Abstract
Inhibition of leukocyte migration into target organs has long been an attractive, though challenging, basis for anti-inflammatory strategies. However, to date, the manipulation of leukocyte rolling along blood vessels has not yielded successful new therapies. An important study may now open new avenues in this exciting field of anti-inflammatory therapies by introducing a putative inhibitor of poly-N-acetyllactosamine biosynthesis that affects selectin ligand activity and shows efficacy in a rodent skin inflammation model.
Tissue-specific localization of T cells is a requirement for immune surveillance in the skin and in addition plays a pivotal role in the pathogenesis of numerous inflammatory skin disorders. Indeed, the evidence that T cells are crucial factors in mediating psoriasis, allergic contact dermatitis, atopic dermatitis, and cutaneous T cell lymphomas is so strong that these diseases are now considered as T cell–mediated dermatoses (1). Consequently, insight into mechanisms of T cell recruitment to the skin (and other target organs) may lead to novel anti-inflammatory therapies, and the subject is therefore of particular interest.
Selectin and selectin ligand interactions mediate leukocyte rolling along the endothelium
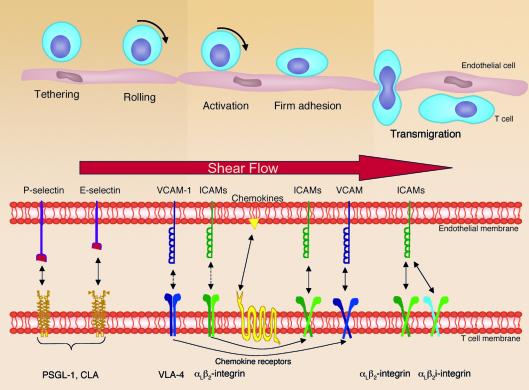
The multistep cascade of T cell migration has been well described (Figure 1), and the molecular basis for T cell skin homing has been reviewed recently (2). The first steps of T cell localization to all tissues include leukocyte tethering and rolling along the vessel wall, which is mediated primarily by interactions between selectin and selectin ligand (3). A number of studies have demonstrated the pivotal role of E- and P-selectin for leukocyte rolling as well as their overlapping and mutually compensating functions (4). Therefore, it was not surprising that a neutralizing antibody solely against E-selectin was found to be without beneficial effects in a recent clinical trial (5). Probably for these reasons, the development of a potent, but E-selectin–specific low–molecular weight antagonist called ESA-2 (6) was stopped. The lesson learned from these findings was that potent and clinically active selectin antagonists have to interfere with at least two of the three selectins (E, P, and L) in order to show in vivo efficacy. Some such antagonists have been reported recently (7).
Figure 1.
The multistep process of the interactions between lymphocytes and endothelial cells leading to T cell migration into skin. Selectin and selectin ligand interactions allow lymphocyte rolling along the endothelial lining. Lymphocyte chemokine receptors thereby come into contact with chemokines displayed by the endothelium; e.g., via proteoglycans leading to activation of integrins. This is necessary for subsequent firm adhesion of lymphocytes to the endothelium and the final transmigration through the blood vessel wall. VLA-4, very late antigen-4.
Synthesis of sialyl Lewis X is essential for selectin ligand binding
The tetrasaccharide sialyl Lewis X (sLeX), which is closely related to cutaneous lymphocyte-associated antigen (CLA), binds to all three selectins and appears to be an interesting drug target. Its expression by T cells requires the action of multiple glycosyltransferases such as core 2 β1,6-N-acetylglucosaminyltransferase-I, N-acetylglucosamine-6-O-sulfotransferase, β1,4-galactosyltransferases (GalTs), the α2,3-sialyltransferase (ST3GalIV), and α1,3-fucosyltransferases IV and VII (FucTIV and FucTVII). ST3GalIV, FucTIV, and FucTVII are responsible for synthesizing terminal sialofucosylations that serve as E-selectin ligands expressed by P-selectin glycoprotein ligand-1 (PSGL-1) (8). Thus, these enzymes are potential drug targets and might even be targeted by small molecules in a manner suitable for oral administration. Among the best-characterized enzymes involved in lymphocyte skin homing are FucTIV and FucTVII (9). FucTs catalyze the final step in the synthesis of selectin ligands. Mice deficient in FucTVII are characterized by a marked reduction of E-, P-, and L-selectin ligand activity and impaired leukocyte extravasation into inflamed tissue, indicating a major role for FucTVII in the generation of selectin ligands. In mice deficient for FucTVII and FucTIV, leukocyte rolling is completely absent, and contact hypersensitivity (CHS), which is substantially reduced in FucTVII-deficient mice, is virtually absent (10, 11). Discovery of low–molecular weight antagonists of FucTVII, which plays the dominant role for selectin ligand production, is ongoing but has not yet reached clinical development (12, 13). Inhibition of FucTVII might be challenging, since FucTVII antagonists have to penetrate the cell and Golgi membrane to reach their target. More important is the fact that in humans, FucTIV may synthesize active selectin ligands in the absence of FucTVII, a compensatory mechanism that was observed in one patient carrying a homozygous missense mutation in the FucTVII gene (14).
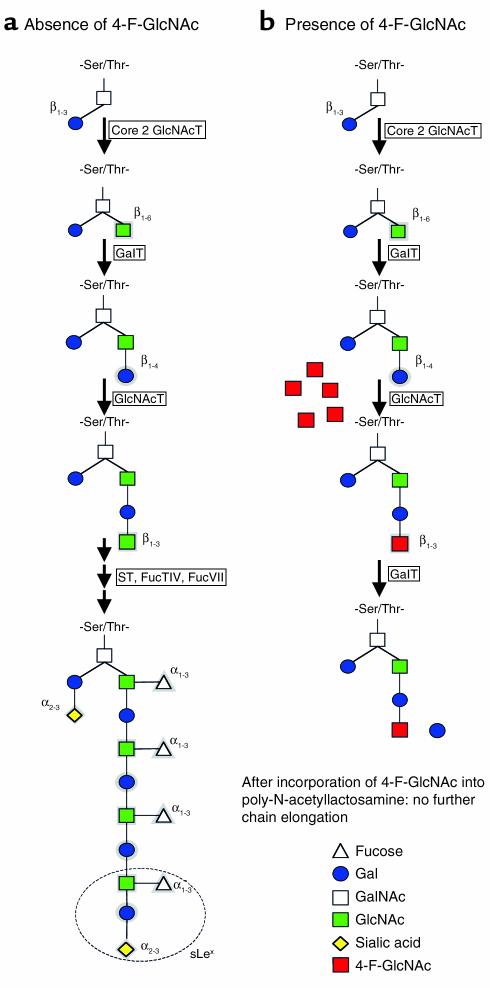
Inhibition of carbohydrate side chain elongation blocks E-selectin ligand expression and cutaneous immune reactions in vivo
In this issue of the JCI, Dimitroff et al. (15) introduce a new strategy for the inhibition of selectin ligand production using the fluorinated analog of N-acetylglucosamine — peracetylated-4-fluorinated-D-glucosamine (4–F–GlcNAc) — as a glycosylation inhibitor. Terminal sLeX structures are expressed on poly-N-acetyllactosamines found on core 2 O- and N-linked glycans displayed by PSGL-1 (Figure 2). Consequently, compounds interfering with the synthesis of poly-N-acetyllactosamine structures should reduce selectin ligand activity and function. Indeed, Dimitroff and colleagues demonstrated previously that 4-F-GlcNAc was directly incorporated into native CLA expressed on human T cells, indicating direct inhibition of poly-N-acetyllactosamine elongation (8). It is thought that after conversion to UDP-4-F-GlcNAc and addition to the poly-N-acetyllactosamine backbone, 4-F-GlcNAc blocks the addition of Gal to its 4-OH, leading to premature chain termination during sLeX generation (8). In the present study, the authors extend their observations by neatly demonstrating blockade of lymphocyte E-selectin ligand expression in vivo and prevention of murine CHS reactions in the presence of 4-F-GlcNAc (15). Most interesting, 4-F-GlcNAc inhibited CHS responses with an extremely high efficacy, which reached or even exceeded that of potent glucocorticosteroids or calcineurin inhibitors. On the other hand, in addition to their importance in cell-cell interaction, carbohydrate side chains on glycoproteins also play a significant role in protein confirmation, transport, and stability. Mice deficient in UDP-Gal:N-acetylglucosamine GalT — the enzyme responsible for elongation of poly-N-acetyllactosaminoglycans — are normal at birth but exhibit growth retardation and reduced life span. It was suggested that GalT, although dispensable during embryonic life, plays critical roles in the regulation of proliferation and differentiation of epithelial cells after birth (16). Currently, it is unclear whether incomplete, temporary inhibition of poly-N-acetyllactosaminoglycan elongation by 4-F-GlcNAc will have similar unwanted effects as observed in GalT-deficient mice. Nevertheless, selective glycosyltransferase inhibitors are expected to be more specific and may cause even fewer side effects. Although this indicates the need for further investigations and suggests that a novel drug is still a long way off, the present investigation has indicated that inhibition of selectin ligand activity can be achieved by antagonizing specific glycosyltransferases with small molecules and leads to anti-inflammatory effects in vivo. Remarkably, 4-F-GlcNAc only inhibited elicitation without modulating the sensitization phase in the CHS model (15) — a phenomenon that was also observed in FucTVII-deficient mice (11). This indicates that these strategies have the potential to be effective not only in preventive approaches but also in therapeutic modalities, which is important for the therapy of established disease. Moreover, the lack of inhibitory effects on the sensitization phase may even be an advantage, since it should allow a triggering of the immune response, which is important for host defense against pathogens.
Figure 2.
(a) Biosynthesis of core 2–type, sLeX-containing O-glycans. sLeX is circled. (b) In the presence of 4-F-GlcNAc, the inhibitor is incorporated into poly-N-acetyllactosamine chains, which finally leads to termination of chain elongation.
Acknowledgments
We gratefully acknowledge helpful discussions with U. Voigtmann. Because of the extent and complexity of the lymphocyte homing field, we could not discuss many interesting studies, and we apologize to those authors whose excellent work could not be cited due to space limitations.
Footnotes
See the related article beginning on page 1008.
Conflict of interest: The authors are employees and stockholders of Schering AG, which is working on developing anti-inflammatory compounds for the treatment of skin diseases.
Nonstandard abbreviations used: sialyl Lewis X (sLeX); cutaneous lymphocyte-associated antigen (CLA); β1,4-galactosyltransferase (GalT); α2,3-sialyltransferase (ST3GalIV); α1,3-fucosyltransferase (FucT); P-selectin glycoprotein ligand-1 (PSGL-1); contact hypersensitivity (CHS); peracetylated-4-fluorinated-d-glucosamine (4-F-GlcNAc).
References
- 1.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N. Engl. J. Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 2.Schon, M.P., Zollner, T.M., and Boehncke, W.H. 2003. The molecular basis of lymphocyte recruitment to the skin — clues for the pathogenesis and selective therapies for inflammatory disorders. J. Invest. Dermatol. In press. [DOI] [PubMed]
- 3.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 4.Labow MA, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Cell. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhushan M, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. Br. J. Dermatol. 2002;146:824–831. doi: 10.1046/j.1365-2133.2002.04743.x. [DOI] [PubMed] [Google Scholar]
- 6.Thoma G, Banteli R, Jahnke W, Magnani JL, Patton JT. A readily available, highly potent E-selectin antagonist. Angew. Chem. Int. Ed. Engl. 2001;40:3644–3647. doi: 10.1002/1521-3773(20011001)40:19<3644::AID-ANIE3644>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Schon MP, et al. Efomycine M, a new specific inhibitor of selectin, impairs leukocyte adhesion and alleviates cutaneous inflammation. Nat. Med. 2002;8:366–372. doi: 10.1038/nm0402-366. [DOI] [PubMed] [Google Scholar]
- 8.Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen expression: implications in modulating lymphocyte migration to skin. Blood. 2003;101:602–610. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- 9.Schottelius AJ, Hamann A, Asadullah K. Role of fucosyltransferases in leukocyte trafficking: major impact for cutaneous immunity. Trends Immunol. 2003;24:101–104. doi: 10.1016/s1471-4906(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 10.Maly P, et al. The alpha(1>, 3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 11.Smithson G, et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J. Exp. Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compain P, Martin OR. Carbohydrate mimetics-based glycosyltransferase inhibitors. Bioorg. Med. Chem. 2001;9:3077–3092. doi: 10.1016/s0968-0896(01)00176-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee LV, et al. A potent and highly selective inhibitor of human alpha-1,3-fucosyltransferase via click chemistry. J. Am. Chem. Soc. 2003;125:9588–9589. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 14.Bengtson P, Lundblad A, Larson G, Pahlsson P. Polymorphonuclear leukocytes from individuals carrying the G329A mutation in the alpha 1,3-fucosyltransferase VII gene (FUT7) roll on E- and P-selectins. J. Immunol. 2002;169:3940–3946. doi: 10.4049/jimmunol.169.7.3940. [DOI] [PubMed] [Google Scholar]
- 15.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J. Clin. Invest. 2003;112:1008–1018. doi:10.1172/JCI200319220. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano M, et al. Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 1997;16:1850–1857. doi: 10.1093/emboj/16.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]