Activation of the NF-κB/Rel transcription family, by nuclear translocation of cytoplasmic complexes, plays a central role in inflammation through its ability to induce transcription of proinflammatory genes (1). This pathway is activated upon appropriate cellular stimulation, most often by signals related to pathogens or stress. Here we will discuss the specificity of various NF-κB proteins, their role in inflammatory disease, the regulation of NF-κB activity by IκB proteins and IκB kinase (IKK), and the development of therapeutic strategies aimed at inhibition of NF-κB.
Functions of individual NF-κB proteins in immune cells
The NF-κB/Rel family includes NF-κB1 (p50/p105), NF-κB2 (p52/p100), p65 (RelA), RelB, and c-Rel (2). Most members of this family (RelB being one exception) can homodimerize, as well as form heterodimers with each other. The most prevalent activated form of NF-κB is a heterodimer consisting of a p50 or p52 subunit and p65, which contains transactivation domains necessary for gene induction.
Studies in knockout mice have shown distinct functions for different members of the NF-κB/Rel family. Various NF-κB proteins play a pivotal role in defense of the host against certain pathogens. Furthermore, lack of RelA leads to embryonic lethality and liver degeneration in knockout mice, whereas mice lacking p50 or RelB are immunodeficient but otherwise develop normally to adulthood. B cells from p50 knockout mice show abnormal mitogen responses and antibody production. RelB plays a role in the development and differentiation of dendritic cells, and a mutation disrupting relB impairs antigen presentation. Other NF-κB proteins, including C-Rel and p52, are also essential for normal immune function. Of interest, unlike either of the corresponding single knockout mice, p50/p52 double knockout mice exhibit impaired development of osteoclasts and B cells (3).
The expression of NF-κB proteins can provide site- and event-specificity in response to a particular stimulus. For instance, IL-1–induced collagenase expression in synoviocytes is primarily activated by p50 homodimers that bind to a critical NF-κB–like binding site (4). Both p50 and p65 play a role in constitutive IL-6 production in rheumatoid arthritis (RA) synovial fibroblasts (5), whereas p65 activation by thrombin regulates ICAM-1 expression in endothelial cells (6). p50 and p65 heterodimers are intimately involved in activation of inflammatory genes by IL-1 or TNF-α in human monocytes, and these effects are blocked by the anti-inflammatory cytokine IL-10 (7). Additional specificity is provided by the expression patterns of other regulatory molecules, including surface receptors to proinflammatory cytokines as well as the hierarchy of IKK and IκB isotypes in each cell lineage (see below). These differences, along with multiple variations in NF-κB–like binding sites that interact with certain NF-κB hetero- and homodimers, permit cells to respond to the external environment at the appropriate time with the correct genes.
Regulation of NF-κB activation by IκB and IKKs
NF-κB exists in the cytoplasm in an inactive form associated with regulatory proteins called inhibitors of κB (IκB), of which the most important may be IκBα, IκBβ, and IκBε. As discussed below, IκBα is associated with transient NF-κB activation, whereas IκBβ is involved in sustained activation. IκBα binds to the p50-p65 heterodimer and the p50 homodimer, although it does not inhibit DNA binding activity of the latter (8). IκBβ inhibits p50-p65 more strongly than it inhibits p50-RelB and p50-c-Rel complexes, whereas IκBα has similar effects on these complexes. In endothelial cells, IκBε is associated with p65 and to a lesser extent with c-Rel, whereas IκBα and IκBβ associate with p65, but not c-Rel (9). Hence, different IκB molecules have distinct and overlapping specificities. The tissue distribution may also differ for various IκBs. Since different IκB molecules might control the regulation of distinct genes in various tissues by inhibiting specific NF-κB subsets, IκB proteins could be attractive targets for specific therapies.
Phosphorylation of IκB, an important step in NF-κB activation, is mediated by IKK. The IKK complex consists of at least three subunits, including the kinases IKK-α and IKK-β (also called IKK-1 and IKK-2, respectively), (10) and the regulatory subunit IKK-γ (11). An inducible form of IKK, known as IKKi, was recently identified in endotoxin-stimulated immune cells (12). IKK activation initiates IκBα phosphorylation at specific NH2-terminal serine residues. Phosphorylated IκBα is then ubiquitinated, which targets it for degradation by the 26S proteasome (13), thereby releasing NF-κB dimers from the cytoplasmic NF-κB–IκB complex and allowing them to translocate to the nucleus. NF-κB then binds to κB enhancer elements of target genes, inducing transcription of proinflammatory genes. IKK also phosphorylates IκBβ and IκBε. Phosphorylation of IκBβ leads to prolonged NF-κB activation (14), which can in part be explained by the delay in IκBβ resynthesis after degradation. IκBε is degraded with relatively slow kinetics like IκBβ, but it may also be resynthesized at a rate comparable with that of IκBα.
In many cell types, IKK resides at a key convergence site for multiple signaling pathways that lead to NF-κB activation (15). IKK-β knockout mice develop liver failure due to hepatocyte apoptosis, especially in the presence of TNF-α (16). Although NF-κB blockade appears to be hepatotoxic during embryonic development, the use of an inducible IκBα super-repressor transgene suggests that NF-κB inhibition is well tolerated in adult mice (17). However, even in that model, NF-κB blockade compromises normal host defense and leaves mice unable to clear opportunistic infections with Listeria monocytogenes. IKK-α knockouts exhibit abnormal morphogenesis. IKK-α appears to be involved in keratinocyte differentiation and formation of the epidermis (18).
Recent data indicate that activation of IKK-β, rather than IKK-α, participates in the primary pathway by which proinflammatory stimuli induce NF-κB function (19). IKK-β is expressed in fibroblast-like synoviocytes and plays a central role in IL-1– and TNF-α–mediated NF-κB activation and expression of proinflammatory genes (15). Similarly, IKK-β specifically regulates NF-κB activation and inflammatory gene transcription in human monocytes and in human CD4+ T lymphocytes. However, it remains to be determined whether IKK-β has a pivotal role in NF-κB activation in all cell types. Upstream of IKK, kinases such as NF-κB–inducing kinase (NIK) and MEKK1 (both members of the mitogen-activated protein kinase kinase kinase [MAPKKK] family) and an IKK-related kinase named NAK (NF-κB–activating kinase) (20) can activate IKK in response to pro-inflammatory stimuli.
Role of the NF-κB pathway in immune responses and inflammation
Studies in transgenic mice whose T cells lack the NF-κB/Rel signaling pathway show that NF-κB plays a role in Th1-dependent delayed-type hypersensitivity responses (21). NF-κB activation increases expression of the adhesion molecules E-selectin, VCAM-1, and ICAM-1, while NF-κB inhibition reduces leukocyte adhesion and transmigration (22). NF-κB activation is also involved in apoptosis, although its role is not always straightforward. For instance, NF-κB activation may lead to induction of apoptosis in some cell types (2), perhaps because it, along with activator protein 1 (AP-1), can induce FasL expression (23). In other lineages like hepatocytes and synovial cells, NF-κB plays an antiapoptotic role (24, 25). Inhibition of NF-κB–induced caspase-8 activation (25) might be one of the mechanisms by which targeting NF-κB may lead to protection against apoptosis. This protective function may be regulated by Akt, which could contribute to the ability of PDGF to serve as a cell survival signal (26).
NF-κB is clearly one of the most important regulators of proinflammatory gene expression. Synthesis of cytokines, such as TNF-α, IL-1β, IL-6, and IL-8, is mediated by NF-κB, as is the expression of cyclooxygenase 2 (Cox-2). Aupperle et al. (15) recently studied the consequences of IKK in primary fibroblast-like synoviocytes isolated from synovium of patients with RA and osteoarthritis. In both groups, immunoreactive IKK protein is abundant in these cells, and IKK-α and IKK-β are constitutively expressed at the mRNA level. IKK function in these cells can be greatly enhanced by TNF-α and IL-1, leading to degradation of endogenous IκBα and nuclear translocation of NF-κB. Activation of this pathway and the consequent induction of IL-6, IL-8, ICAM-1, and collagenase-1 expression depends specifically on IKK-β (15). Thus, transfection with adenoviral constructs encoding an IKK-β dominant-negative mutant prevents TNF-α–mediated NF-κB nuclear translocation and proinflammatory gene expression in synoviocytes, whereas dominant-negative IKK-α mutant has no effect (15).
NF-κB is also able to function in concert with other transcription factors, such as AP-1. For instance, NF-κB translocation can enhance transcription of the collagenase-3 (MMP13) gene in IL-1–stimulated synoviocytes (27). Full expression of collagenase gene transcription, however, depends on the participation of AP-1, whose transcriptional induction follows a distinct pathway involving phosphorylation of the MAPK c-Jun NH2-terminal kinase (JNK), with subsequent phosphorylation of c-Jun. Therefore, the signal transduction cascade after cytokine stimulation of synoviocytes results in the activation of parallel kinase cascades regulating AP-1 and NF-κB. This dual pathway enhances production of some proinflammatory cytokines (like TNF-α), and, more strikingly, increases expression of the destructive enzymes that regulate matrix remodeling (28). Hence, AP-1 and NF-κB are simultaneously activated in IL-1 or TNF-α–stimulated synoviocytes as well as the intimal synovial lining of RA patients (29), which is the site in vivo where matrix metalloproteinases (MMPs) are produced. Coordinate stimulation of NF-κB and AP-1 can thereby contribute to bone and cartilage destruction in the joint.
NF-κB activation in animal models of inflammation
Animal models of inflammatory arthritis support the notion that NF-κB activation plays a pathogenic role in vivo. For instance, increased synovial NF-κB binding precedes the development of clinical joint involvement in murine collagen-induced arthritis (CIA) and gradually increases during the evolution of disease (29) (Figure 1). Much of this binding activity appears to be due to p50, which has been implicated in collagenase-3 transcription and could contribute, along with locally activated AP-1, to extracellular matrix resorption. Synovial NF-κB activation also occurs within a few days after immunization in rat adjuvant arthritis (30). Selective activation of NF-κB in normal rats by intra-articular transfer of a functional IKK-β gene leads to synovial inflammation and clinical signs of arthritis (31). Conversely, reduction of NF-κB nuclear translocation and clinical synovitis was observed in adjuvant arthritis in rats after an intra-articular injection with a dominant-negative adenoviral IKK-β construct (31). The central role of NF-κB in inflammation has also been shown in rats with streptococcal cell wall–induced arthritis (24) and in mice with CIA (29, 32).
Figure 1.
Activation of NF-κB in murine CIA. Mice were immunized with type II collagen on days 0 and 20 and boosted with lipopolysaccharide on day 28. Electromobility shift assays were performed on nuclear extracts of ankle joints at various time points during the evolution of disease. The right side of the gel shows NF-κB binding to labeled consensus NF-κB oligonucleotide, while the left side shows lack of binding to a mutant NF-κB oligonucleotide. Note that NF-κB binding activity was observed as early as 10 days after immunization, even though clinical arthritis was not observed until approximately day 30. Con, positive control.
NF-κB has also been implicated in several other tissue-specific models of inflammation. NF-κB activation induces production of proinflammatory cytokines in nephritic glomeruli, resulting in experimental glomerulonephritis in rats (33). IL-2 knockout mice develop an inflammatory colitis that is marked by increased IL-1 expression (34). These changes are accompanied by enhanced NF-κB activation in colonic epithelium, although it is not certain whether the increases in proinflammatory cytokine production are the cause or result of NF-κB binding. In allergen-induced asthma, loss of the p50 locus in knockout mice ablates the eosinophilic airway response (35). These animals produce reduced levels of the Th2 cytokine IL-5, along with MIP-1α and MIP-1β, which are implicated in the recruitment of mononuclear cells to the site of inflammation. Similarly, c-Rel knockout mice have decreased airway hyperresponsiveness and eosinophil infiltration as well as lower levels of serum IgE in an allergen-induced asthma model (36). These observations support the notion that NF-κB plays an important role in many different inflammatory disorders.
NF-κB activation in human inflammatory diseases
NF-κB is highly activated at sites of inflammation in diverse diseases (Table 1) and can induce transcription of proinflammatory cytokines, chemokines, adhesion molecules, MMPs, Cox-2, and inducible nitric oxide (iNOS). In RA, for example, NF-κB is overexpressed in the inflamed synovium (29), where its activity may enhance recruitment of inflammatory cells and production of proinflammatory mediators like IL-1, IL-6, IL-8, and TNF-α. Both p50 and p65 have been localized to nuclei in synovial lining cells as well as mononuclear cells in the sublining regions. Electrophoretic mobility shift assays demonstrate that NF-κB binding to DNA is much greater in RA compared with osteoarthritis controls, consistent with increased proinflammatory cytokine production in the former (29).
Table 1.
NF-κB–activation inflammatory disease
Inflammatory airway disease in humans has also been associated with cytokine and adhesion molecule expression. This correlates with activation of NF-κB in bronchial biopsies from asthma patients (37). Increased NF-κB activity with nuclear localization was observed especially in airway epithelial cells, where there is abundant expression of proinflammatory cytokines, chemokines, iNOS, and Cox-2.
Helicobacter pylori–associated gastritis is also marked by increased NF-κB activity in gastric epithelial cells (38). The number of NF-κB positive cells correlates with the degree of gastritis. Similarly, there is evidence of NF-κB activation in inflammatory bowel disease, where lamina propria macrophages display activated p50, c-Rel, and especially p65. In vitro treatment of these cells with antisense p65 oligonucleotides reduced proinflammatory cytokine production (39). Neurological disease (see article by Mattson and Camandola, this series, ref. 40) and inflammation associated with atherosclerosis (see article by Collins and Cybulsky, this series, ref. 41) are also mediated, in part, by NF-κB.
NF-κB–directed therapies in animal models and in human disease
The identification of NF-κB as a key player in the pathogenesis of inflammation suggests that NF-κB–targeted therapeutics might be effective in diseases like RA. This view is supported by the effects of various therapeutic strategies aimed at blocking NF-κB activity. As noted above, inhibition of IKK-β activity markedly ameliorates adjuvant-induced arthritis (31). NF-κB blockade with NF-κB decoy oligonucleotides, either by direct injection or by viral gene transfer, inhibits the development of streptococcal cell wall–induced arthritis and CIA in rats (24, 42), perhaps by increasing apoptosis and suppressing cytokine gene expression. Inhibition of NF-κB in T cells in transgenic mice overexpressing IκBα resulted in decreased incidence and severity of CIA (43). In agreement with these observations, treatment with a T-cell specific NF-κB inhibitor significantly decreased arthritis severity in CIA in mice (32). NF-κB–directed therapy is also effective in a model of inflammatory bowel disease induced by 2, 4, 6,-trinitrobenzene sulfonic acid. Local application of p65 antisense oligonucleotides blocks both clinical and histologic evidence of disease activity (44). Thus, the feasibility and efficacy of specific inhibition of NF-κB activity has been shown in several animal models of inflammatory disease.
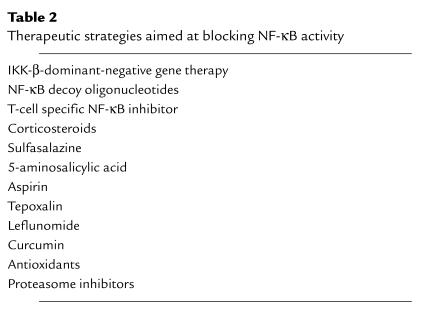
A variety of drugs used to treat human inflammatory disease have effects on NF-κB activity (Table 2; see article by Yamamoto and Gaynor, this series, ref. 45). For example, some of the effects of corticosteroids, used in the treatment of inflammatory bowel disease, asthma, psoriasis, and RA, are probably mediated through the inhibition of NF-κB activation. Other examples of small molecules include sulfasalazine and its salicylate moiety 5-aminosalicylic acid, aspirin, and other nonsteroidal anti-inflammatory drugs, and leflunomide. Sulfasalazine and leflunomide block nuclear translocation of NF-κB through inhibition of IκBα degradation. This might be caused by a direct effect on IKK or upstream signals. Aspirin appears to function as a competitive inhibitor of IKK-β. These drugs are, however, not specific, and require relatively high concentrations to achieve effective NF-κB inhibition. Agents that block proximal cytokines, such as IL-1 and TNF-α, also limit NF-κB activation and inhibit the inflammatory cascade. Blocking individual cytoplasmic components of the IKK activation pathway, including TRADD, RIP, TRAF-2, and TRAF-6, might in part have similar effects, although this remains to be shown in animal models and human disease. Moreover, these molecules may not be essential for NF-κB activation.
Table 2.
Therapeutic strategies aimed at blocking NF-κB activity
IKK-β is essential for TNF-α– and IL-1–induced NF-κB activation and therefore represents a potential target for strategies aimed at inhibiting proinflammatory gene expression for the treatment of a variety of inflammatory disorders, especially RA (46). It is conceivable that blockade of specific subsets of the NF-κB/Rel family could be used to inhibit inflammatory disease. The ability to identify individual components as key to a particular disease will be essential to developing specific therapeutics (like IKK-β in RA synoviocytes). In inflammatory bowel disease, for instance, the apparent importance of p65 suggests that therapeutic agents directed at this protein might be useful.
Of some concern is the potential for toxicity since NF-κB blockade could result in liver apoptosis, especially in the presence of TNF-α. This issue is clearly relevant in RA where TNF-α overproduction is a hallmark of the disease. The ultimate benefit of such targeted therapy will depend on the delicate balance between suppressing inflammation and interfering with normal cellular functions. By selectively targeting specific NF-κB subunits, IκB proteins, or kinases that have a degree of tissue specificity, one might attain therapeutic efficacy and minimize systemic toxicity.
References
- 1.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 3.Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Miyazawa K, Mori A, Yamamoto K, Okudaira H. Constitutive transcription of the human interleukin-6 gene by rheumatoid synoviocytes: spontaneous activation of NF-kappa B and CBF1. Am J Pathol. 1998;152:793–803. [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman A, Anwar KN, True AL, Malik AB. Thrombin-induced p65 homodimer binding to downstream NF-kappa B site of the promoter mediates endothelial ICAM-1 expression and neutrophil adhesion. J Immunol. 1999;162:5466–5476. [PubMed] [Google Scholar]
- 7.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Nabel GJ. A new member of the I kappaB protein family, I kappaB epsilon, inhibits RelA (p65)-mediated NF-kappaB transcription. Mol Cell Biol. 1997;17:6184–6190. doi: 10.1128/mcb.17.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiecker M, Darius H, Liao JK. A functional role of I kappa B-epsilon in endothelial cell activation. J Immunol. 2000;164:3316–3322. doi: 10.4049/jimmunol.164.6.3316. [DOI] [PubMed] [Google Scholar]
- 10.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 15.Aupperle KR, et al. NF-kB regulation by IkB kinase in primary fibroblast-like synoviocytes. J Immunol. 1999;163:427–433. [PubMed] [Google Scholar]
- 16.Li ZW, et al. The IKKbeta subunit of IkappaB Kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavon I, et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat Med. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 19.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 20.Tojima Y, et al. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 21.Aronica MA, et al. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116–5124. [PubMed] [Google Scholar]
- 22.Chen CC, Rosenbloom CL, Anderson DC, Manning AM. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of I kappa B-alpha phosphorylation. J Immunol. 1995;155:3538–3545. [PubMed] [Google Scholar]
- 23.Kasibhatla S, et al. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 24.Miagkov AV, et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 26.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 27.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 29.Han ZN, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kappa B regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- 30.Tsao PW, et al. The effect of dexamethasone on the expression of activated NF-kappa B in adjuvant arthritis. Clin Immunol Immunopathol. 1997;83:173–178. doi: 10.1006/clin.1997.4333. [DOI] [PubMed] [Google Scholar]
- 31.Tak PP, et al. IkB kinase-2 (IKK-2) is a key regulator of synovial inflammation. Arthritis Rheum. 1999;42:S400. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 32.Gerlag DM, et al. Effect of a T cell specific NF-kB inhibitor on in vitro cytokine production and collagen-induced arthritis. J Immunol. 2000;165:1652–1658. doi: 10.4049/jimmunol.165.3.1652. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai H, et al. Activation of transcription factor NF-kappa B in experimental glomerulonephritis in rats. Biochim Biophys Acta. 1996;1316:132–138. doi: 10.1016/0925-4439(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, de Villiers WJ, Lee EY, McClain CJ, Varilek GW. Increased nuclear factor-kappaB activation in colitis of interleukin-2-deficient mice. J Lab Clin Med. 1999;134:378–385. doi: 10.1016/s0022-2143(99)90152-x. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, et al. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan CE, et al. NF-kappa B/Rel transcription factors: c-Rel promotes airway hyperresponsiveness and allergic pulmonary inflammation. J Immunol. 1999;163:6827–6833. [PubMed] [Google Scholar]
- 37.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 38.van Den Brink GR, et al. Expression and activation of NF-kappa B in the antrum of the human stomach. J Immunol. 2000;164:3353–3359. doi: 10.4049/jimmunol.164.6.3353. [DOI] [PubMed] [Google Scholar]
- 39.Neurath MF, et al. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann NY Acad Sci. 1998;859:149–159. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattson, M.P., and Camandola, S. 2001. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 41.Collins, T., and Cybulsky, M.I. 2001. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 42.Tomita T, et al. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappa B decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999;42:2532–2542. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999;163:1577–1583. [PubMed] [Google Scholar]
- 44.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, Y., and Gaynor, R.B. 2001. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 46.Firestein GS, Manning AM. Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum. 1999;42:609–621. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]