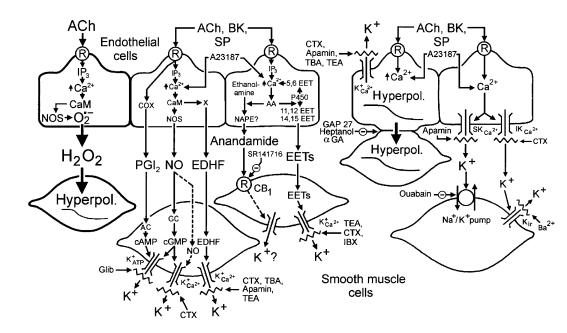
In a recent issue of the JCI, Matoba et al. (1) argue that hydrogen peroxide (H2O2) derived from endothelial nitric oxide synthase (eNOS) acts as an endothelium-derived hyperpolarizing factor (EDHF) in small mesenteric arteries of the mouse. Their data are convincing, and if their conclusion is correct, H2O2 now joins nitric oxide (NO) (2, 3), superoxide anions (4), and potassium ions (5) on the list of small inorganic molecules that carry regulatory messages from the endothelium to vascular smooth muscle cells and so contribute to intercellular signaling in the vascular wall. The effect of H2O2 adds another layer to our already complex description of endothelium-dependent hyperpolarization (Figure 1; see refs. 6–8).
Figure 1.
Multiplicity of mechanisms leading to endothelium-dependent hyperpolarization. The mechanism proposed by Matoba et al. (1) is highlighted. Substances such as acetylcholine (ACh), bradykinin (BK), and substance P (SP), through the activation of M3-muscarinic, B2-bradykinin, and NK1-neurokinin receptor subtypes, respectively, and agents that increase intracellular calcium, such as the calcium ionophore A23187, release endothelium-derived hyperpolarizing factors. CaM, calmodulin; COX, cyclooxygenase; EET, epoxyeicosatrienoic acid; IP3, inositol trisphosphate; GC, guanylate cyclase; NAPE, N-acylphosphatidylethanolamine; Hyperpol., hyperpolarization; NOS, NO synthase; O2•–, superoxide anions; PGI2, prostacyclin; P450, cytochrome P450 monooxygenase; R, receptor; X, putative EDHF synthase. SR141716 is an antagonist of the cannabinoid CB1 receptor subtype (CB1). Glibenclamide (Glib) is a selective inhibitor of ATP-sensitive potassium channels (K+ATP). Tetraethylammonium (TEA) and tetrabutylammonium (TBA) are nonspecific inhibitors of potassium channels when used at high concentrations (> 5 mM), while at lower concentrations (1–3 mM) these drugs are selective for calcium-activated potassium channels (K+Ca2+). Iberiotoxin (IBX) is a specific inhibitor of large conductance K+Ca2+. Charybdotoxin (CTX) is an inhibitor of large conductance K+Ca2+, intermediate conductance K+Ca2+ (IKCa2+), and voltage-dependent potassium channels. Apamin is a specific inhibitor of small conductance K+Ca2+ (SKCa2+). Barium (Ba2+), in the micromolar range, is a specific inhibitor of the inward rectifier potassium channel (Kir). GAP 27 (an eleven–amino acid peptide possessing conserved sequence homology to a portion of the second extracellular loop of connexin), 18α-glycyrrhetinic acid (αGA), and heptanol are gap junction uncouplers. Adapted from ref. 7.
H2O2 and vascular relaxation
Matoba et al. (1) conclude that eNOS generates superoxide anions, which are converted by superoxide dismutase (SOD) to H2O2. This mediator then acts on ion channels on the vascular smooth muscle, increasing K+ conductance and causing hyperpolarization and relaxation of the vascular smooth muscle (9–11). Early evidence that H2O2 can participate in endothelium-dependent relaxation came from work with canine coronary arteries, where the generation of superoxide anions leads to catalase-sensitive vascular relaxation. Because this response, as observed using arterial rings maintained ex vivo, is more evident in rings with than in those without endothelial cells (12), these experiments suggested that H2O2 facilitates endothelium-dependent relaxation. The present findings imply that this facilitation was due to transformation in the endothelial cells of superoxide anions into H2O2. Hence, the study by Matoba et al. (1) now helps to establish H2O2’s causal role in this response. One exciting conclusion of this study is that H2O2 is generated by the action of the eNOS. The proposal that eNOS can generate oxygen-derived free radicals is not unprecedented (13–15), but the notion that this happens physiologically, in normal mouse arteries exposed to acetylcholine, is novel. These conditions differ dramatically from those in which NO synthase has been observed to produce oxygen-derived free radicals, namely the absence of normal substrates or cofactors (13, 14).
H2O2 clearly differs from all other putative mediators of endothelium-dependent hyperpolarization. At least in the small mesenteric arteries studied by Matoba et al. (1), myoendothelial gap junctions are not required as a conduit for H2O2’s EDHF activity. Moreover, its activity can be abolished by using a suitable combination of K+ channel blockers, a hallmark of EDHF-mediated responses (5–8). By these criteria, H2O2 appears to be an EDHF in its own right.
The field of EDHF research is mired with species differences and with functional differences between small and large blood vessels (see refs. 5–8). For this reason, it is not clear whether the present findings, made in small arteries of the mouse, can be extended to the small and large blood vessels of other species. For example, in coronary arteries of the dog, catalase, which abolishes EDHF-mediated response in the experiments of Matoba et al. (1), has minimal, if any, effects on the response to acetylcholine (12). This is a most important issue, as it may well be that in the vascular periphery the effect of EDHFs on vascular tone overwhelms that of endothelium-derived NO, thus controlling peripheral resistance and arterial blood pressure. If so, the cardiovascular scientific community, which took fifteen years to accept that NO participates in endothelium-dependent regulation of vascular tone, may have to adjust itself to the idea that this gaseous mediator is not the only such endothelium-derived regulator.
Reactive oxygen species reconsidered
This report also reopens the broader question of the regulatory role of oxygen-derived free radicals in general, particularly in endothelium-dependent responses. So far, the perception has been that those radicals, in particular superoxide anions, contribute mainly to destructive processes. This is attributed to the scavenging effect of superoxide anions on endothelium-derived NO (16), which leads to the production of the ill-reputed peroxynitrite. In addition, both superoxide anions and H2O2 can be transformed into hydroxyl radicals, which can catalyze the production of vasoconstrictor prostanoids. These mediators act directly on vascular smooth muscle cells to induce contraction (17). While this activity is thought of as pathological, superoxide anions in large cerebral arteries and certain other blood vessels may play a valuable role in the response to stretch to allow for vascular autoregulation (4, 18).
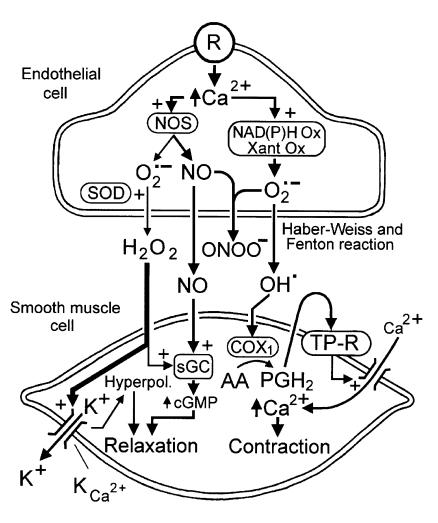
The identification of H2O2 as an EDHF provides a satisfying explanation of the negative feedback (see, for example, ref. 19) exerted by NO on endothelium-dependent relaxation, at least in small mesenteric arteries of the mouse (1). Since NO and superoxide anions (the precursor of H2O2) react spontaneously (16), these two products of eNOS would be expected to suppress each other’s effects — although the product of their interaction, peroxynitrite, is itself vasoactive. The physiological effects of NO and superoxide anions may depend crucially on their local concentrations and their intracellular site of formation. In order to accumulate as H2O2, endothelial superoxide anions must first escape interacting with NO molecules; under normal conditions, at least in some blood vessels, it appears that a small amount of superoxide anions survives for long enough to be acted on by SOD and generate H2O2. However, in other healthy vessels, such as the large cerebral arteries (4, 18), and under pathological situations in many blood vessels, the production of superoxide anions (largely from sources other than eNOS) may overwhelm the production of NO. Under these conditions, H2O2 production will be limited only by the availability of SOD, and endothelium-derived peroxynitrite, prostanoids, and possibly isoprostanes may tip the balance toward vasoconstriction (Figure 2). In addition, massive scavenging of NO will unleash the production and permit the action of endothelin-1, accelerating the evolution toward pathology (20).
Figure 2.
Proposed interactions between NO and superoxide anions (O2•–) in the regulation of endothelium-dependent responses. NO synthase (NOS) produces both NO and superoxide anions. Under normal circumstances, and in most arteries, the production of NO predominates, and NO scavenges the small amounts of superoxide anion formed. In the small mesenteric artery of the mice, the superoxide anions that escape the scavenging by NO are transformed by SOD to H2O2, which diffuses to the vascular smooth muscle and causes its hyperpolarization (Hyperpol.) by opening of a K+ conductance (KCa2+). NO activates soluble guanylate cyclase (sGC) to produce more cGMP. In arteries such as the canine cerebral arteries, or the aortas of hypertensive or diabetic animals, other sources of production of superoxide anions (e.g., NAD(P)H oxidase [NAD(P)H Ox]) or xanthine oxidase (Xant Ox) are activated when the intracellular Ca2+ concentration increases. The large quantities of superoxide anions formed scavenge most or all of the NO, leading to the production of peroxynitrite (ONOO–). In addition, superoxide anions can be transformed to hydroxyl radicals, which diffuse to the vascular smooth muscle and induce the production of vasoconstrictor endoperoxides (PGH2) and prostanoids (and possibly isoprostanes). The latter activate TP receptors (TP-R) that are coupled positively to the contractile process.
Explaining physiological differences among blood vessels
The multiplicity of pathways defined so far to explain endothelium-dependent hyperpolarization (Figure 1) implies a delicate equilibrium between several signaling molecules produced by the endothelial cells. The importance of the various mediators that influence this vascular response may vary across blood vessels of different species, anatomical origin, or size, and may depend on their differential synthesis or secretion by endothelial cells. Such an explanation focuses on putative heterogeneity among endothelial cells in these different settings, but it is equally plausible that endothelial cells are generally similar in their secretion of vasoactive factors in response to increased intracellular Ca2+ concentration (Figure 1) and that the explanation for heterogeneous vascular physiology lies elsewhere. H2O2 and the other vasoactive factors may take on greater or lesser importance in different settings depending on their diffusion properties, their stability in the local environment, and the sensitivity of the vascular smooth muscle to their effects. Differences in responsiveness of vascular smooth muscle to these mediators may be determined by its genetic background and its chronic local environment. A strong hint indicating the importance of vascular smooth muscle comes from the long-standing observation that exogenous NO, given to cardiac patients in the form of nitrates, relaxes large coronary arteries, but not small coronary arteries in which EDHF-mediated responses are prominent. For the patient, this differential response offers the advantage that nitrates, unlike some other dilators, do not divert blood flow from the healthy myocardium. For the endothelial biologist, fascinated by the quest for the identity of EDHF, it prompts the humbling thought that it is the smooth muscle, not the endothelial cell, that determines which endothelium-derived factors are important in a given blood vessel.
References
- 1.Matoba T, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2017. [PubMed] [Google Scholar]
- 3.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- 5.Edwards G, Dora KA, Gardener MJ, Garland CJ. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoutte, P.M., editor. 1998. Endothelium-derived hyperpolarizing factor. Harwood Academic Publishers. Amsterdam, The Netherlands. 335 pp.
- 7.Vanhoutte, P.M., editor. 1999. Endothelium-dependent hyperpolarizations. Harwood Academic Publishers. Amsterdam, The Netherlands. 433 pp.
- 8.Vanhoutte, P.M., editor. EDHF 2000. Harwood Academic Publishers. Amsterdam, The Netherlands. In press.
- 9.Beny JL, von der Weid PY. Hydrogen peroxide: an endogenous smooth muscle cell hyperpolarizing factor. Biochem Biophys Res Commun. 1991;176:378–384. doi: 10.1016/0006-291x(91)90935-z. [DOI] [PubMed] [Google Scholar]
- 10.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- 11.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 12.Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium and responsiveness of vascular smooth muscle. Am J Physiol. 1986;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- 13.Stroes E, et al. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez-Vivar J, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaesemeyer WH, Ogonowski AA, Jin L, Caldwell RB, Caldwell RW. Endothelial nitric oxide synthase is a site of superoxide synthesis in endothelial cells treated with glyceryl trinitrate. Br J Pharmacol. 2000;131:1019–1023. doi: 10.1038/sj.bjp.0703665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor(s) Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 17.Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- 18.Katusic Z, Shepherd JT, Vanhoutte PM. Endothelium-dependent contraction to stretch in canine basilar arteries. Am J Physiol. 1987;21:H671–H673. doi: 10.1152/ajpheart.1987.252.3.H671. [DOI] [PubMed] [Google Scholar]
- 19.Olmos L, Mombouli JV, Illiano S, Vanhoutte PM. cGMP mediates the desensitization to bradykinin in isolated canine coronary arteries. Am J Physiol. 1995;268:H865–H870. doi: 10.1152/ajpheart.1995.268.2.H865. [DOI] [PubMed] [Google Scholar]
- 20.Vanhoutte PM. Say NO to ET. J Auton Nerv Syst. 2000;81:271–277. doi: 10.1016/s0165-1838(00)00126-0. [DOI] [PubMed] [Google Scholar]