activity (15% of TM20; Fig. 2) was found. This level is evidently insufficient to produce visible syncytia in the qualitative and quite insensitive syncytia assay used previously. TM16, which retains one Gly residue (12), possessed normal syncytia forming activity in HeLa cells (M. Whitt, personal communication).
The possibility remained that the shortened TM domain of TM14 might be inherently incapable of mediating fusion, regardless of sequence. To test this possibility, an Ile → Gly substitution was made in TM14, at position 6 from the cytoplasmic end, to form TM14 I-G (Fig. 1). Introduction of this single Gly residue restored 80% of wild-type fusion activity to a protein possessing a 14-residue TM domain (Fig. 2). Thus, there is sequence specificity in TM domain function, an appropriately located Gly residue is required, and a 14-residue TM domain can be competent for fusion.
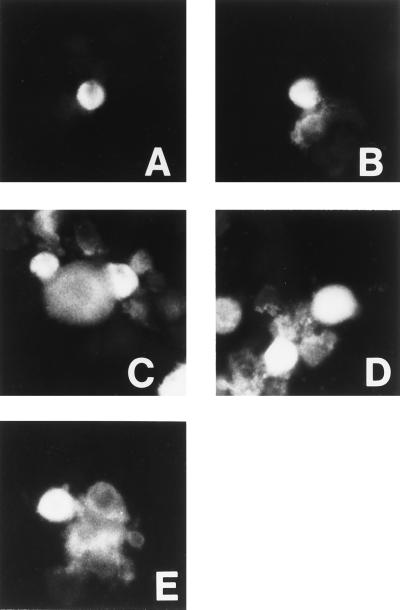
Studies with GPI-linked influenza HA had shown that the TM domain was not required for hemifusion (1, 2). It was therefore of interest to determine whether the inactive or poorly active VSV G protein mutants—L6,10; A6,10; and TM14—were capable of hemifusion. To test this, another VSV fusion assay was developed in which insect cells (High Five) were used as target cells. These were loaded with the lipid dye octadecylrhodamine (R18) and allowed to bind to HeLa cells expressing the mutant VSV G proteins. After a brief exposure to low pH to activate the G protein, R18 dye transfer occurred from the High Five cells to the HeLa cells expressing L6,10 or A6,10 or TM14 (Fig. 3 C–E) and to cells expressing TM20 (Fig. 3B). Dye transfer did not occur in control HeLa cells transfected with a pGEM vector lacking any G protein gene (Fig. 3A) or if the low pH step was omitted (data not shown), showing that the transfer was protein- and pH-dependent. Microscopic examination revealed no marked differences in the number of lipid mixing events mediated by cells expressing TM 20 or mutant proteins. Loss of the essential Gly residues, thus, had no apparent effect on the G protein-mediated interactions between the external leaflets of the reacting membranes.
Figure 3.
R18 dye transfer induced by low pH from dye-loaded High Five cells into HeLa cells expressing the following constructs: (A) pGEM alone. (B) TM20. (C) TM14. (D) A6,10. (E) L6,10. R18 transfer, indicative of lipid mixing, has occurred in all cells except those in A. (×200.)
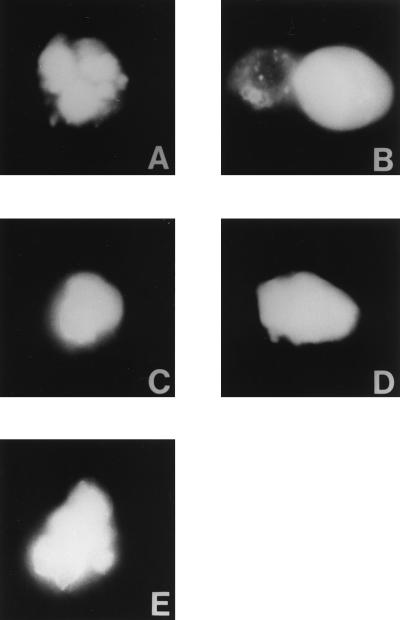
In a variation of the R18 assay, the High Five cells were loaded with the soluble dye lucifer yellow. Unexpectedly, substantial amounts of this dye were present in the cell cytoplasm after loading, as well as in small vacuoles. Transfer of lucifer yellow into the HeLa cells would indicate mixing of cytoplasmic aqueous contents and, hence, complete fusion. As expected, transfer of dye into cells transfected with TM20 was observed (Fig. 4B), but no transfer occurred into cells expressing the mutants L6,10 or A6,10 or TM14 (Fig. 4 C–E). These observations confirmed the findings shown in Fig. 2 and served as an additional control for Fig. 3 against the possibility of nonspecific leakiness from the High Five target cells.
Figure 4.
Lucifer yellow dye transfer induced by low pH from dye-loaded High Five cells into HeLa cells expressing the following constructs: (A) pGEM alone. (B) TM20. (C) TM14. (D) A6,10. (E) L6,10. Lucifer yellow transfer, indicative of aqueous compartment mixing, has occurred only in cells in B. (×400.)
Because a TM-domain Gly residue was thus shown to be required for mediation of complete fusion by the VSV G protein, we wondered whether specific TM residues might be required in other viral fusion reactions as well. Accordingly, the TM domain sequences of 28 viral fusion proteins (Table 1) were compared with those of 19 viral nonfusion proteins (Table 2). Fusion proteins were chosen to represent a broad range of enveloped virus classes without overrepresenting specific ones, e.g., through the use of many serotypes. These were compared with nonfusion membrane proteins from the same viruses, excluding ion channels.
Table 1.
TM domain sequences of viral fusion proteins
| Name | TM domain | Cyto. | Ref. |
|---|---|---|---|
| VSVInd | SWK SSIASFFFIIGLIIGLFLVL | RVG | 19 |
| VSVNJ | GWR SSLMGVLAVIIGFVILMFLI | KLI | 19 |
| VSVChan | WKE SLAAGVVLILVVVLIYGVL | RCF | 19 |
| VSVPiry | WRE SVMAIVGIVLLIVVTFLAI | KTV | 19 |
| RabiesCVS | WGK YVLMTAGAMIGLVLIFSLMTWC | RRA | 20 |
| RabiesSAD | WGK YLLLSAGALTALMLIIFLMTCC | RRV | 21 |
| SV5 | TTS VLSIIAICLGSLGLILIILLSVVVW | KLL | 22 |
| hRSV | STN IMITTIIIVIIVILLSLIAVGLLLYC | KA | 22 |
| HPIV1 | NTE STQIIIIIIVCILIIIICGILYYLY | RVR | 23 |
| HPIV3 | STT IIIVLIMIIILFIINVTIIIIAV | KYY | 24 |
| NDV | STS ALITYIVLTIISLVFGILSLVLACYLMI | KQK | 25 |
| Measles | STS IVYILIAVCLGGLIGIPALICCC | RGR | 26 |
| RindV | NIK GVSVTNTGYILVGVGLIAVVGILIITCCC | KKR | 27 |
| Sendai | SRE TVITIIVVMVVILVVIIVIVIVLY | RLK | 22 |
| Mumps | SSK IGAIIVAALVASILSIIISLLFCFWAYIAT | KEI | 28 |
| SFV | VQK ISGGLGAFAIGAILVLVVVTCIGL | RR | 29 |
| RRV | VQR LASGLGGLALIAVVVLVLVTCITM | RR | 30 |
| Sindbis | LFA LFGGASSLLIIGLMIFACSMMLTST | RR | 30 |
| VEEV | SK TAWTWLTSLLGGSAVIIIIGLVLATIVAMYVLT | NQK | 31 |
| WNV | SDR TIAMTFLAVGGILMFLSVNVHA | DTG | 32 |
| YFV | NTR NMTMSMSMILVGVIMMFLSLGVGA | DQG | 32 |
| DEN2 | NSR STSLSVSLVLVGVVTLYLGAMVQA | DSG | 32 |
| Flu A | GVY QILAIYSTVASSLVLLVSLGAISFWMCS | NGS | 33 |
| Flu B | DNH TIILLYYSTAASSLAVTLMIAIFIVYMVS | RDN | 34 |
| Flu C | YSD PFYWGSSLGLAITAANLMAALVISGIAIC | RTK | 34 |
| MoMuLV | SPWFTTLISTIMGPLIVLLMILLFGPCILN | R | 4 |
| HIV1 | YIK IFIMIVGGLVGLRIVFAVLSIVN | RVR | 35 |
| Uukuniemi | WLR AIWAILGGTVSLIIGVVIIYMVFTLCL | KVK | 36 |
SV5, simian virus 5; hRSV, human respiratory syncytial virus; HPIV, human parainfluenza virus; NDV, Newcastle disease virus; RindV, Rinderpest virus; SFV, Semliki Forest virus; RRV, Ross River virus; VEEV, Venezuelan equine encephalitis virus; WNV, West Nile virus; YFV, yellow fever virus; Den, Dengue; Flu, influenza; MoMuLV, Moloney murine leukemia virus; HIV, human immunodeficiency virus; Cyto., cytoplasmic sequence.
Table 2.
TM domain sequences of viral nonfusion proteins
| Name | TM Domain | Cyto. | Ref. |
|---|---|---|---|
| Flu A NA | KLH LGINLGLNVIGILVAITGIVLATASTCLI | KQN | 37 |
| Flu B NA | SFK LLVDSYLLYSLSASVYLSLLVGGSTLLL | TLT | 38 |
| NDV HN | VLD SPTSAGMSYVLSAVSTALTVVTLLLIAI | RFI | 39 |
| HPIV3 HN | SK ISNILVIIFVISLLVLIITWLKYTI | KNT | 40 |
| HPIV1 HN | DQK IILDICLIMIIFSLITHMTTAILLWI | HTK | 41 |
| SV5 HN | LSE YLISISLALLTCLFILTTT | RFL | 42 |
| hRSV G | KHN ASAIFIIAAIILSTSIIMALISLTI | QAV | 43 |
| Sendai HN | YQR ASIIICIIVTAISLAWQTFSLILLWT | DAK | 44 |
| Mumps HN | SQR ILQGLTVIVLILTVAQVSLVLI | RFC | 45 |
| Measles HN | HLR IGAIALLGILSLFMVFLVALLVYP | REI | 46 |
| Rinderpest H | HLR IGAIALLGVLSLFMVFLVALLMYP | REI | 47 |
| SFV E2 | LYP AATGVSAVVGMSLLALISIFASCYMLVAA | RSK | 30 |
| RRV E2 | LYP AATIAAVSGASLMALLTLAATCCMLATA | RRK | 30 |
| Sindbis E2 | RHP VYTILAVASATVAMMIGVTVAVLCAC | KAR | 30 |
| VEEV E2 | YHR YPMSTILGLSICAAIATVSVAASTWLFC | RSR | 31 |
| WNV M | MQR VVFAILLLLVAAPAYS | 32 | |
| YFV M | TQR VVIALLVLAVGPAYS | 32 | |
| DEN2 M | FQR ALIFILLTAVAPSMT | 32 | |
| Uukuniemi G1 | KEQ VLILVAVSSLCILLLASVL | RAL | 36 |
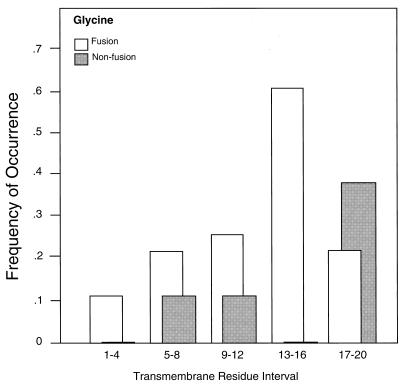
The greatest difference in residue abundance between these two groups of proteins was Gly, with a 2.6-fold greater abundance in the fusion compared with the nonfusion TM domains (P = 0.004; Table 3). One or more Gly residues were present in positions 1–20 (from the cytoplasmic end) of the TM domains of 24 of the 28 fusion proteins surveyed, compared with only 9 of 19 nonfusion proteins. Further, the distribution of the Gly residues was quite different in the fusion and nonfusion TM domains, with most of those in the fusion proteins located in the interior of the TM domain, whereas those in the nonfusion proteins were more abundant close to the external surface (Fig. 5). This observation suggests that TM domain Gly residues might be important for fusion by other viral proteins as well. Other possibly noteworthy differences were the greater abundances of Met, Cys, and β-branched amino acid residues in the TM domains of the fusion proteins, although the small sample size precluded a determination of statistical significance for any of these except Ile (P = 0.02; Table 3).
Table 3.
Mole percent amino acid residues in the TM domains of fusion and nonfusion proteins
| Residue | mol %
|
Ratio fus/nonfus | P value | |
|---|---|---|---|---|
| Fusion | Nonfusion | |||
| Ala | 8.8 | 14.0 | 0.6 | 0.07 |
| Arg | 0.2 | 0 | — | |
| Asn | 0.9 | 0 | — | |
| Cys | 4.1 | 2.7 | 1.5 | |
| Gln | 0.2 | 0 | — | |
| Gly | 7.9 | 3.0 | 2.6 | 0.004 |
| His | 0.2 | 0.5 | 0.4 | |
| Ile | 19.7 | 13.7 | 1.4 | 0.02 |
| Leu | 19.2 | 22.8 | 0.8 | |
| Lys | 0 | 0.2 | — | |
| Met | 5.6 | 3.6 | 1.6 | |
| Pro | 0.5 | 1.4 | 0.4 | |
| Ser | 6.8 | 9.3 | 0.7 | |
| Thr | 3.9 | 8.8 | 0.4 | 0.004 |
| Val | 15.1 | 11.8 | 1.3 | |
| Try | 0.7 | 1.4 | 0.5 | |
| Tyr | 2.0 | 2.7 | 0.8 | |
Figure 5.
Frequency of occurrence of Gly residues in specific locations within the TM domains of fusion and nonfusion proteins listed in Tables 1 and 2. Location intervals are numbered from the cytoplasmic end of the TM domain as shown in Fig. 1.
DISCUSSION
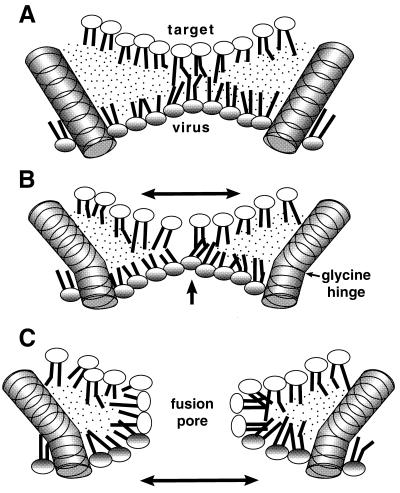
The results reported in this article provide insights into the mechanism of the involvement of the fusion protein TM domain in viral fusion. An intact TM domain is required for complete reaction by several virus fusion proteins (1–8), but the reason for this remains unclear. The TM domain is known to function late in the fusion process, subsequent to formation of a hemifusion diaphragm (1–3). Rearrangement of this lipidic (3, 10) and inherently unstable (9, 11) reaction intermediate into a fusion pore constitutes a major late step in fusion, and this is likely to be potentiated by the TM domains. However, these are topologically excluded from the hemifusion diaphragm, which consists of the unmixed cytoplasmic leaflets of the two reacting membranes (Fig. 6). At least three HA trimers are required for each fusion event (48), and a similar stoichiometry for VSV G protein may be assumed. The picture emerges of numerous TM domains clustered around the edge of a hemifusion diaphragm, facilitating its rearrangement into a fusion pore.
Figure 6.
Model for the participation of a TM Gly residue in fusion. (A) Rigid TM domains are embedded in the viral (lower) membrane and positioned around a hemifusion diaphragm, composed of the inner leaflets of the two reacting membranes. Because hemifusion has already occurred, a single outer leaflet (not shown) surrounds the structure shown in three dimensions. (B) Same as A, but with TM domain containing a bend around a Gly “hinge.” This could result in compression (increased negative curvature) of the viral leaflet and expansion of the target (upper) leaflet of the hemifusion diaphragm. (C) The perturbed lipids rearrange to form a more stable fusion pore. Expansion occurs in the direction indicated by the arrows.
The requirement of a Gly residue in the TM domain of the VSV G protein for fusion activity has been established in this report. This finding indicates that the TM domain does not function solely as a hydrophobic “membrane anchor” but must possess specialized functions in mediating viral fusion.
Gly residues play important and unique roles in certain nonfusion properties of TM domains, most notably in helix–helix interactions. Certain TM Gly residues have been shown to essential for dimerization of glycophorin A (49–51) and for helix–helix interactions and viability in the coat protein of bacteriophage M13 (52). However, it seems unlikely that the Gly residues of the G protein TM domain are involved in such specific interactions, for several reasons. (i) The major trimerization domains of native G protein reside in the large external portion of the molecule; GPI-linked G protein trimerizes normally (53), as does TM14 (12). (ii) Although Gly residues are common in TM domains of viral fusion proteins (Fig. 5 and Table 3), the rest of the glycophorin dimerization motif is absent. (iii) The activities of the single-substitution mutants A6, A10, and TM14 I-G (Fig. 3) indicate that a single Gly residue can function equally well in a variety of sequence positions. In contrast, each Gly residue in the interaction domains of both glycophorin A and the M13 coat protein is essentially immutable and unmovable. These considerations make it most probable that the Gly residue is required for some aspect of the interaction between the TM domain and the fusing lipids, as suggested above, and not for interactions between adjacent TM domains.
A likely possibility is suggested by the observation that Gly residues can destabilize α-helices in both aqueous and membrane environments (54, 55). TM domains tend, in general, to adopt a helical conformation (56–60), but introduction of Gly residues into a model helical TM domain markedly decreased its helicity both in micellar and membrane environments (55). The Gly residue might thus confer a measure of deformability to an appropriate TM helix. Bends formed around this flexible “hinge,” perhaps induced by forces acting elsewhere in the molecule, could destabilize the hemifusion diaphragm and catalyze fusion pore formation (Fig. 6). This could be aided by interaction with amphipathic sequences immediately cytoplasmic to the TM domain, which have been implicated in fusion reactions of VSV (8) and other enveloped viruses (61, 62).
A very recent study of the fusion requirements of VSV G protein (8) has drawn conclusions opposite to those stated herein. Chimeric G proteins were constructed in which the TM domain was replaced by TM domains from four nonfusion proteins. In each case, essentially complete syncytia forming activity was retained by the chimeric protein, suggesting that the TM domain, although essential, required no sequence specificity. It is noteworthy, however, that of the four TM domains used, three possessed Gly residues. The fourth possessed internal Met and Cys residues, which, as suggested below, may be important in those TM domains lacking Gly. These results (8) therefore do not contradict the results herein, although they do suggest that TM requirements for fusion can be met by a variety of sequences, including some in the TM domains of nonfusion proteins. This provides a further argument against specific interactions between TM domains being required for the fusion reaction. In the same study, G protein chimeras in which the cytoplasmic and TM domains were both replaced lost fusion activity, indicating involvement also of the cytoplasmic domain (8).
Comparisons of the amino acid residues present in the TM domains of fusion (Table 1) and nonfusion proteins (Table 2) revealed some differences (Fig. 5 and Table 3). Most notably, Gly residues were present in 24 of the 28 fusion protein sequences and were concentrated in the interior of the TM domain, especially in the TM residue interval 13–16 from the cytoplasmic side (Fig. 5; P = 0.0005 for this interval). Although this is not the location of the TM Gly residues in VSV G protein (Fig. 1), Gly residues in all these positions could mediate helix deformations sufficient to destabilize a quasistable hemifusion diaphragm. The sequence comparison thus suggests that TM Gly residues in many viral fusion proteins might perform similar functions.
However general the requirement for a TM Gly residue may prove to be, alternate mechanisms for carrying out the Gly-dependent step in fusion must exist, because 4 of the 28 TM sequences listed in Table 1 lack such residues altogether. It may be interesting in this regard that 3 of the 4 of the Gly-less TM domains possess internal Met residues and the fourth possesses a Cys residue. These appear to be the second and third most enriched residues in fusion protein TM domains (Table 3), although the small sample size precludes statistical significance. These sequence comparisons may be helpful in focusing the search for required residues in the TM domains of other viral fusion proteins.
An interesting effect of TM domain length on fusion activity can be inferred from the results shown in Fig. 2. TM14, lacking Gly, exhibited significant fusion activity, whereas mutants possessing 20-residue TM domains lacking Gly did not (15% for TM14 vs. 3% for L6,10 and A6,10). Further, introduction of a single Gly conferred higher activity to the 14-residue TM than to the 20-residue TM domain (80% for TM14 I-G vs. about 50% for A6 or A10). It thus appears that the shorter TM domain actually enhances fusion activity to some extent, possibly by distorting the surrounding bilayer and thus destabilizing the hemifusion diaphragm. Alternatively, the immediate environment of the Gly residue in TM14 I-G may be more favorable than that surrounding the Gly residues in TM20 (residues FGI vs. IGL).
More sensitive fusion assays were essential for the experiments reported herein. (i) The reporter gene activation assay (17) has been previously used to identify coreceptors for HIV (63, 64). This assay provides an enormous increase in sensitivity over visual identification of syncytia, which has been the traditional criterion for cell–cell fusion. The reporter gene activation assay also permits accurate quantification, so that partial activities exhibited by different mutant proteins can be compared. (ii) The development of the High Five cell line as a suitable VSV G protein target has provided the basis for convenient assays of both fusion and hemifusion for this nonhemolytic virus, comparable to the erythrocyte-based assays used in studies of influenza HA (1, 2). The functionally important TM sequence elements of most of the proteins listed in Table 1 should now be amenable to detailed characterization using minor modifications of available fusion and hemifusion assays.
ABBREVIATIONS
- TM
transmembrane
- VSV
vesicular stomatitis virus
- HA
hemagglutinin
- GPI
glycerylphosphatidylinositol
References
- 1.Kemble G W, Danieli T, White J M. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 2.Melikyan G B, White J M, Cohen F S. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melikyan G B, Brener S A, Ok D C, Cohen F S. J Cell Biol. 1997;136:995–1005. doi: 10.1083/jcb.136.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ragheb J A, Anderson F. J Virol. 1994;68:3207–3219. doi: 10.1128/jvi.68.5.3207-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salzwedel K, Johnston P B, Roberts S J, Dubay J W, Hunter E. J Virol. 1993;67:3264–3273. doi: 10.1128/jvi.67.9.5279-5288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss C D, White J M. J Virol. 1993;67:7060–7066. doi: 10.1128/jvi.67.12.7060-7066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleverley D Z, Geller H M, Lenard J. Exp Cell Res. 1997;233:288–296. doi: 10.1006/excr.1997.3573. [DOI] [PubMed] [Google Scholar]
- 8.Odell D, Wanas E, Yan J, Ghosh H P. J Virol. 1997;71:7996–8000. doi: 10.1128/jvi.71.10.7996-8000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nussler F, Clague M J, Herrmann A. Biophys J. 1997;73:2280–2291. doi: 10.1016/S0006-3495(97)78260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernomordik L V, Leikina E, Frolov V, Bronk P, Zimmerberg J. J Cell Biol. 1997;136:81–93. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel D P. Biophys J. 1993;65:2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams G A, Rose J K. Cell. 1985;41:1007–1015. doi: 10.1016/s0092-8674(85)80081-7. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:365–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 14.Rose J K, Buonocore L, Whitt M A. Biotechniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 15.Fuerst T R, Niles E G, Studier F W, Moss B. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 17.Nussbaum O, Broder C C, Berger E A. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitt M A, Zagouras P, Crise B, Rose J K. J Virol. 1990;64:4907–4917. doi: 10.1128/jvi.64.10.4907-4913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brun G, Bao X, Prevec L. Intervirology. 1995;38:274–282. doi: 10.1159/000150451. [DOI] [PubMed] [Google Scholar]
- 20.Seif I, Coulon P, Rollin P E, Flamand A. J Virol. 1985;53:926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conzelmann K K, Cox J H, Schneider L G, Thiel H J. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 22.McGinnes L W, Morrison T G. Virus Res. 1986;5:343–356. doi: 10.1016/0168-1702(86)90028-6. [DOI] [PubMed] [Google Scholar]
- 23.Merson J R, Hull R A, Estes M K, Kasel J A. Virology. 1988;167:97–105. doi: 10.1016/0042-6822(88)90058-x. [DOI] [PubMed] [Google Scholar]
- 24.Spriggs M K, Olmsted R A, Venkatesan S, Coligan J E, Collins P L. Virology. 1986;152:241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- 25.Chambers P, Millar N S, Emmerson P T. J Gen Virol. 1986;67:2685–2694. doi: 10.1099/0022-1317-67-12-2685. [DOI] [PubMed] [Google Scholar]
- 26.Buckland R, Gerald C, Barker R, Wild T F. J Gen Virol. 1987;68:1695–1703. doi: 10.1099/0022-1317-68-6-1695. [DOI] [PubMed] [Google Scholar]
- 27.Tsukiyama K, Yoshikawa Y, Yamanouchi K. Virology. 1988;164:523–530. doi: 10.1016/0042-6822(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 28.Elango N, Varsanyi T M, Kovamees J, Norrby E. J Gen Virol. 1989;70:801–807. doi: 10.1099/0022-1317-70-4-801. [DOI] [PubMed] [Google Scholar]
- 29.Garoff H, Frischauf A-M, Simons K, Lehrach H, Delius H. Nature (London) 1980;288:236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- 30.Dalgarno L, Rice C M, Strauss J H. Virology. 1983;129:170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- 31.Kinney R M, Johnson B J, Brown V L, Trent D W. Virology. 1986;152:400–413. doi: 10.1016/0042-6822(86)90142-x. [DOI] [PubMed] [Google Scholar]
- 32.Deubel V, Kinney R M, Trent D W. Virology. 1986;155:365–377. doi: 10.1016/0042-6822(86)90200-x. [DOI] [PubMed] [Google Scholar]
- 33.Veit M, Kretzschmar E, Kuroda K, Garten W, Schmidt M F G, Klenk H-D, Rott R. J Virol. 1991;65:2491–2500. doi: 10.1128/jvi.65.5.2491-2500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakada S, Creager R S, Krystal M, Aaronson R P, Palese P. J Virol. 1984;50:118–124. doi: 10.1128/jvi.50.1.118-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willey R L, Rutledge R A, Dias S, Folks T, Theodore T, Buckler C E, Martin M A. Proc Natl Acad Sci USA. 1986;83:5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raonnholm R, Pettersson R F. Virology. 1987;160:191–202. doi: 10.1016/0042-6822(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 37.Air G M, Webster R G, Colman P M, Laver W G. Virology. 1987;160:346–354. doi: 10.1016/0042-6822(87)90005-5. [DOI] [PubMed] [Google Scholar]
- 38.Shaw M W, Lamb R A, Erickson B W, Briedis D J, Choppin P W. Proc Natl Acad Sci USA. 1982;79:6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millar N S, Chambers P, Emmerson P T. J Gen Virol. 1986;67:1917–1927. doi: 10.1099/0022-1317-67-9-1917. [DOI] [PubMed] [Google Scholar]
- 40.Elango N, Coligan J E, Jambou R C, Venkatesan S. J Virol. 1986;57:481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorman W L, Gill D S, Scroggs R A, Portner A. Virology. 1990;175:211–221. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- 42.Hiebert S W, Paterson R G, Lamb R A. J Virol. 1985;54:1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satake AM, Coligan J E, Elango N, Norrby E, Venkatesan S. Nucleic Acids Res. 1985;13:7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shioda T, Iwasaki K, Shibuta H. Nucleic Acids Res. 1986;14:1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovamees J, Norrby E, Elango N. Virus Res. 1989;12:87–96. doi: 10.1016/0168-1702(89)90056-7. [DOI] [PubMed] [Google Scholar]
- 46.Alkhatib G, Briedis D J. Virology. 1986;150:479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- 47.Tsukiyama K, Sugiyama M, Yoshikawa Y, Yamanouchi K. Virology. 1988;160:48–54. doi: 10.1016/0042-6822(87)90042-0. [DOI] [PubMed] [Google Scholar]
- 48.Danieli T, Pelletier S L, Henis Y I, White J M. J Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemmon M A, Flanagan J M, Treutlein H R, Zhang J, Engelman D M. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie K R, Prestegard J H, Engelman D M. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 51.Lemmon M A, Treutlein H R, Adams P D, Brunger A T, Edelman D M. Nat Struct Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Glibowicka M, Joensson C, Deber C M. J Biol Chem. 1993;268:4584–4587. [PubMed] [Google Scholar]
- 53.Crise B, Ruusala A, Zagouras P, Shaw A, Rose J K. J Virol. 1989;63:5328–5333. doi: 10.1128/jvi.63.12.5328-5333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cantor C R, Schimmel P R. Biophysical Chemistry. San Francisco: Freeman; 1980. , Part I, pp. 300–305. [Google Scholar]
- 55.Li S-C, Deber D M. Nat Struct Biol. 1994;1:368–374. doi: 10.1038/nsb0694-368. [DOI] [PubMed] [Google Scholar]
- 56.Singer S J. Adv Protein Chem. 1962;17:1–68. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- 57.Lenard J, Singer S J. Proc Natl Acad Sci USA. 1966;56:1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henderson R, Unwin P N. Nature (London) 1975;257:28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- 59.Diesenhofer J, Epp O, Miki K, Huber R, Michel H. Nature (London) 1985;316:618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 60.Lemmon M A, Engelman D M. Q Rev Biophys. 1994;27:157–218. doi: 10.1017/s0033583500004522. [DOI] [PubMed] [Google Scholar]
- 61.Freed E O, Martin M A. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Januszeski M M, Cannon P M, Chen D, Rozenberg Y, Anderson W F. J Virol. 1997;71:3613–3619. doi: 10.1128/jvi.71.5.3613-3619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkhatib G, Broder C C, Berger E A. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]