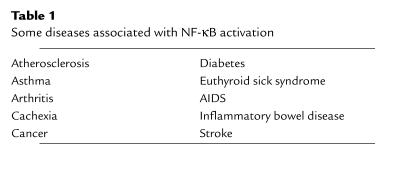
Beginning with its discovery in 1986 and continuing through the present, the transcription factor NF-κB has attracted widespread interest based on its unusual regulation, the variety of stimuli that activate it, the diverse genes and biological responses that it controls, the striking evolutionary conservation of structure and function among family members, and its apparent involvement in a variety of human diseases (Table 1). Importantly, and consistent with the last point, NF-κB has been shown to be the target of several anti-inflammatory and anticancer drugs.
Table 1.
Some diseases associated with NF-κB activation
Discovered by David Baltimore’s group (1), NF-κB was shown to be ubiquitously expressed and regulated in an unusual manner in that it could be activated by phorbol esters in the presence of protein synthesis inhibitors. The basis for this type of regulation ultimately was shown to involve the interaction of NF-κB with an inhibitor protein known as IκB (reviewed in refs. 2, 3). Cloning of the NF-κB subunits has revealed a family of proteins exhibiting a conserved central region known as the Rel homology domain, which is involved in DNA binding, interactions with IκB molecules, and dimerization. There are presently five members of the immediate NF-κB family in mammals: p50/p105, p65/RelA, c-Rel, RelB, and p52/p100. The p50 and p52 proteins are derived from precursor proteins or by cotranslational mechanisms from the appropriate mRNA. Although many dimeric forms of NF-κB have been detected, the classic form of NF-κB is the heterodimer of the p65/RelA and p50 subunits. The cloning of the first form of IκBα was facilitated by the observed homology between it and the COOH-terminal region of the p105 of NF-κB (2). Other forms of IκB have been identified, including IκBβ and IκBε (3). IκBβ interacts with similar NF-κB subunits, but its degradation is specifically associated with persistent NF-κB activation (3). An interesting member of the IκB family is Bcl-3, which functions through interactions with certain NF-κB subunits to promote transcription (2, 3). Three proteins related to NF-κB have been identified in Drosophila. These are Dorsal, Dif, and Relish, which are involved in expression of genes controlling dorso-ventral patterning in development and the fly immune response. Drosophila has an IκB homologue known as Cactus, whose interactions with Dorsal are under control of Toll, a homologue of the IL-1 receptor (2, 3).
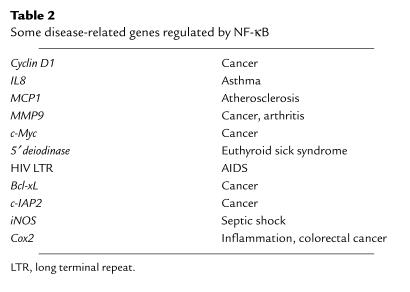
NF-κB can be activated within minutes by a variety of stimuli, including inflammatory cytokines such as TNF-α and IL-1, T-cell activation signals, growth factors, and stress inducers. The activation of NF-κB is normally associated with induction of phosphorylation of IκB, followed by its degradation by the proteasome and nuclear translocation (2, 3). Recently, a kinase complex know as the IκB kinase (IKK) has been identified that, when activated, phosphorylates IκBα on serines 32 and 36 (reviewed in ref. 4). Additionally, NF-κB regulation involves phosphorylation of NF-κB subunits, promoting transcriptional activity (see ref. 3). In the nucleus, NF-κB binds to target DNA elements and positively regulates the transcription of genes involved in immune and inflammatory responses, cell growth control, and apoptosis. Genes encoding cytokines, cytokine receptors, cell adhesion molecules, chemoattractant proteins, and growth regulators are positively regulated by NF-κB (Table 2). Genes regulated by NF-κB include those encoding IL-2, IL-6, IL-8, the IL-2 receptor, the IL-12 p40 subunit, VCAM-1, ICAM-1, TNF-α, IFN-γ, and c-Myc (2, 3). Consistent with the regulation of genes involved in the immune and inflammatory response, mice null for several of the NF-κB subunits show defects in clearing bacterial infection along with defects in B- and T-cell functions (see ref. 3). Surprisingly, the knockout of the p65/RelA subunit dies at day 16 of development from extensive liver apoptosis, revealing a role for NF-κB in controlling cell death (5). One can also assume that there is redundancy in the NF-κB system, since the combined p50 and p52 knockout showed osteopetrosis with a block in osteoclast differentiation, whereas the individual p50 or p52 knockouts show no such defect (6, 7).
Table 2.
Some disease-related genes regulated by NF-κB
The ability of NF-κB to be activated by inflammatory cytokines such as TNF-α and to regulate genes involved in inflammatory function raised the question of whether NF-κB dysregulation would be associated with inflammatory disease. Indeed, as discussed by Tak and Firestein (this Perspective series), NF-κB is activated in the inflamed synovium of rheumatoid arthritis patients (8) as well as in the synovium of animal models of this disease (9). Interestingly, inhibition of NF-κB inhibited the inflammation in a bacterial cell wall model for arthritis (9). The fact that NF-κB regulates TNF-α expression and is a key effector of this cytokine is consistent with the development of therapies aimed at blocking TNF as a therapy for rheumatoid arthritis. NF-κB activation is assumed to lie at the heart of other inflammatory diseases, such as asthma (1), and has been shown to be required for development of inflammatory bowel disease in an animal model (10). Thus, the ability of NF-κB to activate transcription of genes encoding cell adhesion molecules (ICAM-1, VCAM-1, E-selectin) and chemoattractant proteins (monocyte chemoattractant protein-1 [MCP-1]) would lead to the recruitment of inflammatory cells to the lung, a hallmark of asthma (11). Additionally, NF-κB appears to be an effector, downstream of TNF-α, in euthyroid sick syndrome (12) and in cachexia (13). The Perspective by Tak and Firestein in this series focuses on the role of NF-κB in inflammatory disorders, whereas the Perspective by Zhang and Ghosh focuses on the key role of NF-κB in promoting innate immune responses. These two articles clearly illustrate the “good and evil” aspects of NF-κB whereby NF-κB is required for immunological mechanisms but detrimental when it is dysregulated.
Consistent with its essential role in inflammation, NF-κB is known to be the target of anti-inflammatory compounds (see ref. 11). Thus, it has been reported that aspirin and other nonsteroidal anti-inflammatory drugs block NF-κB (14–16). Glucocorticoids such as prednisone have been shown to block NF-κB activation by different mechanisms in different cell types (11, 17). In fact, other anti-inflammatory compounds used in therapy have been shown to inhibit NF-κB. For example, gold compounds used as antiarthritic therapies were shown to block NF-κB activation (18). The development and use of NF-κB inhibitors in a variety of disorders are the subject of the Perspective by Yamamoto and Gaynor.
Many of the same issues regarding NF-κB activation and inflammation can be extended to NF-κB and its potential involvement with atherogenesis. NF-κB is known to be activated in endothelial cells by oxidized LDLs (19) and by fluid shear stress (20), key initiating and progression mechanisms in the process of atherosclerosis. Recruitment of monocytes and their extravasation into the subendothelial space is a key event in atherogenesis (21) and is likely to be regulated by NF-κB, as is the induction of proliferation of vascular smooth muscle cells (22). Consistent with these in vitro studies are results showing that NF-κB is activated in the atherosclerotic lesion (23). The complex issues surrounding a potential role of NF-κB in atherogenesis will be dealt with in depth by Collins and Cybulsky in this Perspective series. Interestingly, dietary compounds that are known to block the initiation of atherogenesis, as well as other diseases such as cancer, are now known to inhibit NF-κB activation (for example, see ref. 24).
One of the earliest observations regarding NF-κB was that it is a transcriptional regulator of HIV (reviewed in refs. 2, 3). Two NF-κB sites in the HIV long terminal repeat (LTR) have been proposed to be involved in viral transcription and replication (25). Interestingly, it has been reported that HIV infection induces NF-κB activation, which may suppress HIV-induced apoptosis in infected myeloid cells (26). Many other viruses have co-opted NF-κB for their use (27), presumably because of the inducibility of this transcription factor and because of its ability to regulate cell cycle, DNA replication, and apoptosis. Consistent with this, inhibition of NF-κB blocked the ability of herpes simplex virus to replicate (28). Most viruses encode proteins that are capable of activating NF-κB. For example, the LMP-1 protein of Epstein-Barr virus activates NF-κB (29) in a manner similar to the mechanism used by the TNF receptor (30). Thus, NF-κB activation by viral infection is required for viruses to induce proliferative responses, to replicate their genetic material, and to induce pathogenic responses. The various roles for NF-κB in virus-associated mechanisms and pathology are discussed in the Perspective by Hiscott and his colleagues.
A relatively recent observation is that NF-κB is a critical regulator of apoptosis. Studies in this area were based on the original findings of Beg, Baltimore, and colleagues (31), who showed that the p65/RelA knockout mouse died embryonically from extensive liver apoptosis. In response to many normal physiological stimuli (such as TNF), NF-κB is activated and suppresses apoptotic potential through the transcriptional activation of genes whose products block apoptosis (reviewed in ref. 32). The involvement of NF-κB with apoptosis has significant disease implications, as discussed below. Curiously, NF-κB has been found to be associated with antiapoptotic as well as proapoptotic mechanisms (32). For example, NF-κB activation appears to induce apoptosis in cells exposed to hydrogen peroxide (33). Consistent with the latter observation and with evidence that reactive oxygen species activate NF-κB, loss of one of the NF-κB subunits can block cell death in an ischemia/reperfusion stroke model (34). In these regards, NF-κB can be most easily viewed as a stress response factor that controls whether a cell will live or die. The nature (or strength) of the stimulus and the cell type involved may determine whether NF-κB leads to cell survival or cell death. In one of the Perspectives in this series, I discuss in more detail the complex roles of NF-κB in apoptosis.
NF-κB and NF-κB–like factors have been described in the nervous system (35). Importantly, NF-κB appears to control nervous system development, and its regulation is controlled by neurotransmitters and neurotrophic factors. Consistent with its role in regulating apoptosis, NF-κB serves a cell survival role in neurons in response to cell injury through the upregulation of antiapoptotic and antioxidant genes. The complex roles of NF-κB in the neurons and the potential pharmacological intervention in specific diseases, such as Alzheimer’s disease and stroke, are discussed in the Perspective by Mattson and Camandola.
Several reports demonstrate that NF-κB is activated by oncoproteins, including oncogenic forms of Ras as well as viral oncoproteins, such as LMP-1 of Epstein-Barr virus and HTLV-I Tax (reviewed in refs. 36–38). More recently it has been shown that NF-κB is required for several of these oncoproteins to induce cellular transformation. Inhibition of NF-κB blocks cell transformation induced by oncogenic Ras and blocks tumor formation induced by Bcr-Abl (see ref. 36). NF-κB likely participates in oncogenesis both by suppressing apoptosis and by inducing cell proliferation. Thus, activation of NF-κB by oncogenic Ras suppresses Ras-induced apoptosis. Inhibition of NF-κB in conjunction with oncogenic Ras expression leads to apoptosis (39). The ability of NF-κB to control cell proliferation depends in part on its ability to transcriptionally activate cyclin D1 expression (36).
Because apoptosis is the primary mechanism of tumor cell killing by radiation and by chemotherapy, the observations that the activation of NF-κB by TNF suppresses apoptotic potential generated interest in the effect of NF-κB on the efficacy of cancer therapies. Indeed, suppression of NF-κB activation significantly enhances cell killing in culture in response to these treatments (40). In tumor models, NF-κB is activated in tumor cells in response to chemotherapy, and inhibition of NF-κB by viral expression of IκB (41) or by a small molecule inhibitor of NF-κB leads to significant enhancement in the apoptotic response of the chemotherapy (J. Cusack, R. Liu, and A. Baldwin, Jr., unpublished observations). In fact, complete elimination of experimental tumors can be achieved with optimal dosing regimens. The relevance of inhibition of NF-κB as an adjuvant approach to cancer therapy is covered in my Perspective on NF-κB and oncogenesis.
The experimental results outlined above and this Perspective series indicate that NF-κB is a primary effector of human disease. For this reason, numerous efforts are underway to develop safe inhibitors of NF-κB to be used in treatment of both chronic and acute disease situations. Many scientists question whether a factor that is required for basic immune responses can be effectively targeted to inhibit associated disease characteristics. Clearly, the most logical use of NF-κB inhibitors will be in acute situations, where short-term therapy is needed. Thus, inhibition of NF-κB during stroke or in cancer treatment would provide the least likelihood of side effects targeting immune function. However, the fact that long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids can be tolerated and that these compounds block NF-κB provides evidence that long-term use of NF-κB inhibitors is a valid strategy. The development of specific NF-κB inhibitors should reduce side effects associated with drugs such as NSAIDs and glucocorticoids and offer significant potential for the treatment of a variety of human diseases.
References
- 1.Sen R, Baltimore D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a post-translational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin AS. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, May M, Kopp E. NF-κB and Rel proteins: evolutionarily conserved mediators of the immune response. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 5.Beg A, Sha W, Bronson R, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 6.Franzoso G, et al. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iotsova V, et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 8.Marok R, et al. Activation of transcription factor NF-κB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- 9.Miagkov A, et al. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurath M, Petterson S, Buschenfelde K, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the RelA subunit of NF-κB abrogates established experimental colitis. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 11.Barnes P, Karin M. NF-κB: a pivotal transcription factor in chronic inflammatory disease. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya T, et al. A potential role of activated NF-κB in the pathogenesis of euthyroid sick syndrome. J Clin Invest. 2000;106:393–402. doi: 10.1172/JCI7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttridge D, Mayo M, Madrid L, Wang C-Y, Baldwin A. NF-κB-induced post-transcriptional loss of MyoD mRNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Yamamoto Y, Gaynor R. The anti-inflammatory agents aspirin and salicylate inhibit the activity of the IκB kinase β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Yin M, Lin K, Gaynor R. Sulindac inhibits the activation of the NF-κB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 16.Wahl C, Liptay S, Adler G, Schmid R. Sulfasalazine: a potent and specific inhibitor of NF-κB. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bosschler K, et al. Glucocorticoids repress NF-κB-driven genes by disturbing the interaction of p65 with the basal machinery, irrespective of co-activator levels in the cell. Proc Natl Acad Sci USA. 2000;97:3919–3924. doi: 10.1073/pnas.97.8.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traber K, et al. Anti-rheumatic compound aurothioglucose inhibits TNFα-induced HIV-1 replication in latently infected OM10.1 and Ach2 cells. Int Immunol. 1999;11:143–150. doi: 10.1093/intimm/11.2.143. [DOI] [PubMed] [Google Scholar]
- 19.Cominacini L, et al. Oxidized LDL binding to ox-LDL receptor 1 in endothelial cells induces the activation of NF-κB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 20.Khachigan L, Resnick N, Gimbrone M, Collins T. NF-κB functionally interacts with the PDGF B chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berliner J, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 22.Bellas R, Lee J, Sonenshein G. Expression of a constitutive NF-κB-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J Clin Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand K, et al. Activated transcription factor NF-κB is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes-McNary M, Baldwin A. Chemopreventive properties of trans-resveratrol are associated with inhibition of the IκB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 25.Alcami J, et al. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLuca C, Petropoulos L, Zmeureanu D, Hiscott J. Nuclear IκBβ maintains persistent NF-κB activation in HIV-1 infected myeloid cells. J Biol Chem. 1999;274:13010–13016. doi: 10.1074/jbc.274.19.13010. [DOI] [PubMed] [Google Scholar]
- 27.Mosialos G. The role of Rel/NF-κB proteins in viral oncogenesis and the regulation of viral transcription. Semin Cancer Biol. 1997;8:121–129. doi: 10.1006/scbi.1997.0063. [DOI] [PubMed] [Google Scholar]
- 28.Patel A, et al. Herpes simplex type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication. Virology. 1998;147:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 29.Paine E, Scheinman R, Baldwin A, Raab-Traub N. Expression of LMP1 in epithelial cells leads to activation of a select subset of NF-κB/Rel family proteins. J Virol. 1995;69:4572–4576. doi: 10.1128/jvi.69.7.4572-4576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sylla B, et al. EBV transforming protein LMP1 activates NF-κB through a pathway that includes NIK and IKKα and IKKβ. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beg A, Shaw W, Bronson R, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;396:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 32.Barkett M, Gilmore T. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 33.Dumont A, et al. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- 34.Schneider A, et al. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 35.Moerman A, Mao X, Lucas MM, Barger S. Characterization of a neuronal κB-binding factor distinct from NF-κB. Brain Res Mol Brain Res. 1999;67:303–315. doi: 10.1016/s0169-328x(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 36.Rayet B, Gelinas C. Aberrant Rel/NF-κB genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 37.Sun S-C, Ballard D. Persistent activation of NF-κB by the Tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 38.McFarland E, Izumi K, Mosialos G. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 39.Mayo M, et al. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 40.Wang C-Y, Mayo M, Baldwin A. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 41.Wang C-Y, Cusack J, Liu R, Baldwin A. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]