Abstract
It has been suggested that increased collagenase-3 (MMP-13) activity plays a pivotal role in the pathogenesis of osteoarthritis (OA). We have used tetracycline-regulated transcription in conjunction with a cartilage-specific promoter to target a constitutively active human MMP-13 to the hyaline cartilages and joints of transgenic mice. Postnatal expression of this transgene resulted in pathological changes in articular cartilage of the mouse joints similar to those observed in human OA. These included characteristic erosion of the articular cartilage associated with loss of proteoglycan and excessive cleavage of type II collagen by collagenase, as well as synovial hyperplasia. These results demonstrate that excessive MMP-13 activity can result in articular cartilage degradation and joint pathology of the kind observed in OA, suggesting that excessive activity of this proteinase can lead to this disease.
Introduction
Degenerative joint diseases including osteoarthritis (OA) are common, particularly in the elderly. Early signs of OA include progressive loss from articular cartilage of the proteoglycan aggrecan, reflected by a loss of safranin O staining, excessive damage to type II collagen, and general degeneration and fibrillation of the cartilage surface, resulting ultimately in a loss of articular cartilage (1).
One of the primary targets of this disease is type II collagen, the major structural collagen found in articular cartilage in healthy individuals. There is ordinarily a strict balance between the production of type II collagen and degradation of this protein by catabolic enzymes during normal remodeling of cartilage (1). Pathological conditions such as OA are characterized by a loss of this balance with increased proteolysis (1–5) and upregulation of the synthesis of type II procollagen (5) and aggrecan (6).
Matrix metalloproteinases (MMPs) comprise a family of zinc-dependent enzymes that degrade extracellular matrix components. MMPs are synthesized in articulating joints by synovial cells and chondrocytes. In mature articular cartilage, chondrocytes maintain the cartilage-specific matrix phenotype. Elevated expression of MMPs is associated with cartilage degradation (1). MMP-13, also known as human collagenase-3, is thought to play an important role in type II collagen degradation in articular cartilage and especially in OA (4, 7–9). Type II collagen is the preferred substrate for MMP-13 (4, 7, 10). Expression and contents of MMP-1 (collagenase-1) and MMP-13 (7, 11, 12), expression of MMP-8 (collagenase-2), and collagenase activity (4, 8) are upregulated in human OA cartilage.
Spontaneous development of focal sites degeneration has been described in aging guinea pigs (13). Sublines of the inbred STR/ORT strain of mice also develop spontaneous OA with aging (14). Mice exhibit upregulated expression of MMP-13 and collagenase activity is upregulated in focal lesions (15). In guinea pigs, MMP-1 and MMP-13 are also upregulated in OA lesions associated with increased collagenase activity (16).
Abnormalities in the structure of human type II (17), type IX (18), or type XI (19) collagens (which together constitute the collagen fibril) can each lead to the early development of a familial osteoarthritis. Thus loss of structural integrity of collagen fibrils due to a molecular abnormality can lead to the development of OA.
In this study, we have investigated the role of MMP-13 in the pathogenesis of OA by expressing postnatally a constitutively active mutant of human MMP-13 in transgenic mice, using tetracycline-regulable gene expression targeted specifically to chondrocytes. We used this regulable system because MMPs are known to be required during embryogenesis (20) and because collagenase-3 can play an essential role in matrix remodeling and development of the growth plates as well as in other skeletal tissues as indicated by deletion of Cbfa1 (21, 22), a transcription factor for MMP-13 (23, 24).
We show that MMP-13 transgenic animals exhibit joint pathology that strongly resembles OA. This provides direct evidence in support of a role for this proteinase in the pathology of this disease.
Methods
Generation of transgene constructs.
The CPE-tTA construct was created by subcloning a 1,600-bp Hind III/Nde I, collagen type II promoter, and an 1,800-bp Bam HI enhancer fragment (25, 26) into BS(SK-) (Stratagene, La Jolla, California, USA). The tTA gene, a 1,025-bp Eco RI/Bam HI fragment, was excised from pUHG15-1 (27) and cloned 3′ of the collagen promoter. The tetO7-MMP-13* construct was created by excising the tetO7 promoter region (Xho I-Eco RI) from pUHD10-3 (27) and cloned 5′ to an Sal I-Eco RI MMP-13* cDNA fragment. A mutation resulting in a proline → valine substitution at amino acid 99 was generated in the MMP-13 cDNA (28; referred to as MMP-13*) by site-directed mutagenesis using the pAlter vector (Promega Corp., Madison, Wisconsin, USA). Both constructs contain the SV40 splice and poly (A)n Xba I–Nco I region (750 bp) pcDNA I (Invitrogen Corp., San Diego, California, USA) 3′ of the gene. The β-galactosidase reporter gene (CPE-lacZ construct) containing a nuclear localization signal was constructed as follows: an Xba I/Bgl II fragment, containing the nuclear β-galactosidase structural gene, a β-globin intron, and a poly (A) signal, was excised from plasmid pNlacF (29) and cloned 3′ of the collagen II promoter/enhancer.
Generation of transgenic mice.
A Kpn I/Not I 5,268-bp CPE-tTA fragment and a Xho I/Not/ I 2783 bp tetO7-MMP-13* fragment were purified by CsCl centrifugation and comicroinjected into the FVB/N strain in equal molar amounts. A Kpn I/Not I 7,000-bp CPE-lacZ fragment was also purified and injected into fertilized eggs as described elsewhere (30). Founder animals were first identified by PCR and then Southern blot hybridization. The tTA-encoding transgene was identified by using primers corresponding to the tTA sequence (CGAGGGCCTGCTCGATCTCC) and sequences encoding the 3′ untranslated region (GGCATTCCACCACTGCTCCC). The resulting PCR product was 584 bp in size. The MMP-13* -encoding transgene was identified using primers corresponding to sequences encoding MMP-13* (GAGCACCCTTCTCATGACCTC) and the 3′ untranslated region (GGCATTCCACCACTGCTCCC). The resulting PCR product was 731 bp in size. The lacZ-encoding transgene was identified using primers corresponding to the nuclear localization signal of the β-galactosidase gene (GTTGGTGTAGATGGGCGCATCG) and the collagen promoter (GCGGGGTCTCAGGTTACAGCC). The resulting PCR product was 673 bp in size. Southern blot analysis of tail DNA digested with Bam HI/Nco I or Pvu II/Nco I and hybridized to the 3′ untranslated region under high stringency conditions was performed to confirm the PCR results. The number of copies of transgene DNA that integrated into the genome was determined by Taqman quantitative PCR according to manufacturer’s specifications (Perkin-Elmer Applied Biosystems, Foster City, California, USA). Transgenic lines were generated by mating founder animals to FVB/N wild-type mice and the subsequent generations were identified by PCR.
Doxycycline treatment.
A stock solution of doxycycline (Dox) (Sigma Chemical Co., St. Louis, Missouri, USA) was prepared as 100 mg/ml in 50% ethanol, diluted to its final concentration of 1.0 mg/ml in acidic drinking water, and changed on a daily basis (31).
Whole embryo staining for β-galactosidase (lacZ) activity.
Wild-type females were plugged by transgenic males harboring the CPE-lacZ construct. On embyronic day 16 (E16), the females were sacrificed, and the embryos were stained for β-galactosidase activity as described elsewhere (30). Before fixation, tails from the E16 embryos were removed, digested, and analyzed for transgene transmission by PCR.
Expression analysis via RT-PCR.
Transgene expression was assayed by RT-PCR. Total RNA was isolated from tissues following homogenization in Trizol (Life Technologies Inc., Gaithersburg, Maryland, USA). First-strand synthesis was generated using the Superscript preamplification kit by Life Technologies Inc. Briefly, 5 μg of total RNA was treated with DNase I for 15 minutes at RT, inactivated by the addition of 2 μl of 25 mM EDTA, and heated to 65°C for 15 minutes. Subsequently, the RNA was annealed to 0.5 μg oligo dT and reverse transcribed according to manufacturer’s specifications. PCR analysis of the cDNA was done using the following primers sets: tet activator: CGC CCA GAA GCT AGG TGT AGA G and CGG CCA TAT CCA GAG CGC CG; MMP-13*: GCC CTC TGG CCT GCT GGC TCA TG and CAG GAG AGT CTT GCC TGT ATC CTC. The resulting PCR products are 859 bp and 648 bp, respectively. To test the integrity of the mRNA and the efficiency of the RT, each PCR reaction also contained the following c-fos primer set: 5′-AGG AGG GAG CTG ACA GAT ACA CTC C-3′ and 5′-AGG CCA CAG ACA TCT CCT CTG G-3′. PCR analysis was performed on the cDNA using Taq-gold (Perkin-Elmer Applied Biosystems) for 10 minutes at 95°C, followed by 35 cycles of 60 seconds at 96°C, 90 seconds at 67°C, and 60 seconds at 72°C. A final 12-minute extension was done at 72°C. Reaction products were run on 2.0% agarose gels and visualized by ethidium bromide staining.
Histology.
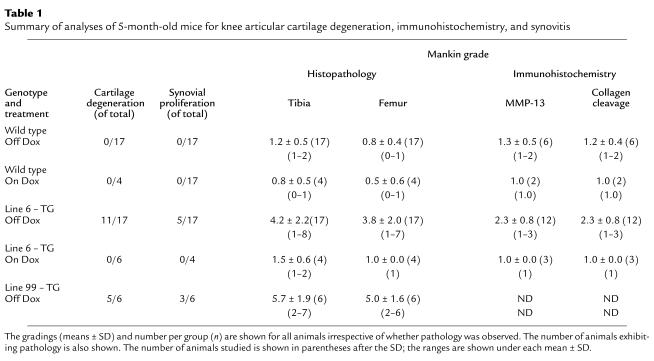
After sacrifice by cervical dislocation or carbon dioxide asphyxiation, mouse hind knee joints were fixed in 10% phosphate-buffered formalin for further processing and analysis. After fixation, the joints were decalcified in 14% EDTA for 7–10 days, dehydrated in graded alcohol, and embedded longitudinally or coronally in paraffin. Serial sections (5 μm thick) were cut through the whole knee joint. For each animal, the left knee was sectioned coronally and right knee was sectioned in sagittally. Sections included adjacent bone tissue, which was examined to ensure uniformity of section thickness. Sections of some of the left knees were used for immunohistochemistry (see Table 1) and stained with hematoxylin and eosin (H&E), and adjacent sections stained with Fast Green and Safranin O (32). The sections were stained either in-house or by American Histo Labs (Gaithersburg, Maryland, USA). Sections stained with Safranin 0 and Fast Green were used to assess cartilage degeneration by using the Mankin grading system (33).
Table 1.
Summary of analyses of 5-month-old mice for knee articular cartilage degeneration, immunohistochemistry, and synovitis
Immunohistochemistry.
The rabbit polyclonal antibodies that we used recognized a human MMP-13 specific peptide sequence (Pro-Asn-Pro-Lys-His-Pro-Lys-Thr-Pro-Glu-Lys). A rabbit antiserum was prepared to this peptide conjugated to a carrier protein as described (4). An anti-neoepitope rabbit polyclonal antibody (COL2-3/4C short peptide) (Gly-Pro-Hyp-Gly-Pro-Gln-Gly) against type II collagen primary cleavage site (4) was also used. A mouse mAb recognizing type X collagen specific peptide sequence (Tyr-Asp-Pro-Arg-Thr-Gly-Ile-Phe-Thr) (34) was employed. Selected sections from the same knee joint used for staining (described earlier here) were deparaffinized in xylene and rehydrated in graded ethanol and PBS. Sections were treated with 0.3% H2O2/methanol for 30 minutes at room temperature and then digested with chondroitin ABC lyase (0.2 U/ml) (ICN Biomedicals Inc., Aurora, Ohio, USA) for 1 hour at 37°C to remove chondroitin sulfate. After blocking with normal horse serum for 30 minutes, primary antibody was incubated with the sections for 1.5 hours at room temperature. Sections were washed three times, for 10 minutes each time, with PBS. The binding of antibody to epitope was detected using a biotinylated secondary antibody and an avidin-linked peroxidase system (Vectastain Universal Elite ABC kit, PK-6200; Vector Laboratories Inc., Burlingame, California, USA). Controls comprised samples with the antibody-peroxidase system but without primary antibody. Staining was graded as follows: 1 = limited stain within chondrocyte lacunae; 2 = moderate stain in lacunae and perilacuna sites; and 3 = intense stain in lacunae, extracellular matrix, and at and close to surface of articular cartilage.
Results
Generation of transgenic mice and tissue-specific expression of the transgene.
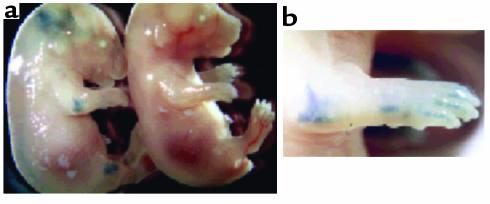
Two genes were introduced into mice to yield tetracycline-regulated expression of MMP-13 in hyaline cartilages, such as in the articulating joints of these transgenic animals. One transgene expressed the tetracycline-regulated transcriptional transactivator (tTA) specifically in chondrocytes under the control of the type II collagen promoter (26, 27). Tissue-specific expression of this promoter was investigated by examining expression of a β-galactosidase reporter gene in a separate transgenic line. Figure 1a shows whole-mount staining for β-galactosidase activity in E16 transgenic mouse embryos expressing this transgene. Blue staining is evident in joints throughout the body, including the ankles, knees, hips, phalanges, wrists, elbows, shoulders, and vertebrae. In addition, cartilage that has not ossified to bone at this stage of development (e.g., some of the facial and rib bones and jaw joint) also stained blue. No staining was observed in nontransgenic littermates. These data demonstrate the correct tissue-specificity of the type II collagen promoter.
Figure 1.
Transgenic mice expressing β-galactosidase under the control of the rat type II collagen promoter. (a) Whole-mount staining for β-galactosidase activity on E16 embryos. The embryos show staining of the transgenic compared with a wild-type embryo. (b) Enlargement of the elbow and front paw.
The second transgene encoded a mutant form of MMP-13 (ref. 28; MMP-13*, see later here) driven by the tet07 promoter (27, 35), which is transactivated by the tTA. Normally, MMPs are synthesized as precursors (i.e., procollagenases or zymogens) whose enzymatic activity is triggered by proteolytic removal of the proregion after secretion. The requirement for proteolytic processing can be circumvented by the use of a variant of the enzyme that is enzymatically active when uncleaved. This constitutively active MMP-13 variant (MMP-13*) contains a proline to valine substitution (PRCGVPDV→PRCGVVDV) at amino acid 99 in a conserved regulatory region that is important for controlling enzyme latency. The same substitution in the proregion of human MMP-1 and MMP-3 has also been shown to produce enzymes that are constitutively active in vivo (36–38).
The tTA and MMP-13* constructs were comicroinjected into fertilized mouse embryos, and four transgenic lines containing both transgenes were identified by PCR and verified by Southern blot. Quantitative Taqman PCR showed that line 6 contained approximately six to eight copies and three to four copies, respectively, of the tTA transgene and the MMP-13* transgene, whereas line 8 contained two copies and one copy, respectively; line 42 contained 34–37 copies and 17–18 copies, respectively; and line 99 contained about 31–33 copies and 19–20 copies, respectively.
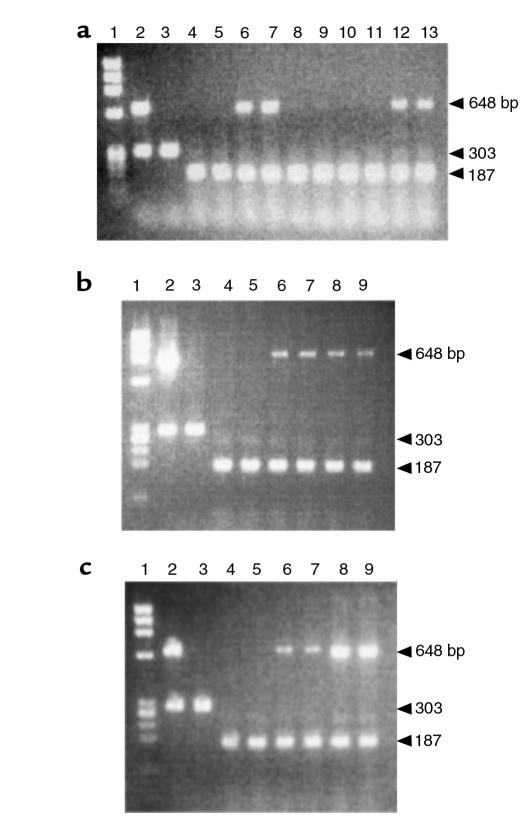
Expression of the tTA and MMP-13* transgenes was initially evaluated in the hind knee joints of 4-week-old mice. Amplification of c-fos mRNA was used as a positive control for each reaction. In mice removed from Dox, Figure 2a shows expression of the MMP13* transgene (648 bp) in lines 6 (lanes 6–7) and 99 (lanes 12–13); however, no MMP13* expression was detected in the nontransgenic controls (lanes 4–5) or in lines 8 (lanes 8–9) and 42 (lanes 10–11). Figure 2b shows an 890-bp fragment resulting from amplification of a tTA-specific primer set. The tTA transgene was expressed in transgenic mice with (lanes 6–7) or without Dox treatment (lanes 8–9), but was not expressed in the nontransgenic controls (lanes 4–5). This result is consistent with the tetracycline-responsive system because the tTA is constitutively expressed and not expected to fluctuate in the presence of Dox or MMP13* expression.
Figure 2.
Expression profile of the tTA and MMP-13* RNA by RT-PCR. (a) Amplification of MMP13* in each of the transgenic lines. Lane 1: φx174 Hae III MW markers; lane 2: PCR amplification of transgenic (line 6) genomic DNA; lane 3: PCR amplification of nontransgenic genomic DNA; lane 4: a wild-type mouse maintained on Dox; lane 5: a wild-type mouse off Dox; lanes 6–7, 8–9, 10–11, and 12–13: heterozygous lines 6, 8, 42, and 99 (4 weeks) removed from Dox at birth, respectively (duplicate). (b and c) Amplification of tTA (b) and MMP-13* (c) cDNA from total RNA. Lanes 1–5: same as in a; lanes 6–7: transgenic mouse (4 weeks) maintained on Dox (duplicate); lanes 8–9: transgenic mouse (4 weeks) removed from Dox at birth (duplicate). The 859-bp fragment and the 648-bp fragment represent the tTA RNA and the MMP-13* RNA, respectively. Each reaction was run using c-fos primers as an internal control, spliced mRNA yielding 187 bp, and unspliced mRNA 303 bp. Note, no bands were detected in corresponding lanes containing RNA for PCR that was not treated with M-MLV RT (data not shown). Moreover, PCR fragments were transferred to a nylon membrane and hybridized to a tTA or MMP-13 transgene-specific probe to verify the identity of the PCR product (data not shown).
Figure 2c shows a 648-bp fragment resulting from amplification of an MMP-13–specific primer set that is specific for human MMP-13. The primer set does not hybridize to the mRNA encoding the endogenous mouse homologue, MMP-13, indicated by the absence of any signal in the nontransgenic RNA. MMP-13* encoding mRNA was not expressed in the nontransgenic controls (lanes 4–5). Lanes 6–7 show the unstimulated level of MMP-13* mRNA expression in mice maintained on Dox. By contrast, lanes 8 and 9 reveal approximately fourfold stimulation of MMP-13* mRNA expression in joints after removal of Dox from the drinking water. Expression of the tTA and the MMP-13* transgene was not observed by RT-PCR in other tissues including brain, heart, liver, kidney, spleen, and skeletal muscle (data not shown). Under the appropriate experimental conditions, we show later here that lines 6 and 99 develop severe articular lesions, synovial hyperplasia, and other OA pathologies. Consistent with the lack of transgene expression, lines 8 and 42 showed no obvious pathologies. Therefore, the focus of this article is largely on line 6 but with supportive data from line 99.
Phenotypic characterization of transgenics.
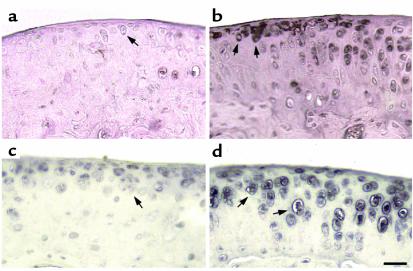
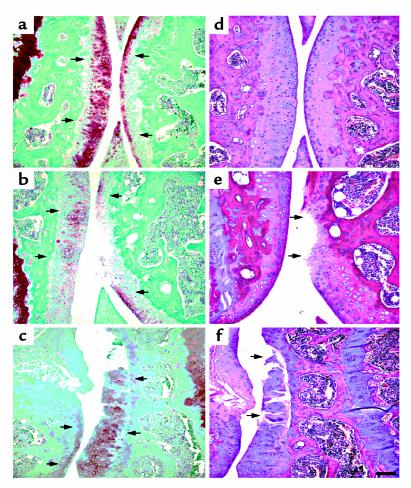
Both wild-type and transgenic mice were maintained on Dox throughout pregnancy. Because we are looking at the effect of MMP-13* expression on the articular cartilage in the absence of Dox, it is appropriate to compare both wild-type mice to transgenic mice after removal of Dox. In both immunohistochemical and histology studies, there was no significant effect of Dox in wild-type mice maintained on Dox compared with those not on Dox (Table 1). Immunohistochemistry was performed on sections taken from selected 5-month-old mice removed from Dox at weaning (21 days). In serial sections, antibodies to human MMP-13 (which cross-react with mouse MMP-13) and to the collagenase-generated neoepitope in the primary cleavage site in type II collagen clearly revealed the presence of increased MMP-13 (Figure 3b) and excessive cleavage of type II collagen (Figure 3d) in similar sites at or close to the surface of articular cartilage of the transgenic mice (Table 1). Staining for MMP-13 (Figure 3a) and collagenase activity (Figure 3c) were less detectable in the wild-type controls (Table 1). In transgenics, MMP-13* was detected mainly at and close to the articular surfaces (Figure 3b) of chondrocytes, including those close to the articular surface (arrows). Moreover, upregulated expression of MMP-13 was found throughout the articular cartilage; however, greater amounts of MMP-13 expression was observed in sites where focal lesions formed, e.g., not covered by meniscus. As we have demonstrated here, our antibody does not distinguish between human and mouse MMP-13. However, the close correspondence between MMP-13 staining and expression of the human transgene suggests that the antibody detects primarily human MMP-13.
Figure 3.
Photomicrographs of immunohistochemical analysis of knee joints from 5-month-old mice. The left panels (a and c) represent sagittal sections of the tibia from wild-type mice, and the right panels (b and d) represent sagittal sections of the tibia from heterozygous line 6 mice. Expression of MMP-13* and its activity was confirmed by staining for MMP-13 and its activity. Section from a wild-type (a) and a transgenic (b) mouse stained with the rabbit anti-human MMP-13 polyclonal antibody; and adjacent sections from a wild-type (c) and a transgenic (d) mouse stained with the COL2-3/4Cs antibody that detects the primary collagenase cleavage site in type II collagen. The arrows represent staining in pericellular sites around chondrocytes. Scale bar = 30 μm.
The cleavage of type II collagen by MMP-13 in vivo generates two fragments, referred to as 3/4 (TCA) and 1/4 (TCB). The polyclonal antibody, COL2-3/4Cs, recognizes a unique amino acid sequence neoepitope in the COOH-terminal end of the large 3/4 fragment of the α1(II) chain of type (II) collagen generated by cleavage by collagenase (4). Figure 3d shows knee joint sections stained for type II collagen cleavage by collagenase using this COL2-3/4Cs antibody. In comparison to wild-type animals, the joint sections taken from transgenics showed a significant amount of pericellular and intracellular staining associated with the chondrocytes (Table 1) of the more superficial articular cartilage (Figure 3d, arrows). This result provides direct evidence for the presence of a significant increase in collagen II cleavage associated with increased presence of MMP-13, reflecting increased proteolytic activity of MMP-13* in the joints of transgenic animals. Such an increase in staining of cleaved and denatured type II collagen in more superficial sites around chondrocytes is also seen in osteoarthritis in humans (ref. 3; A.R. Poole et al., manuscript in preparation) and in naturally developing osteoarthritis in mice (15).
The loss of safranin O staining, and development of cartilage degeneration involving proteoglycan loss, was also examined in the articular cartilage of transgenic mice. Table 1 shows a summary of the data for the right knees. In Figure 4a, the articular cartilage from a wild-type animal shows strong safranin O staining, whereas tissue from line 6 (Figure 4b) and line 99 (Figure 4c) exhibit a significant depletion of safranin O staining in the articular cartilage, including areas around a deep focal lesion (Figure 4b, arrows). These data are summarized in detail in Table 1. As an internal control, the growth plate of both the control and the transgenic animals showed intense safranin O staining throughout the growth plate (data not shown). Serial sections from line 6 (Figure 4e) and line 99 (Figure 4f) stained with H&E revealed more clearly the deep focal articular lesion and articular degeneration, respectively, which was not seen in the wild type (Figure 4d). Furthermore, it is unlikely that expression of the tTA alone in the articular cartilage is responsible for degenerative changes, as no phenotype was observed in transgenic animals from line 6 maintained on Dox.
Figure 4.
Photomicrographs of the articular cartilage of the tibia (right) and femur (left), taken from 5-month-old mice removed from Dox at weaning (21 days). (a–c) Sagittal sections from a wild-type (a) and a heterozygous (b) line 6 and a coronal section from homozygous line 99 (c) stained with safranin O. (d–f) A serial section from each animal stained with H&E. The arrows in a, b, and c represent areas of safranin O staining, whereas the arrows in e and f point to focal lesions and degradation of the articular cartilage. Scale bar = 100 μm.
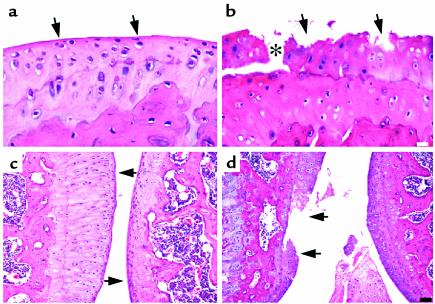
Further assessment of articular cartilage degeneration due to transgene expression revealed that, in contrast to joint tissue from the wild-type mouse (Figure 5a), which shows a relatively smooth articular surface, transgenic animals exhibited a considerable loss of cartilage with focal erosions at the articulating surface (Figure 5b). The asterisk in Figure 5b shows a fissure in the cartilage that splits along the tidemark at the junction with the calcified cartilage. In addition, the chondrocytes appear smaller and less dense throughout the articular cartilage, and empty lacunae are indicative of cell death. Together, these changes resulted in increased Mankin grades reflected by the analyses shown in Table 1.
Figure 5.
Photomicrographs of sagittal sections of the hind knee joint stained with H&E at high magnification (a and b) and at low magnification (c and d) from heterozygous line 6. Sections of the tibia articular cartilage from wild-type (a and c) and transgenic (b and d) mice are shown. In c and d, the tibia is on the left and the femur is on the right. The arrows in a and c point to the smooth articular surface, whereas the arrows in b and d point to the degraded articular surface. Analysis was performed on 5-month-old mice removed from Dox at 21 days. The white scale bar in b represents 30 μm, and the black scale bar in d represents 100 μm.
The wild-type mouse shown in Figure 5c has articular cartilages that are intact, whereas in another transgenic animal from line 6, a focal erosion has developed and progressed into a lesion extending to the tidemark (Figure 5d). Analysis of these animals focused on the hind knee joints. Other joints, however, including the spine and phalanges from two transgenic animals, also showed evidence of joint degeneration (data not shown). Focal lesions were observed in sites not covered by the menisci, suggesting a mechanical component is involved in the lesion formation. Furthermore, there is evidence of more MMP-13 cleavage where the lesions are present.
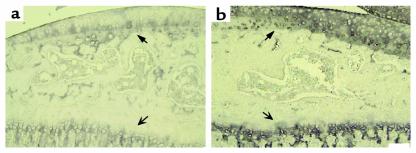
Type X collagen is not normally detected in healthy articular cartilage, but it is expressed in OA lesions in mice (33, 39) and human (40). Therefore its expression was investigated in the transgenic mice. In preliminary studies of both the wild-type and transgenic animals, type X collagen was observed in the hypertrophic zone but not in the resting zone of the growth plate (Figure 6, a and b, open arrows). The wild-type animals also stained for type X collagen in the calcified articular cartilage adjacent to subchondral bone and at the junction of the tidemark with the uncalcified articular cartilage (Figure 6a). Of three transgenic animals examined, all showed a marked increase in expression of type X throughout the matrix of the articular cartilage both below and above the tidemark, including the upper and middle zones, as well as the articular surface irrespective of joint damage (Figure 6b). In the menisci, another region containing type II collagen, an increase in type X collagen was detected in the transgenic mice but not in the wild-type animals (data not shown).
Figure 6.
Photomicrographs to show localization of type X collagen in the tibia articular cartilage and growth plate from sagittal sections of a wild-type (a) and heterozygous (b) line 6 mouse. The bold arrows represent the articular cartilage, whereas the open arrows indicate the growth plate. Analysis was performed on simultaneously stained sections taken from 3-month-old mice removed from Dox at 21 days. Scale bar = 100 μm.
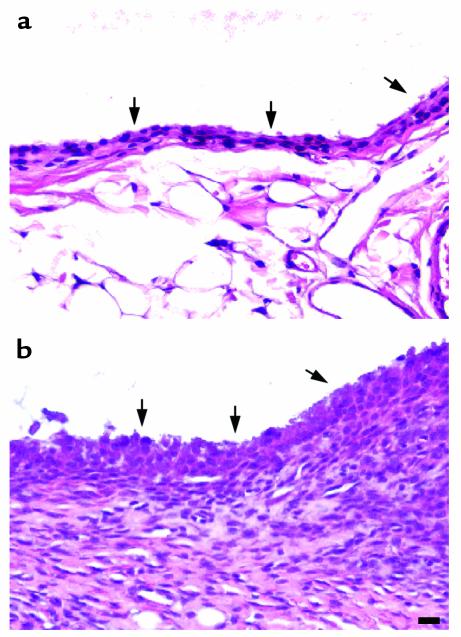
We also observed an effect of MMP-13 transgene expression on the synovium. In Figure 7a, the synovial lining layer from the wild-type animal appears healthy and normal. However, marked synovial proliferation and fibroblast hyperplasia, and some angiogenesis but no inflammatory cell infiltration, were observed in the synovia from some of the arthritic joints (Figure 7b; Table 1). The arthritis varied from moderate hyperplasia to severe as shown in Figure 7b and Table 1. In line 6, synovial proliferation was observed in three mice in which cartilage damage was pronounced. Yet it was also seen in two other mice in which no obvious cartilage changes were detectable. In line 99, all three mice showing proliferation exhibited cartilage pathology.
Figure 7.
Photomicrographs to show synovial hyperplasia in 5-month-old mice removed from Dox at weaning. Sagittal sections were cut to show the synovium from a wild-type (a) and a heterozygous (b) line 6 mouse stained with H&E. The arrows point to the synovial membrane. Scale bar = 30 μm.
A summary of the number of transgenic animals from line 6 showing the most prominent osteoarthritic articular cartilage pathology is shown in Table 1. A total of 11 of 17 mice expressing the transgene showed evidence of fibrillation and lesions in knee joints (Mankin grade ≥ 3.0) (Table 1). Six of these animals also exhibited synovial hyperplasia. No such pathology was observed in wild-type mice or in mice in which the MMP-13 transgene expression was suppressed by Dox.
In both line 6 (n = 17) and line 99 (n = 6), the arthritis when present in both knee joints (n = 3) was bilateral. Similar types of changes were observed in both lines, although there was a greater degree of penetrance in line 99 (five of six). Moreover, in both lines, damage to the articular cartilage was noted in all compartments; however, there was a tendency toward increased damage in the medial compartment of the tibia.
Discussion
This study reveals that postnatal expression of constitutively active human MMP-13 in the articular cartilage of transgenic mice leads to the degeneration of articular cartilage, in the manner observed in human OA. In lines 6 and 99, the penetrance of this phenotype was 60% and 83%, respectively. The variability could be due to differences in the expression levels of MMP-13* possibly resulting from (a) variable kinetics of activation of the type II collagen promoter; (b) differences in copy number of each transgene; (c) differences in the chromatin architecture of the transgene integration site (41); (d) differences in the degree of physical activity among the mice; and/or (e) differences in the levels of tissue inhibitor of metalloproteases (TIMP). In comparison to line 6, the enhanced incidence of pathology observed in line 99 maintained on Dox is consistent with higher levels of baseline MMP13* expression, which, in turn, is consistent with the higher copy number. However, in line 6, the pathology was only observed in transgenic animals removed from Dox. Although the induction of MMP-13* in line 6 represents only a modest fourfold increase in gene expression, it was sufficient to elicit OA symptoms over several months. Increased expression in utero during development would probably be lethal, as withdrawal of females from Dox before mating resulted in a 50% loss of transgenic embryos, presumably reflecting embryonic lethality induced by excessive MMP-13* expression. Interestingly, transgenic pups that survived such pregnancies were generally asymptomatic. This may have been the result of partial silencing of the transgene in these animals by some unknown mechanism, allowing survival to term. Moreover, excessive expression of the tTA is known to have lethal side effects (42). Attempts to generate homozygous line 6 mice, which would presumably express even higher levels of tTA than would heterozygotes, were unsuccessful. This lethality could be due to increased MMP-13* expression, although it was possible to derive homozygous line 99 animals, which, given that this line has a higher penetrance, might be expected to produce even higher MMP-13* levels.
One potential complication in using this approach comes from the fact that tetracycline and its analogs can act as inhibitors of MMPs and specifically of collagenases (43, 44). Dox has been shown to not only inhibit collagenase and gelatinase activity in vivo (45) but also to downregulate expression of MMP-1, MMP-8, and MMP-13 (12). This raises the possibility that treatment of transgenic mice with Dox might indirectly influence endogenous MMP gene expression. To address this question, we measured serum levels of Dox in animals receiving a 1.0 mg/ml dose of this drug in their drinking water, which is sufficient to repress the tetO7 promoter. Serum levels did not exceed 2.6 μM under these conditions, which is about five to tenfold lower than the concentration at which inhibition of MMPs has been observed. Treatment with Dox may, however, not only suppress MMP-13 via the activation/repression of the MMP-13* transgene but may also inhibit the activity of MMP-13* directly, thus providing the possibility of a second level of control. However, even if Dox does affect the activity of the MMP-13* transgene protein in a significant way, it does not alter the fact that we have a line of mice that develop OA in the months after removal of Dox from their drinking water.
Our immunohistochemical analysis provides evidence to indicating that the degenerative changes observed histologically are accompanied by molecular changes seen in OA in humans and animals. The excessive cleavage of type II collagen by collagenase seen in human and mouse OA (4, 15) in association with increased staining for MMP-13 seen in human disease (W. Wu et al., manuscript in preparation) is clearly observed in these mice containing the MMP-13* transgene. This was accompanied by a loss of staining with safranin O, reflecting loss of the proteoglycan aggrecan, which is also observed in OA cartilage in association with damage to type II collagen fibril about which the proteoglycan aggregate is organized. Thus, damage to collagen would be expected to lead to, and is associated with the loss of, aggrecan (3). Another feature of human and mouse OA is enhanced expression of type X collagen. In healthy tissue, during development, growth, and aging, this protein is synthesized in the growth plate during ossification, as well as in the calcified articular cartilage, whereas in human and mouse OA, it is strongly upregulated, in parallel with MMP-13 (34, 39, 40, 46). Affected MMP-13* transgenics exhibited increased staining for type X collagen extending throughout the articular cartilage. Thus, the degenerative changes we observed in articular cartilage are characteristic of these seen in OA in humans and mice, and induction of type X expression is linked to overexpression of MMP-13 and type II collagen cleavage as observed in the growth plate where these changes occur as part of development (47).
Synovial hyperplasia is also often associated with OA (48). Of the MMP-13* transgenics that developed OA, about half showed synovial proliferation and fibroblast hyperplasia. Joint histopathology characteristic of rheumatoid arthritis, including neutrophils, T-cells and macrophages, infiltration, and pannus formation (49) were not observed in the MMP-13* transgenic. The cause of this hyperplasia is unclear. It may be a result of a response to cartilage degradation, although there was no recognizable relationship between the degree of degeneration and synovial cell proliferation and synovial cell proliferation since, and it was observed in some but not all animals expressing the transgene and was seen in some animals in which cartilage damage was not apparent.
There was no evidence for the development of immunity to this transgene in that intra-articular expression was not associated with the infiltration of neutrophils, lymphocytes, and monocytes. The synovitis when observed represented a nonimmune cellular proliferation (these immune cells were absent), although there was evidence for angiogenesis. The reason for the lack of immunity to a human MMP-13 may result from the early postnatal expression of the transgene resulting in the induction of immune tolerance. It is well known that it is during this immediate postnatal period that tolerance can be induced for the life of the animal (50).
Degenerative joint pathologies were observed only when Dox was removed in the adolescent mouse (21 days); withdrawal of mice greater than 2 months of age from Dox treatment did not result in any noticeable phenotype during the period of observation (up to 5 months). This is probably due to the fact that the type II collagen gene is more strongly expressed during early growth, although it is upregulated in OA cartilage when synthesis of this molecule is upregulated (5). Therefore, given that damage is related to expression of the collagenase transgene under the control of the type II promoter, damage to articular cartilages is probably self-driven in the adult transgenic mouse with OA as a result of the induction of degeneration of articular cartilage that is ordinarily accompanied by upregulation of type II procollagen synthesis in OA (5).
The observation that overt lesions were often localized and in sites where meniscal coverage is absent also suggests that other factors may determine lesion development, such as compartmental differences in mechanical loading. Such focal lesions are observed in aging in human knees and exhibit features of early OA lesions, such as increased collagenase cleavage of type II collagen (G. Webb and A.R. Poole, manuscript in preparation). In aging wild-type mice, compartmental differences in lesion development are also observed, which are associated with increased cleavage of collagenase although they are more localized than we observed in the transgenic mice (15).
These studies provide an in vivo demonstration of the capacity of overexpression of active MMP-13 alone to induce degenerative changes of the kind observed in human OA. These human lesions are themselves closely associated with excess activity of collagenases (4, 8). This present study provides in vivo evidence in support of an important role for MMP-13 in causing the pathology of OA. However, whether the damage that is caused is all directly induced by MMP-13 or may involve a stimulation of the expression of endogenous MMP-13 and/or other MMPs remains to be established.
These MMP-13* transgenic mice should provide a valuable model for the study of OA and its treatment. Further studies are in progress with the model to investigate how rapidly OA develops and the degree of damage that causes an irreversible pathology.
Acknowledgments
We thank H. Bujard and M. Gossen for the tet-regulable plasmids, W. Horton for the rat type II collagen promoter and helpful discussions, C. Lopez-Otin for the human MMP-13 cDNA, and Gillian Murphy for recombinant human MMP-13. We also thank Isabelle Pidoux, Lisa Prior, Catherine Roth, Michele Sharr, Yijin She, Sheri Sturgis, and Paul Swinton, for their technical assistance and Paula Carabelli for her administrative assistance. Robin Poole was funded by Shriners Hospitals for Children, Medical Research Council of Canada, Canadian Arthritis Network, the NIH (AG13857), and Wyeth Ayerst.
Footnotes
The present address for Lisa A. Neuhold, John Kulik, Philip Babij, and Louis J. DeGennaro is: Molecular Genetics Division, Genetics Institute, Andover, Massachusetts, USA.
The present address for William Wu is: Massachusetts General Hospital, Arthritis Unit, Boston, Massachusetts, USA.The present address for C. Billinghurst is: Department of Clinical Sciences, Colorado State University, Ft. Collins, Colorado, USA.The present address for T. Meijers is: Canadian Arthritis Network, Mt. Sinai Hospital, Toronto, Ontario, Canada.
References
- 1.Poole, A.R., Alini, M., and Hollander, A. 1995. Cellular biology of cartilage degradation. In Mechanisms and models in rheumatoid arthritis. B. Henderson, R Pettifer, and J. Edwards, editors. Academic Press. New York, New York, USA. 163–204.
- 2.Hollander AP, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollander AP, et al. Increased damage to type II collagen in osteoarthritic cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billinghurst RC, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson F, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole AR, Ionescu M, Swan A, Dieppe PA. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis. J Clin Invest. 1994;94:25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell PG, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlberg L, et al. Collagenase-mediated cleavage of type II collagen is selectively enhanced in osteoarthritis cartilage and can be arrested with a synthetic inhibitor which spares collagenase-1 (MMP-1) Arthritis Rheum. 2000;43:673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Billinghurst RC, et al. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43:664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Knauper V, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 11.Shlopov BV, Smith GN, Jr, Cole AA, Hasty KA. Differential patterns of response to doxycycline and transforming growth factor beta 1 in the down-regulation of collagenase in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999;42:719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Shlopov BV, et al. Osteoarthritic lesions. Involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 13.Watson PJ, Hall LD, Malcolm A, Tyler JA. Degenerative joint disease in the guinea pig. Use of magnetic resonance imaging to monitor progression of bone pathology. Arthritis Rheum. 1996;39:1327–1337. doi: 10.1002/art.1780390810. [DOI] [PubMed] [Google Scholar]
- 14.Das-Gupta EP, Lyons TJ, Hoyland JA, Lawton DM, Freemont AJ. New histological observations in spontaneously developing osteoarthritis in the STR/ORT mouse questioning its acceptability as a model of human osteoarthritis. Int J Exp Pathol. 1993;74:627–634. [PMC free article] [PubMed] [Google Scholar]
- 15.Stoop R, et al. Type II collagen degradation in spontaneous osteoarthritis in C57Bl/6 and BALB/c mice. Arthritis Rheum. 1999;42:2381–2389. doi: 10.1002/1529-0131(199911)42:11<2381::AID-ANR17>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Huebner JL, Otterness IG, Freund EM, Caterson B, Kraus VB. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998;41:877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Ala-Kokka L, Baldwin CT, Moskowitz RW, Prockop DJ. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci USA. 1990;87:6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paassilta P, et al. Col 9A3: a third locus for multiple epiphyseal dysplasia. Am J Hum Genet. 1999;64:1036–1044. doi: 10.1086/302328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vikkula M, et al. Autosomal dominant and recessive osteochondrodysplasias associated with the COL 11A2 locus. Cell. 1995;80:431–437. doi: 10.1016/0092-8674(95)90493-x. [DOI] [PubMed] [Google Scholar]
- 20.Chin JR, Werb Z. Matrix metalloproteinases regulate morphogenesis, migration, and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development. 1997;124:1519–1530. doi: 10.1242/dev.124.8.1519. [DOI] [PubMed] [Google Scholar]
- 21.Jiminez MJG, et al. Collagenase-3 is a target for Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol. 1999;19:4431–4442. doi: 10.1128/mcb.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inada M, et al. Maturational disturbance of chondrocytes in Cbfa-1-deficient mice. Devel Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Porte D, et al. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene. 1999;19:667–678. doi: 10.1038/sj.onc.1202333. [DOI] [PubMed] [Google Scholar]
- 24.Selvamurugan N, Chou W-Y, Pearman AT, Pulmati MR, Partridge NC. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem. 1998;273:10647–10657. doi: 10.1074/jbc.273.17.10647. [DOI] [PubMed] [Google Scholar]
- 25.Kohno K, Sullivan M, Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985;260:4441–4447. [PubMed] [Google Scholar]
- 26.Horton W, Miyashita T, Kohno K, Hassell JR, Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci USA. 1987;84:8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freije JMP, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 29.Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- 30.Hogan, B., Constantini, F., and Lacy, E. 1996. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press. Plainview, New York, USA. 497 pp.
- 31.Schultze N, Burki Y, Lang Y, Certa U, Bluethmann H. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat Biotechnol. 1996;14:499–503. doi: 10.1038/nbt0496-499. [DOI] [PubMed] [Google Scholar]
- 32.Peter M, et al. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol. 1990;71:19–31. [PMC free article] [PubMed] [Google Scholar]
- 33.Mankin MJ, Dorfman H, Lippiello L, Zarius A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53A:523–537. [PubMed] [Google Scholar]
- 34.van der Kraan PM, Vitters EL, Meijers THM, Poole AR, van den Berg WB. Collagen type I antisense and collagen type IIA messenger RNA is expressed in adult murine articular cartilage. Osteoarthritis Cartilage. 1998;6:417–426. doi: 10.1053/joca.1998.0145. [DOI] [PubMed] [Google Scholar]
- 35.Furth PA, et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Lopez R, Nicholson R, Gesnel MC, Matrisian LM, Breathnach R. Structure-function relations in the collagenase family member transin. J Biol Chem. 1988;263:11892–11899. [PubMed] [Google Scholar]
- 37.Park AJ, et al. Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the “cysteine switch”. J Biol Chem. 1991;266:1584–1590. [PubMed] [Google Scholar]
- 38.Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eerola I, et al. Type X collagen, a natural component of mouse articular cartilage: association with growth, aging, and osteoarthritis. Arthritis Rheum. 1998;41:1287–1295. doi: 10.1002/1529-0131(199807)41:7<1287::AID-ART20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 40.von der Mark K, et al. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 41.Henikoff S. Conspiracy of silence among repeated transgenes. Bioessays. 1998;20:532–535. doi: 10.1002/(SICI)1521-1878(199807)20:7<532::AID-BIES3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenwald RA, et al. Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol. 1987;14:28–32. [PubMed] [Google Scholar]
- 44.Greenwald RA, et al. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone. 1998;22:33–38. doi: 10.1016/s8756-3282(97)00221-4. [DOI] [PubMed] [Google Scholar]
- 45.Yu LP, Jr, et al. Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis Rheum. 1992;35:1150–1159. doi: 10.1002/art.1780351007. [DOI] [PubMed] [Google Scholar]
- 46.Girkontaite I, et al. Immunolocalization of type II collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996;15:231–238. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 47.Mwale, F., et al. The assembly and degradation of types II and IX collagens associated with expression of the hypertrophic phenotype. Dev. Dyn. In press. [DOI] [PubMed]
- 48.Altman R, Gray R. Inflammation in osteoarthritis. Clin Rheum Dis. 1985;11:353–368. [PubMed] [Google Scholar]
- 49.Freemont, A.J. 1995. Histopathology of the rheumatoid joint. In Mechanisms and models in rheumatoid arthritis. B. Henderson, J.C.W. Edwards, and E.R. Pettipher, editors. Academic Press. London, United Kingdom. 83–113.
- 50.Qin Y, Sun D, Goto M, Meyermann R, Wekerle H. Resistance to experimental autoimmune encephalomyelitis by neonatal tolerization to myelin basic protein versus regulation of autoaggressive lymphocytes. Eur J Immunol. 1989;19:373–380. doi: 10.1002/eji.1830190223. [DOI] [PubMed] [Google Scholar]