Abstract
Recent studies support the concept that IGF-binding protein-5 (IGFBP-5) stimulates bone formation, at least in part, via IGF-independent mechanisms. To evaluate this hypothesis further, we evaluated in vitro and in vivo effects of IGFBP-5 on bone formation parameters using the IGF-I knockout (KO) mouse. Treatment of serum-free cultures of osteoblast clones derived from IGF-I KO mice with recombinant human IGFBP-5 increased both proliferation and alkaline phosphatase (ALP) activity in a dose-dependent manner, an effect comparable to that seen with IGF-I. IGF-II levels from media conditioned by osteoblasts derived from IGF-I KO mouse were below those detectable by RIA. To eliminate possible actions of IGF-II, if any was produced by osteoblasts derived from IGF-I knockout mice, the IGFBP-5 effect was studied in the presence of exogenously added IGFBP-4, a potent inhibitor of IGF-II actions in bone cells. Addition of IGFBP-4 blocked IGF-I– but not IGFBP-5–induced cell proliferation in osteoblasts derived from IGF-I knockout mice. Consistent with in vitro results, a single local injection of IGFBP-5 to the outer periosteum of the parietal bone of IGF-I KO mice increased ALP activity and osteocalcin levels of calvarial bone extracts. The magnitudes of IGFBP-5–induced increases in ALP and osteocalcin in parietal bone extracts of IGF-I KO mice were comparable to those seen in C3H mice. In contrast to IGFBP-5, local administration of IGFBP-4 had no significant effect on bone formation in C3H and IGF-I KO mice. These results provide the first direct evidence to our knowledge that IGFBP-5 functions as a growth factor that stimulates its actions in part via an IGF-independent mechanism.
Introduction
The IGFs are produced by many tissues, such as brain, thyroid, liver, muscle, kidney, blood, and bone, and modulate growth in these tissues (1). Thus, IGFs have been proposed to play a significant role in these tissues in an autocrine/paracrine manner. In bone, a number of in vitro and in vivo findings demonstrate that IGFs are important regulators of bone formation. IGFs stimulate proliferation and differentiation of osteoblasts (2), as well as of chondrocytes (3). IGFs have also been shown to increase production of several bone matrix proteins and decrease collagen degradation in osteoblasts (4). Mice lacking functional IGF-I genes exhibit severe impairment of bone growth (5). IGF-I administration to humans caused an acute increase in serum levels of bone formation marker proteins (6). These studies show that IGFs play an important role in the regulation of bone formation.
The functions of IGFs depend not only on the amount of IGF produced but also on the level of IGF-binding proteins (IGFBPs) (7–10). IGFBPs modulate the half-life and activity of IGFs. In bone, the individual IGFBPs either inhibit or potentiate IGF effects on osteoblasts (7, 10–14). Of the six high-affinity IGFBPs that are known to circulate in blood and that are produced by osteoblasts, IGFBP-5 has several unique features that suggest that it is a key component of the IGF system in bone. IGFBP-5 is the most abundant IGFBP stored in bone, having a high specific binding affinity for hydroxyapatite and extracellular matrix proteins, thereby fixing it and its bound IGFs within bone (15–18). We have previously proposed that the local release of these sequestered IGFs and IGFBP-5 in bone could provide the mechanism by which osteoclastic bone resorption during remodeling gives rise to a coupled increase in bone formation. Therefore, the significant age-related decline in the skeletal content of IGF-I, which is positively correlated with decreasing IGFBP-5, could contribute in part to the age-related impairment in the coupling of bone formation to resorption (16, 17, 19). IGFBP-5 is also unique, in that it is the only IGFBP that has been shown to consistently stimulate osteoblast cell proliferation in vitro (18, 20–23).
Consistent with the in vitro studies, we found that systemic administration of IGFBP-5 increased bone formation parameters in mice (23). In these studies, we found that IGFBP-5 treatment did not significantly increase serum IGF-I, suggesting that the effects of IGFBP-5 on bone are not mediated by increasing circulating IGF-I (23). In regard to the mechanism by which IGFBP-5 stimulates bone formation, recent studies suggest that the mitogenic effects of IGFBP-5 may in part be independent of IGFs and mediated through IGFBP-5’s own signal transduction pathway (7, 24). IGFBP-5 binds to specific sites on osteoblast cell surfaces, induces phosphorylation of its putative receptor (24), and contains nuclear localization signal (25). On the basis of these findings and the findings that the relative concentration of IGFBP-5 in the conditioned medium of human osteoblasts is an order of magnitude higher than that of IGFs (26), we proposed the hypothesis that IGFBP-5 functions as a growth factor and acts in part via an IGF-independent mechanism. In the present study, we therefore evaluated in vitro and in vivo effects of IGFBP-5 on bone formation parameters using the IGF-I knockout (KO) mouse as a model.
Methods
α-MEM, Ham’s F12 medium, and DMEM were purchased from Life Technologies Inc. (Gaithersburg, Maryland, USA) and Mediatech Inc. (Herndon, Virginia, USA). FBS and Fe-supplemented bovine calf serum (CS) were from Hyclone (Logan, Utah, USA). BSA and paranitrophenyl phosphate was purchased from Fluka (Buchs, Switzerland). All other chemicals were enzyme grade and purchased from Fisher Scientific (Tustin, California, USA) or Sigma Chemical Co. (St. Louis, Missouri, USA). Recombinant human IGF-I was a gift from Upjohn/Pharmacia (Stockholm, Sweden). Recombinant human IGFBP-5 was expressed in Escherichia coli and purified as described previously (7). Recombinant human IGFBP-5 was a gift from K. Lang (Roche Diagnostics GmbH, Penzeberg, Germany). Recombinant human IGFBP-4 was expressed in E. coli and purified as described previously (27). Neither IGFBP-5 nor IGFBP-4 preparations used in these studies contained detectable levels of either IGF-I or IGF-II.
Osteoblast cell culture
Osteoblast cells used were isolated by collagenase digestion from calvariae of newborn C3H/HeJ mice as described previously (28). The cells released were washed in DMEM + 10% calf serum and plated in the same media in 10-cm plates. Cells at passage two were used for cell proliferation and alkaline phosphatase (ALP) studies.
IGF-I KO clonal cell lines
Osteoblasts were isolated from calvaria of newborn IGF-I KO and wild-type mice and cultured in a 1:1 mixture of DMEM and Ham’s F12 media with 10% FCS. Cells were grown to 50–80% confluence and split 1:3 for 26–32 passages, during which cells spontaneously immortalized. Cells were then plated at clonal densities in 10-cm culture dishes, and individual clones were picked using glass cloning cylinders. Clones that proliferated well were expanded and cells were stored cryogenically. Cells for experiments were thawed and grown several passages in α-MEM with 10% CS, then used for the in vitro experiments. Eighty percent confluent cultures were incubated in serum-free media for 48 hours, and the media from osteoblasts derived from wild-type and IGF-I KO mice were used for measurement of IGF-I and IGF-II levels.
IGF-I KO mice
Breeder mice heterozygous for the IGF-I KO allele were kindly supplied by A. Efstratiadis (Columbia University, New York, New York, USA). IGF-I–null pups were identified by the characteristic small size at birth and failure to grow after birth (5), as well as by PCR analysis using IGF-I forward primer (5′-CCACAGGCTATGGCTCCAGCATTC-3′), IGF-I reverse primer (5′-GTCAGTGTGGCGCTCGGCAC-3′), and neo reverse primer (5′-ATCCATCTTGTTCAATGGCCGATCCC-3′) yielding a 450-bp product for the disrupted IGF-I gene and 160-bp product for the wild-type gene. Heterozygous litter mates were genotyped and used for breeding, and the wild-type litter mates were used as control animals in this study.
In vitro experiments
The biologic activity of the IGFBP-5 preparations was established by cell proliferation using the alamarBlue assay, a measure of cell number (AccuMed International Inc., Westlake, Ohio, USA). Briefly, cells were seeded into 96-well plates at 2,000 cells per well in 50 μl of DMEM or α-MEM containing 0.1% CS and 0.1% BSA. Twenty-four hours later, the media was removed, the cell layers were rinsed with PBS, and 100 μl of 10 ng/ml IGF-I or different concentrations (0.1–100 ng/ml) of IGFBP-5 in DMEM or α-MEM containing 0.1% BSA was added to each well. The medium was replaced 48 hours later with 100 μl of 10% alamarBlue diluted in phenol red free DMEM or α-MEM. The fluorescence was determined 4 hours later using a fluorescent plate reader (Fluorolite 1000; Dynex Technologies Inc., Chantilly, Virginia, USA).
For assessment of ALP specific activity, cells were treated as already described here for alamarBlue assay, but incubated 72 hours after the addition of factors. At the end of the incubation, media were removed, and the cell layers were rinsed with PBS and extracted with 0.1 ml of a 0.01% solution of Triton X-100 in 25 mmol/l NaHCO3 buffer (pH 7.5) (29).
In vivo experiments
C3H/HeJ mice purchased from The Jackson Laboratories (Bar Harbor, Maine, USA) and IGF-I KO mice were housed in a controlled environment with 12-hour light/dark cycles at 70°F with food and water ad libitum. The IGF-I and IGFBP-5 doses were determined from a study published previously reporting the effect of IGF-I local administration on bone formation in parietal bones (30). In all experiments, mice were grouped according to their weight. At the end of each experiment, the mice were euthanized by ethrane inhalation and decapitation; calvariae were collected and stored at –70°C until biochemical measurements were performed. The experimental procedures performed in this study are in compliance with NIH guidelines for the care and use of laboratory animals and approved by the Animal Studies Subcommittee at the Jerry L. Pettis Veterans Administration Medical Center.
Experiment 1: Local effect of IGFBP-5 or IGF-I in C3H mice.
On day 1, 7-week-old female C3H/HeJ mice received 0, 0.05, or 0.25 nmol of IGFBP-5, or equimolar doses of IGF-I (n = 8 animals per group). Each mouse received a single 10-μl aliquot of treatment administered via a Hamilton syringe (Reno, Nevada, USA) to the outer periosteum of the right parietal bone (31, 32). Five days later (day 6), the mice were euthanized.
Experiment 2: Local effect of combined IGFBP-5 and IGF-I in C3H mice.
On day 1, 7-week-old female C3H/HeJ mice received 0, 0.002, 0.01, or 0.05 nmol of IGFBP-5, and/or equimolar doses of IGF-I (n = 6 animals per group). Each mouse received 10 μl of treatment administered as in experiment 1. before administration of the mixture, IGF-I was incubated with an equimolar dose of IGFBP-5 for 1 hour at room temperature. On day 6, the mice were euthanized.
Experiment 3: Local effect of IGFBP-5 or IGF-I in IGF-I KO mice.
On day 1, 7-week-old IGF-I–/– mice and wild-type littermates received vehicle (PBS), 0.0125 nmol/g body weight of IGFBP-5 (0.05 nmol/mouse for IGF-I–/– mice and 0.25 nmol/mouse for wild-type mice), or an equimolar dose of IGF-I (n = 5 or 6 animals per group). Each mouse received 10 μl of treatment administered as in experiment 1 (see earlier discussion). The IGFBP-5 and IGF-I doses used in this experiment were selected based on the results from experiment 1. On day 6, the mice were euthanized.
Experiment 4: Local effect of IGFBP-4 in C3H mice.
To compare local effects of IGFBP-5 with IGFBP-4, 7-week-old C3H/HeJ mice received vehicle, 0.05 nmol of IGFBP-4, and/or an equimolar dose of IGF-I (n = 8 animals per group). Each mouse received 10 μl of treatment administered as in experiment 1 (see earlier discussion). Before administration, the IGF-I was incubated with an equimolar dose of IGFBP-4 for 1 hour at room temperature. On day 6, the mice were euthanized.
Experiment 5: Local effect of IGFBP-4 in IGF-I KO mice.
On day 1, 9-week-old IGF-I–/– mice and wild-type littermates received vehicle or 0.0125 nmol/g body weight of IGFBP-4 (0.05 nmol/mouse for IGF-I–/– mice and 0.25 nmol/mouse for wild-type mice) (n = 6 animals per group). Each mouse received 10 μl of treatment administered as in experiment 1 (see earlier discussion). The IGFBP-4 dose in this experiment was equimolar to the IGFBP-5 dose in experiment 3. On day 6, the mice were euthanized.
Bone collection.
Parietal bones were dissected out of each carcass and cleaned of soft tissue carefully, in order to not destroy the periosteum. Each bone was rinsed in PBS at 4°C for 24 hours, followed by extraction in 0.01% Triton X-100 at 4°C for 72 hours as described previously (30).
Biochemical assays
IGF-I and IGF-II RIAs.
IGF-I and IGF-II levels were measured by RIAs after separation of IGFBPs by Bio-Spin separation using Bio-Gel P10 (Bio-Rad Laboratories Inc., Hercules, California, USA) in the presence of 1M acetic acid as described previously (1). This method was validated previously for complete separation of IGFBPs from IGFs in serum and other biologic fluids. The assay for IGF-I utilizes human recombinant IGF-I as a standard and tracer and rabbit polyclonal antiserum (1). The assay for IGF-II utilizes human recombinant IGF-II as a standard and tracer and a mouse mAb (1). Intra- and interassay coefficient of variation (CV) was less than 10% for both of these assays. The sensitivity of the IGF-I and IGF-II RIAs was 20 and 50 pg/ml, respectively. To increase sensitivity, conditioned media samples were concentrated 60× by speed vac centrifugation before Bio-Spin separation.
Osteocalcin RIA.
Osteocalcin levels were determined by using rabbit antibody made against mouse osteocalcin synthetic peptide as described previously (33). The sensitivity of the assay was 19 ng/ml. The intra- and interassay CV was less than 10%.
ALP activity.
The ALP activity of the cells and bone extracts was determined as described previously (34). ALP activity of the bone extracts was expressed as milliunits per milligram of protein or as milliunits per milligram dry weight of bone.
Total protein levels.
Protein concentration was determined by Bradford assay using a commercial kit (Bio-Rad Laboratories Inc.).
Statistical analysis
Statistical analysis of the data was performed by t test or Fisher’s protected least significant difference method (post hoc test) for multiple comparisons in a one-way ANOVA as appropriate. P values less than 0.05 were considered significant.
Results
IGF-I and IGF-II levels in conditioned media and serum samples
As expected, IGF-I levels in the serum and conditioned medium of osteoblasts from IGF-I KO mice were undetectable. Serum and conditioned medium of osteoblasts from 7- to 9-week-old wild-type littermate mice contained approximately 300 ng/ml and 1 ng/ml IGF-I, respectively.
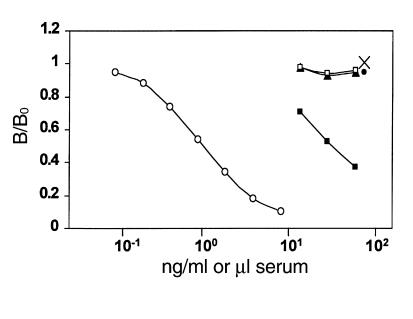
It is known that a developmental switch from IGF-II to IGF-I expression occurs in rodents during early postnatal life. To determine whether adult mouse serum contains IGF-II and osteoblasts derived from postnatal mice produce IGF-II, we also measured IGF-II levels in the serum and conditioned medium of osteoblasts derived from IGF-I KO and corresponding wild-type littermate mice. Figure 1 shows that serum from 7-day-old wild-type mice displaced IGF-II tracer in parallel with the IGF-II standard used in this study. The amount of IGF-II contained in the serum from 7-day-old wild-type mice was estimated as 637 ng/ml. In contrast, serum from IGF-I KO adult (56-day-old) mice or their corresponding wild-type littermate mice contained no detectable IGF-II levels as measured by RIA (<10 ng/ml). In addition, IGF-II levels in the conditioned medium of osteoblasts derived from IGF-I KO mice or their corresponding wild-type mice were below the detectable limit (<50 pg/ml).
Figure 1.
Displacement of IGF-II tracer from IGF-II antiserum. Bound to free ratios (B/Bo) on the y axis represent the relative levels of radiolabeled tracer bound to antiserum with and without competitor. Competition between the [125I]IGF-II tracer and purified recombinant human IGF-II (open circles), IGF pool from 7-day-old wild-type mice (filled squares), IGF pool from 56-day-old wild-type (filled triangles) or IGF-I KO mice (open squares), and IGF pool from conditioned medium of osteoblasts derived from wild-type (filled circle) or IGF-I knockout mice (×). A total of 50 μl of 1:5 diluted serum or 60× concentrated conditioned medium was applied to Bio-Gel P-10, and the IGFs were separated from IGFBPs by Bio-Spin in 1M acetic acid. The IGF pool was neutralized with an equal volume of 1.2M Tris base before RIA. For serum samples, 10, 20, or 40 μl of IGF pool was subjected to RIA at a final dilution of 1:1,500; 1:750; and 1:375, respectively. For conditioned media samples, 50 μl of IGF pool was subjected to RIA at a final concentration of 1× conditioned medium.
In vitro experiments
To investigate whether IGFBP-5 mediates its effects in part by a mechanism independent of IGF-I, we compared the biologic effects of IGFBP-5 or IGF-I on cell proliferation and ALP activity using mouse calvarial osteoblasts derived from wild-type and IGF-I KO mice in serum-free cultures.
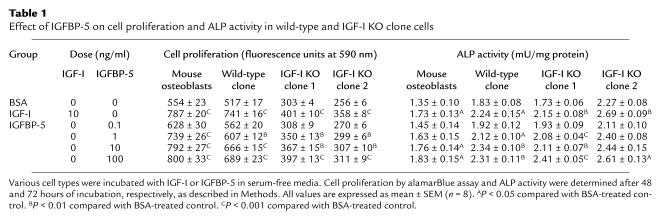
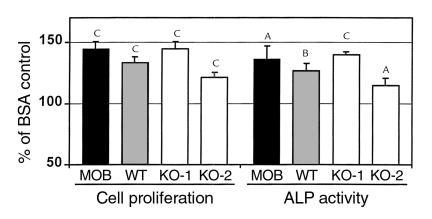
IGFBP-5 treatment increased cell proliferation and ALP activity in a dose-dependent manner in early passage C3H calvaria cells and in IGF-I KO and wild-type cells of the calvaria cell lines that were tested (Table 1; Figure 2). IGFBP-5 at 1 ng/ml and higher concentration increased cell proliferation by 20–40% in all cell groups. IGFBP-5 increased ALP activity in IGF-I KO cells 10–40%, similar to that in the wild-type cell line and early passage C3H osteoblast cultures. IGF-I at 10 ng/ml, as expected, significantly increased cell proliferation by 30–40% and increased ALP activity 20–30 % in osteoblasts derived from wild-type and IGF-I KO mice.
Table 1.
Effect of IGFBP-5 on cell proliferation and ALP activity in wild-type and IGF-I KO clone cells
Figure 2.
Effect of IGFBP-5 on cell proliferation and ALP activity in early passage C3H mouse calvarial osteoblasts (MOB) and in wild-type (WT) and IGF-I knockout (KO-1 and KO-2) cell lines. Cells were seeded at 2,000 cells per well and incubated in media containing 0.1% CS and 0.1% BSA. After 24 hours, the media were completely removed and replaced with the 100 ng/ml of IGFBP-5 in media containing 0.1% BSA (serum-free). After an additional 48 or 72 hours of incubation, cell proliferation by alamarBlue assay and ALP activity of the cells were determined as described in Methods. The values are the mean ± SEM (n = 8). Differences from control were determined by analysis of variance. AP < 0.05, BP < 0.01, and CP < 0.001.
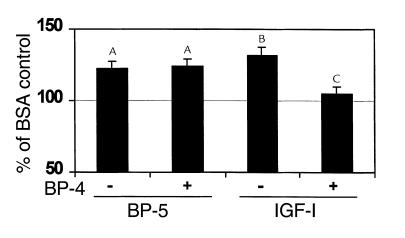
Although the IGF-II level was undetectable in conditioned medium of osteoblasts derived from IGF-I KO mice, IGF-II might be produced in undetectable amounts that were adequate for IGF-dependent IGFBP-5 action. We therefore tested the effects of IGFBP-5 in the presence of IGFBP-4, which would eliminate the actions of IGF-II, if any was produced by osteoblasts derived from IGF-I KO mice. It is known that IGFBP-4 binds IGF-II with tenfold greater affinity than that of IGF receptors and thereby blocks the binding of IGF ligand to its receptor (7). Figure 3 shows that the IGFBP-5–induced increase in proliferation of osteoblasts derived from IGF-I KO mice was not affected by the addition of IGFBP-4 at a dose of 300 ng/ml. In contrast, IGF-I in the amount of 10 ng/ml induced an increase in proliferation of osteoblasts derived from IGF-I KO mice that was completely blocked by the addition of IGFBP-4 under identical culture conditions.
Figure 3.
Effect of IGFBP-4 on IGFBP-5– or IGF-I–induced cell proliferation of osteoblasts derived from IGF-I KO mice. Osteoblasts from IGF-I KO mice (clone 1 cells) were plated as described in Figure 2. IGFBP-4 (300 ng/ml) or vehicle was added for 1 hour before the addition of IGFBP-5 (50 ng/ml) or IGF-I (10 ng/ml) or DMEM/BSA. Forty-eight hours after the addition of effectors, cell proliferation was determined by alamarBlue assay. The fluorescence units at 590 nm in BSA-treated control culture were 347 ± 10 (mean ± SEM; n = 8). The values are the mean ± SEM (n = 8). AP < 0.05 compared with BSA-treated control. BP < 0.01 compared with BSA-treated control. CP < 0.01 versus IGF-I treatment.
On the basis of these findings and the findings that IGFBP-5 increased bone formation in vivo (23), we predicted that IGFBP-5 may have an IGF-I–independent stimulatory effect on bone formation parameters in vivo. We therefore tested the effect of IGFBP-5 on bone formation parameters in vivo using the local parietal bone injection mouse model.
In vivo experiments
Bone formation was evaluated by measuring osteoblast cell products such as ALP and osteocalcin in calvarial bone extracts.
Experiment 1.
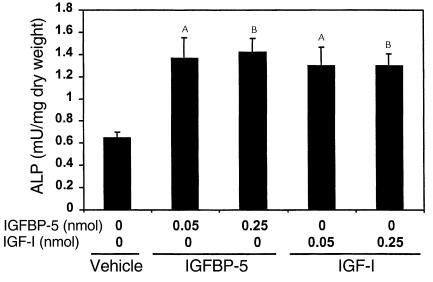
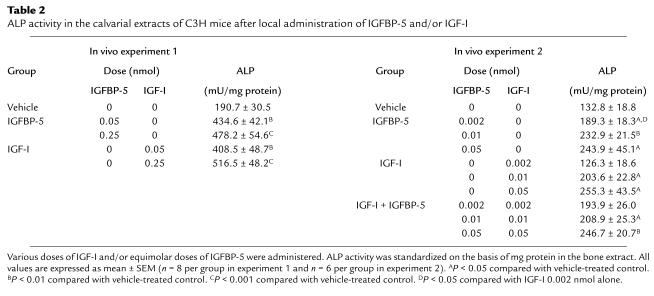
A single local injection of IGFBP-5 to the outer periosteum of the parietal bone increased ALP activity by more than 100% compared with vehicle-treated controls, whether activity was normalized to bone dry weight (Figure 4) or to milligrams of bone extract protein (Table 2). The magnitude of stimulation by IGFBP-5 was comparable to equimolar doses of IGF-I.
Figure 4.
Effect of local injection of IGFBP-5 or IGF-I on ALP activity in parietal bones of C3H mice. Seven-week-old C3H/HeJ mice received 0–0.25 nmol IGFBP-5 or an equimolar dose of IGF-I to the outer periosteum of the right parietal bone. Five days later, the parietal bones were collected and ALP activity in the bone extracts was determined as described in Methods. The values are the mean ± SEM (n = 8). AP < 0.01 compared with vehicle-treated control. BP < 0.001 compared with vehicle-treated control.
Table 2.
ALP activity in the calvarial extracts of C3H mice after local administration of IGFBP-5 and/or IGF-I
Experiment 2.
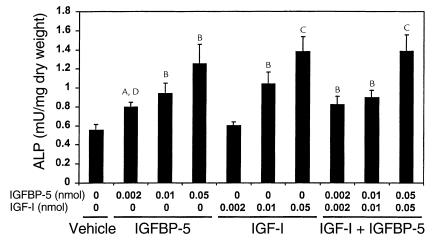
To determine the lowest effective dose of IGFBP-5 to increase bone formation parameters, we tested IGFBP-5 at 0.002, 0.01, and 0.05 nmol/mouse. We found that IGFBP-5 increased ALP activity in a dose-dependent manner with a 100% increase at 0.05 nmol (Figure 5; Table 2). At the lowest dose tested (0.002 nmol/mouse), IGFBP-5 increased ALP activity, but IGF-I did not. IGFBP-5 injected together with IGF-I at this dose increased ALP activity to the same extent as did IGFBP-5 alone. At higher doses, the combination of equimolar doses of IGFBP-5 and IGF-I increased ALP activity to the same extent as either IGFBP-5 or IGF-I alone.
Figure 5.
Effect of local injection of IGFBP-5 and/or IGF-I on ALP activity in parietal bones of C3H mice. Seven-week-old C3H/HeJ mice received 0–0.05 nmol IGFBP-5 and/or an equimolar dose of IGF-I to the outer periosteum of the right parietal bone. Five days later, the parietal bones were collected and ALP activity in the bone extracts was determined as described in Methods. The values are the mean ± SEM (n = 6). AP < 0.05 compared with vehicle-treated control. BP < 0.01 compared with vehicle-treated control. CP < 0.001 compared with vehicle-treated control. DP < 0.05 compared with 0.002 nmol IGF-I alone.
Experiment 3.
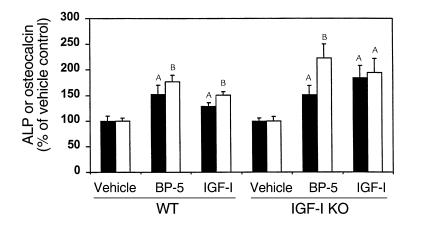
To evaluate whether the IGFBP-5-induced increase in ALP activity in vivo was caused by an IGF-I–independent mechanism, we tested IGFBP-5 effects in IGF-I KO mice and their wild-type littermates. IGFBP-5 treatment increased ALP activity and osteocalcin levels in both IGF-I KO mice and wild-type littermates by 50–120%, when standardized on the basis of dry weight of bone (Figure 6). Similarly, IGF-I also increased ALP activity and osteocalcin levels in both IGF-I KO and wild-type mice.
Figure 6.
Effect of local injection of IGFBP-5 or IGF-I on ALP activity (filled bars) and osteocalcin levels (open bars) in parietal bones of IGF-I knockout (IGF-I KO) mice and their wild-type littermates (WT). Seven-week-old mice received 0.0125 nmol/g body weight of IGFBP-5 or equimolar dose of IGF-I to the outer periosteum of the right parietal bone. Five days later, the parietal bones were collected and the ALP activity and osteocalcin levels in the bone extracts were determined as described in Methods. The values are expressed as a percentage of the vehicle-treated control value, mean ± SEM (n = 5 or 6). The ALP activity in the bone extract of vehicle-treated controls in WT and IGF-I KO mice was 0.33 ± 0.03 and 0.55 ± 0.03 mU/mg dry bone weight, respectively. The osteocalcin levels in the bone extract of vehicle-treated controls in WT and IGF-I KO mice were 0.032 ± 0.003 and 0.047 ± 0.004 ng/mg dry bone weight, respectively. AP < 0.05 compared with vehicle-treated control. BP < 0.01 compared with vehicle-treated control.
The amount of extractable proteins in the parietal bone extract was significantly increased (50–60%) in IGF-I KO mice treated with IGF-I or IGFBP-5 compared with vehicle treated control. Accordingly, the ALP activity and osteocalcin levels when standardized on the basis of extractable protein in IGFBP-5– or IGF-I–treated groups were not significantly different in IGF-I KO mice compared to vehicle-treated control group (data not shown).
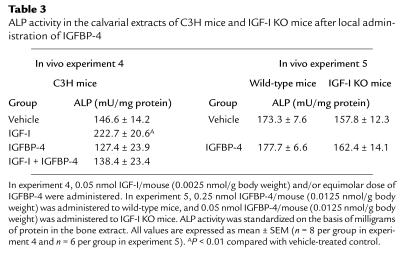
Experiment 4.
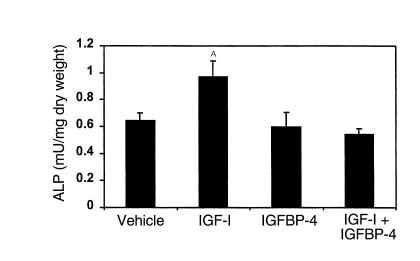
To determine whether the effect of IGFBP-5 to increase bone formation was specific to this binding protein, we tested the local effects of equimolar doses of IGFBP-4, IGFBP-5, and IGF-I in the C3H parietal bone model. IGF-I administration significantly increased ALP activity of parietal bone extract, whereas IGFBP-4 alone had no significant effect (Figure 7; Table 3). In contrast to IGFBP-5 (Table 2), the IGF-I–induced increase in ALP activity in the parietal bones was completely blocked by an equimolar dose of IGFBP-4 (Figure 7; Table 3).
Figure 7.
Effect of local injection of IGF-I and/or IGFBP-4 on ALP activity in parietal bones of C3H mice. Seven-week-old C3H/HeJ mice received 0.05 nmol IGF-I and/or an equimolar dose of IGFBP-4 to the outer periosteum of the right parietal bone. Five days later, the parietal bones were collected and ALP activity in the bone extracts were determined as described in Methods. The values are the mean ± SEM (n = 8). AP < 0.05 compared with vehicle-treated control, with IGFBP-4, and with IGF-1 + IGFBP-4 groups.
Table 3.
ALP activity in the calvarial extracts of C3H mice and IGF-I KO mice after local administration of IGFBP-4
Experiment 5.
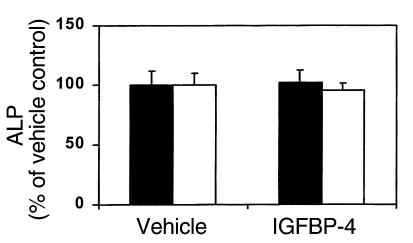
To determine whether the IGFBP-5–induced increase in bone formation parameters in IGF-I KO mice is specific to this IGF-binding protein, we evaluated IGFBP-4 effects in IGF-I KO mice. IGFBP-4 had no significant effect on parietal bone extract ALP activity in IGF-I KO mice and their wild-type littermates (Figure 8; Table 3).
Figure 8.
Effect of local injection of IGFBP-4 on ALP activity in parietal bones of IGF-I KO mice (filled bars) and their wild-type littermates (open bars). Nine-week-old mice received 0.0125 nmol/g body weight of IGFBP-4 to the outer periosteum of the right parietal bone. Five days later, the parietal bones were collected and ALP activity in the bone extracts was determined as described in Methods. The values are expressed as a percentage of the vehicle-treated control value, mean ± SEM (n = 6). The ALP activity in the bone extract of vehicle-treated controls in wild-type and IGF-I KO mice was 0.35 ± 0.03 and 0.59 ± 0.07 mU/mg dry bone weight, respectively.
Discussion
Binding proteins are produced locally by different tissues and could therefore act in local regulation (11, 35). At the local level, IGFBPs could modulate the interactions between IGFs and type I IGF receptors on the cell surface because the affinity of IGFBPs for IGFs is about an order magnitude higher than the affinity of IGF receptors for IGFs (35). Some of the IGFBPs, especially IGFBP-5, has been shown to bind to a number of extracellular matrix proteins and hydroxyapatite, thereby increasing local extracellular IGF concentrations for later release and receptor binding (15–18, 36). Because some IGFBPs inhibit, whereas others (including IGFBP-5) enhance, IGF activities, the actions of locally produced IGFs at a specific site in the body depend on the relative amounts of stimulatory versus inhibitory IGFBPs present at that location.
In previous studies, we found that systemic administration of IGFBP-5 to mice increased bone formation parameters (23). This effect of IGFBP-5 was not associated with increased total IGF-I serum levels or in the fraction of IGF-I in the 50-kDa bioavailable complex, suggesting that IGFBP-5 effects on bone formation are not mediated by changes in circulating level of IGF-I. In contrast to IGFBP-5, systemic administration of IGFBP-4 increases bone formation parameters by an IGF-dependent mechanism (30). Consistent with the stimulatory effect of IGFBP-5 on bone formation parameters in mice are the findings that (a) serum levels of IGFBP-5 exhibit significant positive correlation with bone formation markers and bone density in clinical studies; and (b) growth hormone, one of the major bone anabolic hormones, is a major positive regulator of IGFBP-5 production (37).
In the present study, we provide new in vitro and in vivo evidence supporting the conclusion that IGFBP-5 may function as a growth factor that acts in part via an IGF-independent mechanism. In vitro physiologically relevant concentrations of IGFBP-5 added to serum-free cultures of mouse osteoblast cells derived from IGF-I KO and wild-type mice significantly increased proliferation and ALP activity, a marker of osteoblast differentiation. In vivo, a single local administration of IGFBP-5 to the outer periosteum of parietal bone increased parietal bone levels of bone formation markers ALP and osteocalcin in IGF-I KO and wild-type mice. At the lowest dose tested, IGFBP-5 increased bone formation parameters, although an equimolar dose of IGF-I did not stimulate bone formation parameters significantly. IGFBP-5 and IGF-I were equipotent at higher doses. Both in vitro and in vivo, IGFBP-5 administration increased bone formation parameters in IGF-I KO and wild-type cells or bones with a magnitude that was comparable to that of equimolar dose of IGF-I.
The discovery of IGF-independent activities of IGFBPs has added another layer of complexity to the IGF axis (10, 11, 13). An accumulating body of evidence has revealed that IGFBP-3 has important IGF-independent effects in vitro on growth stimulation of multiple cell types (38, 39). In addition, in vitro studies in our laboratory and others have suggested that IGFBP-5 exerts effects on osteoblasts and osteoclasts independent of IGFs (7, 24, 40). In this study, we found that IGFBP-5 treatment of osteoblasts derived from IGF-I KO mice stimulated both proliferation and alkaline phosphatase specific activity. Furthermore, IGFBP-5 stimulated proliferation of osteoblasts derived from IGF-I KO mice in the presence of IGFBP-4, which is a potent inhibitor of IGF actions in osteoblasts. Because these cells did not contain a functional IGF-I gene, did not produce measurable IGF-II levels, and were treated in serum-free conditions, the results provide the first direct evidence to our knowledge that IGFBP-5 may function as a growth factor by stimulating osteoblasts in the absence of IGFs.
This study also provides the first in vivo evidence for IGF-independent actions of IGFBP-5 to increase bone formation. Our findings that IGFBP-5 has IGF-independent actions, both in vitro and in vivo, strongly supports our hypothesis that IGFBP-5 functions as a growth factor. The effect of IGFBP-5 to increase bone formation parameters in IGF-I KO mice was comparable to effects of IGF-I. Furthermore, this effect was specific for IGFBP-5, as local administration of IGFBP-4 under identical conditions did not influence bone formation in wild-type or IGF-I KO mice. Local administration of IGFBP-4, but not IGFBP-5, inhibited the effects of IGF-I administration to increase bone formation parameters. Because adult mouse serum contains and adult mouse osteoblasts produce no detectable IGF-II, our in vitro and in vivo data with IGF-I KO mice provide direct evidence that IGFBP-5 may function as a growth factor that stimulates its actions in part via an IGF-independent mechanism.
The mechanism by which IGFBP-5 may exert its effects on osteoblasts as a growth factor can only be speculated at the present time. In this regard, we and others have shown that osteoblasts contain putative receptors for IGFBP-5 (7, 24). Studies by Andress (24) indicate that the putative IGFBP-5 receptor may function as a serine kinase, as the phosphorylation of serine residues in the 420-kDa IGFBP-5 receptor is stimulated by intact IGFBP-5, IGFBP-5 (1–169), and IGFBP-5 (201–218) but not by IGF-I or TGF-β. Furthermore, the expression of MTS-I, a calcium-binding protein, was regulated by IGFBP-5 but not by IGF-I or TGF-β, thus suggesting that the IGFBP-5 putative receptor is functionally different from that of IGF-I receptor (41). Another mechanism by which IGFBP-5 could exert IGF-independent effects is by transcriptional activation of genes by IGFBP-5 transported into the nucleus via its nuclear localization signal. In this regard, IGFBP-5 contains a nuclear localization sequence, and recent studies demonstrate evidence for nuclear uptake of fluorescently labeled IGFBP-5 in T47D human breast carcinoma cells (25). Further studies are needed to evaluate the involvement of putative IGFBP-5 receptor and/or nuclear localization sequence in IGFBP-5 in mediating IGF-independent growth factor effect on osteoblasts.
In conclusion, our in vitro and in vivo findings on the actions of IGFBP-5 provide new evidence that IGFBP-5 can function as a growth factor independently of the IGFs. Further long-term studies on the effects of IGFBP-5 on bone histomorphometry and bone density are essential to confirm the stimulatory effects of IGFBP-5 on bone formation parameters in mice. Future studies are also needed to evaluate the molecular mechanism by which IGFBP-5 exerts IGF independent effects on osteoblasts.
Acknowledgments
We acknowledge the technical assistance provided by Daniel Bruch, Rongqing Guo, Matthew Husovsky, and Joe Rung-Aroon. This work was supported by funds from the NIH (AR31062 and AR07543), the Veterans Administration, and Loma Linda University. We thank Argiris Efstratiadis for providing us breeding pairs of IGF-I knockout mice for our studies on the effects of IGFBP-5 on bone formation.
References
- 1.Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab. 1995;80:637–647. doi: 10.1210/jcem.80.2.7531716. [DOI] [PubMed] [Google Scholar]
- 2.Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res. 1993;8(Suppl. 2):S565–S572. doi: 10.1002/jbmr.5650081326. [DOI] [PubMed] [Google Scholar]
- 3.Schoenle E, Zapf J, Hauri C, Steiner T, Froesch ER. Comparison of in vivo effects of insulin-like growth factors I and II and of growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 1985;108:167–174. doi: 10.1530/acta.0.1080167. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy TL, Centrella M, Canalis E. Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology. 1989;124:301–309. doi: 10.1210/endo-124-1-301. [DOI] [PubMed] [Google Scholar]
- 5.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 6.Ebeling PR, Jones JD, O’Fallon WM, Janes CH, Riggs BL. Short-term effects of recombinant human insulin-like growth factor I on bone turnover in normal women. J Clin Endocrinol Metab. 1993;77:1384–1387. doi: 10.1210/jcem.77.5.8077337. [DOI] [PubMed] [Google Scholar]
- 7.Mohan S, et al. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 8.LaTour D, Mohan S, Linkhart TA, Baylink DJ, Strong DD. Inhibitory insulin-like growth factor-binding protein: cloning, complete sequence, and physiological regulation. Mol Endocrinol. 1990;4:1806–1814. doi: 10.1210/mend-4-12-1806. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PG, Novak JF. Insulin-like growth factor binding protein (IGFBP) inhibits IGF action on human osteosarcoma cells. J Cell Physiol. 1991;149:293–300. doi: 10.1002/jcp.1041490216. [DOI] [PubMed] [Google Scholar]
- 10.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 11.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 12.Mohan S, Baylink DJ. Insulin-like growth factor (IGF)-binding proteins in serum: do they have additional roles besides modulating the endocrine IGF actions? J Clin Endocrinol Metab. 1996;81:3817–3820. doi: 10.1210/jcem.81.11.8923818. [DOI] [PubMed] [Google Scholar]
- 13.Mohan S. Insulin-like growth factor binding proteins in bone cell regulation. Growth Regul. 1993;3:67–70. [PubMed] [Google Scholar]
- 14.Srinivasan N, Edwall D, Linkhart TA, Baylink DJ, Mohan S. Insulin-like growth factor-binding protein-6 produced by human PC-3 prostate cancer cells: isolation, characterization and its biological action. J Endocrinol. 1996;149:297–303. doi: 10.1677/joe.0.1490297. [DOI] [PubMed] [Google Scholar]
- 15.Jones JI, Gockerman A, Busby WH, Jr, Camacho-Hubner C, Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolas V, et al. An age-related decrease in the concentration of insulin-like growth factor binding protein-5 in human cortical bone. Calcif Tissue Int. 1995;57:206–212. doi: 10.1007/BF00310260. [DOI] [PubMed] [Google Scholar]
- 17.Mohan S, Farley JR, Baylink DJ. Age-related changes in IGFBP-4 and IGFBP-5 levels in human serum and bone: implications for bone loss with aging. Prog Growth Factor Res. 1995;6:465–473. doi: 10.1016/0955-2235(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 18.Bautista CM, Baylink DJ, Mohan S. Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun. 1991;176:756–763. doi: 10.1016/s0006-291x(05)80249-9. [DOI] [PubMed] [Google Scholar]
- 19.Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17:93S–98S. doi: 10.1016/8756-3282(95)00186-h. [DOI] [PubMed] [Google Scholar]
- 20.Andress DL, Birnbaum RS. A novel human insulin-like growth factor binding protein secreted by osteoblast-like cells [erratum 1991, 177:895] Biochem Biophys Res Commun. 1991;176:213–218. doi: 10.1016/0006-291x(91)90911-p. [DOI] [PubMed] [Google Scholar]
- 21.Schmid C, Schlapfer I, Gosteli-Peter MA, Froesch ER, Zapf J. Effects and fate of human IGF-binding protein-5 in rat osteoblast cultures. Am J Physiol. 1996;271:E1029–E1035. doi: 10.1152/ajpendo.1996.271.6.E1029. [DOI] [PubMed] [Google Scholar]
- 22.Slootweg MC, Ohlsson C, van Elk EJ, Netelenbos JC, Andress DL. Growth hormone receptor activity is stimulated by insulin-like growth factor binding protein 5 in rat osteosarcoma cells. Growth Regul. 1996;6:238–246. [PubMed] [Google Scholar]
- 23.Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 24.Andress DL. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates phosphorylation of the IGFBP-5 receptor. Am J Physiol. 1998;274:E744–E750. doi: 10.1152/ajpendo.1998.274.4.E744. [DOI] [PubMed] [Google Scholar]
- 25.Schedlich LJ, Young TF, Firth SM, Baxter RC. Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem. 1998;273:18347–18352. doi: 10.1074/jbc.273.29.18347. [DOI] [PubMed] [Google Scholar]
- 26.Malpe R, Baylink DJ, Linkhart TA, Wergedal JE, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. J Bone Miner Res. 1997;12:423–430. doi: 10.1359/jbmr.1997.12.3.423. [DOI] [PubMed] [Google Scholar]
- 27.Qin X, Strong DD, Baylink DJ, Mohan S. Structure-function analysis of the human insulin-like growth factor binding protein-4. J Biol Chem. 1998;273:23509–23516. doi: 10.1074/jbc.273.36.23509. [DOI] [PubMed] [Google Scholar]
- 28.Linkhart TA, et al. Osteoclast formation in bone marrow cultures from two inbred strains of mice with different bone densities. J Bone Miner Res. 1999;14:39–46. doi: 10.1359/jbmr.1999.14.1.39. [DOI] [PubMed] [Google Scholar]
- 29.Dimai HP, et al. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone. 1998;22:211–216. doi: 10.1016/s8756-3282(97)00268-8. [DOI] [PubMed] [Google Scholar]
- 30.Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- 31.Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989;124:2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 32.Marcelli C, Yates AJ, Mundy GR. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J Bone Miner Res. 1990;5:1087–1096. doi: 10.1002/jbmr.5650051013. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava AK, Castillo G, Wergedal JE, Mohan S, Baylink DJ. Development and application of a synthetic peptide based osteocalcin assay for the measurement of bone formation in mouse serum. Calcif Tissue Int. 2000;66:435–442. doi: 10.1007/s002230001109. [DOI] [PubMed] [Google Scholar]
- 34.Farley JR, Hall SL, Tanner MA, Wergedal JE. Specific activity of skeletal alkaline phosphatase in human osteoblast-line cells regulated by phosphate, phosphate esters, and phosphate analogs and release of alkaline phosphatase activity inversely regulated by calcium. J Bone Miner Res. 1994;9:497–508. doi: 10.1002/jbmr.5650090409. [DOI] [PubMed] [Google Scholar]
- 35.Mohan, S., and Baylink, D.J. 1999. IGF system components and their role in bone metabolism. In Contemporary endocrinology: the IGF system. R. Rosenfeld and C. Roberts, Jr., editors. Humana Press Inc. Totowa, New Jersey, USA. 457–496.
- 36.Nam TJ, Busby WH, Jr, Rees C, Clemmons DR. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–1106. doi: 10.1210/endo.141.3.7386. [DOI] [PubMed] [Google Scholar]
- 37.Thoren M, et al. Serum levels of insulin-like growth factor binding proteins (IGFBP)-4 and -5 correlate with bone mineral density in growth hormone (GH)-deficient adults and increase with GH replacement therapy. J Bone Miner Res. 1998;13:891–899. doi: 10.1359/jbmr.1998.13.5.891. [DOI] [PubMed] [Google Scholar]
- 38.Oh Y, Muller HL, Lamson G, Rosenfeld RG. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. J Biol Chem. 1993;268:14964–14971. [PubMed] [Google Scholar]
- 39.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 40.Kanatani M, Sugimoto T, Nishiyama K, Chihara K. Stimulatory effect of insulin-like growth factor binding protein-5 on mouse osteoclast formation and osteoclastic bone-resorbing activity. J Bone Miner Res. 2000;15:902–910. doi: 10.1359/jbmr.2000.15.5.902. [DOI] [PubMed] [Google Scholar]
- 41.Andress DL. Insulin-like growth factor binding protein-5 (IGFBP-5)-receptor phosphorylation is specific for IGFBP-5 and IGFBP-5 related peptides. Growth Horm IGF Res. 1999;9:319. (Abstr.) [Google Scholar]