Abstract
The release and transfer of zinc from metallothionein (MT) to zinc-depleted sorbitol dehydrogenase (EC 1.1.1.14) in vitro has been used to explore the role of MT in cellular zinc distribution. A 1:1 molar ratio of MT to sorbitol dehydrogenase is required for full reactivation, indicating that only one of the seven zinc atoms of MT is transferred in this process. Reduced glutathione (GSH) and glutathione disulfide (GSSG) are critical modulators of both the rate of zinc transfer and the ultimate number of zinc atoms transferred. GSSG increases the rate of zinc transfer 3-fold, and its concentration is the major determinant for efficient zinc transfer. GSH has a dual function. In the absence of GSSG, it inhibits zinc transfer from MT, indicating that MT is in a latent state under the relatively high cellular concentrations of GSH. In addition, it primes MT for the reaction with GSSG by enhancing the rate of zinc transfer 10-fold and by increasing the number of zinc atoms transferred to four. 65Zn-labeling experiments confirm the release of one zinc from MT in the absence of glutathione and the more effective release of zinc in the presence of GSH and GSSG. In vivo, MT may keep the cellular concentrations of free zinc very low and, acting as a temporary cellular reservoir, release zinc in a process that is dynamically controlled by its interactions with both GSH and GSSG. These results suggest that a change of the redox state of the cell could serve as a driving force and signal for zinc distribution from MT.
The possible functional implications of the zinc cluster structure of metallothionein (MT) have been the subject of much speculation (1–3). In contrast with other zinc proteins, MT is remarkable in that it binds zinc with high thermodynamic stability [Kd = 1.4 × 10−13 M at pH 7.0 for human MT (4)] while exhibiting a kinetic lability that results in facile zinc exchange reactions (5). This unusual combination appears to be a characteristic property of the clusters and, likely, a critical element in their hypothetical function to “[provide] zinc where and when needed and for whatever role” (6). Thus, in MT the protein plays a role in the biological function of zinc, a paradigm quite different from that in most other zinc proteins where zinc plays a role in the biological function of the protein. The tight binding of zinc to MT raises questions of how it is released and whether or not the release is controlled. In this regard, we have identified glutathione disulfide (GSSG) as a cellular ligand that reacts with MT and mobilizes zinc, resulting in the suggestion that the zinc content of MT is linked to the redox state of glutathione in the cell in such a manner that zinc remains bound to MT as long as high thiol reducing power prevails and is released once the redox balance becomes more oxidizing (7). We here extend these findings by studying the role of glutathione-mediated zinc release in the presence of a zinc acceptor such as an apoenzyme. In particular, we show that only one of the seven zinc atoms is transferred from MT to zinc-depleted sorbitol dehydrogenase (SDH; EC 1.1.1.14) in the absence of glutathione and that zinc transfer reactions are regulated dynamically by interactions between MT and the reduced glutathione (GSH)/GSSG couple. Thus, zinc distribution is dependent not on MT alone, but rather on a biochemical system in which MT and glutathione interact. Such a glutathione/MT system allows MT to serve as both a cellular reservoir for zinc and a controlled release system that can supply different amounts of zinc according to demand. Biological specificity of this system seems to be embedded not in the recognition between MT and apoproteins, but rather in signals effecting a change of the cellular redox state and in signals that control the availability of apoproteins.
MATERIALS AND METHODS
Materials.
GSH, GSSG, NAD+, pyridine-2,6-dicarboxylic acid (dipicolinic acid), Coomassie brilliant blue G, 2-carboxy-2′-hydroxy-5′-sulfoformazylbenzene (zincon), and carbonic anhydrase (bovine erythrocyte) were from Sigma; sorbitol and 4-(2-pyridylazo)resorcinol (PAR) were from Aldrich; and 65ZnCl2 (77.7–103.6 GBq/g) was from DuPont/NEN.
Preparation and Characterization of Human MT.
Human MT-1 and MT-2 isoforms were prepared in this laboratory according to established procedures (8) and converted to their apoforms and reconstituted with Zn2+ to result in the respective Zn7-MT isoforms (7). Excess zinc was removed by gel filtration through a Sephadex G-50 fine column (30 × 0.5 cm) and the MT isoforms were characterized by metal analyses, determinations of sulfhydryl groups with dithiodipyridine, and amino acid analyses. Loosely bound zinc in MT solutions was assayed spectrophotometrically at 620 nm with zincon. Addition of 10 μl of 10 mM zincon to 890 μl of 10 mM Tris⋅HCl, pH 8.6, identifies the existence of less than 4% of loosely bound zinc.
Preparation and Characterization of SDH and Zinc-Depleted SDH (apo-SDH).
Sheep liver SDH was obtained as a lyophilized powder from Boehringer Mannheim and stock solutions were prepared by dissolving enzyme in 1 ml of 0.2 M Tris⋅HCl, pH 7.4. Enzyme concentrations were determined with a Coomassie blue protein-dye binding assay with bovine serum albumin as a standard (9).
apo-SDH was prepared in Centricon-10 centrifugal microconcentrators (Amicon), using 0.2 M sodium phosphate, pH 7.0, containing 10 mM dipicolinic acid to remove zinc (10). The apoform had 2.0% residual enzymatic activity compared with the native zinc enzyme and contained 0.015 atom of zinc per subunit. The amount of zinc in native SDH was 1 atom per subunit, in agreement with the expected value (11).
Kinetic Studies of the Reconstitution of apo-SDH.
Different amounts of free zinc, MT, or carbonic anhydrase were incubated with 1.7 μM apo-SDH in 0.2 M Tris⋅HCl, pH 7.4 at 22.5 ± 0.5°C. Aliquots (10 μl) were withdrawn periodically and assayed for enzymatic activity. The assay solution consisted of 50 mM sorbitol and 1.5 mM NAD+ in a total volume of 1 ml of 0.2 M Tris⋅HCl, pH 7.4. Enzyme activity was determined spectrophotometrically (12) by measuring the rate of absorbance change accompanying NAD+ reduction at 25.0 ± 0.5°C. The experimental error between assays did not exceed 5%.
Reconstitution of apo-SDH with 65Zn7-MT-2.
Alquots of reactants (molar ratio of zinc to apo-SDH of 1.0) were incubated at 22.5 ± 0.5°C for 60 min. Reaction mixtures were then separated on a DEAE MemSep-1000 chromatography cartridge (Millipore) using a linear, 10-min gradient from 0 to 75 mM NaCl in 10 mM Tris⋅HCl, pH 8.6, at a flow rate of 5 ml/min. Radioactivity in each fraction was measured by γ-emission spectroscopy with a Searle model 1185 Automatic Gamma System operating at a 0.12- to 1.2-MeV energy range.
Kinetic Studies of the Reaction between MT-2 and PAR.
PAR (100 μM) was incubated with MT (1.3 μM) and the reaction was followed by measuring the increase of absorbance at 500 nm of the Zn(PAR)2 complex (13, 14).
RESULTS
Kinetic Studies of the Reconstitution of apo-SDH with Zinc Ions and MT.
Mammalian SDH is a tetramer of four identical subunits with an overall molecular mass of 152 kDa and one catalytic zinc atom per subunit (11, 15, 16). The reconstitution of apo-SDH with free zinc ions is very fast and reaches 100% within 3 min (Fig. 1, upper curve). Standard kinetic treatment of the slopes of plots of [Zn-SDH]/[Zn] vs. time gives an estimated rate constant for reconstitution of apo-SDH with free zinc of 20,000 M−1⋅s−1 (Table 1). In the cell, the concentration of free zinc is exceptionally low (see Discussion) and, therefore, is likely not a significant source of zinc for the reconstitution of apoproteins.
Figure 1.
Kinetics of the reconstitution of apo-SDH with free zinc (□) and MT-1 (▪). Free zinc (1.7 μM) or MT (0.24 μM) (molar ratio between zinc and apo-SDH of 1.0) was incubated with apo-SDH (1.7 μM) in 0.2 M Tris⋅HCl, pH 7.4. Aliquots (10 μl) were periodically withdrawn from this mixture and assayed for enzymatic activity. Reactivation is expressed as the activity of native SDH recovered.
Table 1.
Rate of zinc transfer to apo-SDH
| Zinc donor | Rate constant, M−1·s−1 |
|---|---|
| Free zinc | 20,000 |
| MT-1 | 24 |
| MT-2 | 16 |
| MT-1 + GSH | 20 |
| MT-1 + GSSG | 79 |
Rate constants were obtained from plots for a second-order reaction: [SDH]/[Zn] = k2[apo-SDH]0t.
Reconstitution of SDH with Zn-MT-1 is much slower (Fig. 1, lower curve) than with free zinc and reaches 17% after 1 hr at equimolar concentrations of apo-SDH and zinc in MT. In addition, a small burst of reactivation occurs with MT-1 at the first time point of the measurements, perhaps because of the traces of zinc in MT that can be dechelated by treatment with zincon (17). MT-2 reactivates apo-SDH to 13% (data not shown) and is therefore slightly less effective in transferring zinc than is MT-1. The second-order rate constants for MT-1 and MT-2 are 24 and 16 M−1⋅s−1, respectively (Table 1). These slightly different rates and degrees of reactivation with MT-1 and -2 are in accord with data reported for carbonic anhydrase and rat liver MT isoforms (18).
Zinc Transfer from Carbonic Anhydrase to apo-SDH.
To determine whether zinc transfer between MT and apo-SDH is merely controlled by the thermodynamic equilibrium between MT and the apoenzyme, carbonic anhydrase was investigated for its potential to transfer zinc to apo-SDH. Bovine carbonic anhydrase binds zinc less tightly [Kd = 1 × 10−12 M at pH 7.0 (19)] than human MT and, therefore, should be a better zinc donor to apo-SDH if zinc transfer is simply controlled by the difference in the zinc binding constants of the apoenzyme and MT. After a 1-hr incubation of apo-SDH and carbonic anhydrase no significant zinc transfer is observed when there is a 7-fold molar excess of carbonic anhydrase in terms of zinc (Fig. 2, lower curve). In contrast, zinc transfer with MT is very efficient (Fig. 2, upper curve). Therefore, zinc transfer from MT must be under kinetic control. High kinetic lability of zinc distinguishes MT from other zinc proteins, so that MT can serve as an efficient source of zinc.
Figure 2.
Kinetics of the reconstitution of apo-SDH (1.7 μM) with 1.7 μM MT-1 (▪) and 11.9 μM carbonic anhydrase (□). Conditions were as described in the legend of Fig. 1.
Reconstitution with Different Ratios of MT and apo-SDH.
SDH activity increases linearly as a function of the ratio of free zinc (Fig. 3A) or of MT-1 (Fig. 3B) to apo-SDH until its zinc content is fully restored. The equivalence point in the titration of apo-SDH with free zinc ions is at 1 atom of zinc per subunit (Fig. 3A). In contrast, one molecule of MT, which contains seven zinc atoms, is needed per subunit of apo-SDH to achieve full reactivation (Fig. 3B). Thus, under these conditions only one of the seven zinc atoms is transferred from MT, which explains why only 17% of the enzymatic activity is restored when equimolar concentrations of enzyme and zinc are employed (Fig. 1).
Figure 3.
Reconstitution of apo-SDH with free zinc (A) and MT-1 (B). Free zinc or MT-1 was incubated with apo-SDH (1.7 μM) under different ratios of zinc to apo-SDH monomers in 0.2 M Tris⋅HCl, pH 7.4, for 30 min (or 60 min for MT-1). Aliquots (10 μl) were withdrawn from this mixture and assayed for enzymatic activity.
Reconstitution of apo-SDH with MT in the Presence of GSSG and/or GSH.
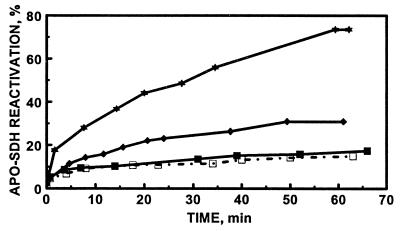
GSSG is known to mobilize all seven zinc atoms from MT at pH 8.6 (7). We therefore tested how GSSG effects the transfer of zinc atoms at pH 7.4. Adding MT to apo-SDH in the presence of GSSG generates activity faster and to a greater extent than in its absence. Thus, incubation of apo-SDH with MT-1 (equimolar zinc) for 1 hr in the presence of GSSG leads to 31% reactivation, almost twice that achieved without GSSG (Fig. 4). Under these conditions the second-order rate constant increases 3-fold to 79 M−1⋅s−1 (Table 1), a comparison based on the assumption that only one zinc atom is released. In fact, however, GSSG releases more than one metal atom. Incubating MT with GSSG for 5 hr prior to the addition of apo-SDH results in a large burst of reactivation up to 44% (data not shown). The reaction does not approach the rate at which free zinc ions interact with apo-SDH, indicating that a step other than the reconstitution of apo-SDH with free zinc becomes rate-limiting. Most likely, this step is the release of zinc from MT.
Figure 4.
Kinetics of the reconstitution of apo-SDH with MT-1 in the presence of GSSG and/or GSH. MT (0.24 μM) (molar ratio between zinc and apo-SDH of 1.0) was incubated with apo-SDH (1.7 μM) in 0.2 M Tris⋅HCl, pH 7.4, in the presence of GSSG and/or GSH. ▪, In the absence of GSH and GSSG; ⧫, in the presence of 3 mM GSSG; ★, in the presence of 3 mM GSSG and 1.5 mM GSH; or □, in the presence of 10 μM GSH. Aliquots (10 μl) were periodically withdrawn from the mixtures and assayed for enzymatic activity.
GSH has been shown to bind to rabbit liver Cd5Zn2-MT (20). We therefore examined the reconstitution of apo-SDH with MT-1 in the presence of GSH. In three independent experiments at most a very slight inhibition of GSH on zinc transfer was observed (Fig. 4).
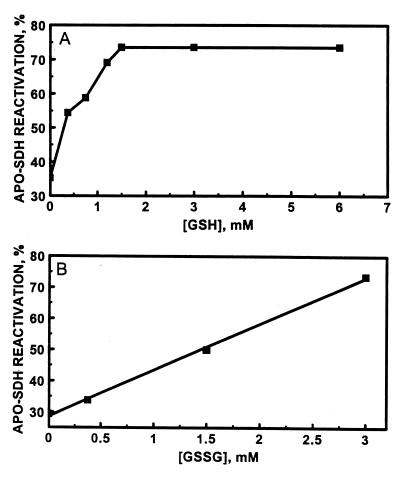
Zinc transfer from MT to apo-SDH was then investigated in the presence of both GSH and GSSG. Neither GSH nor GSSG at the concentrations employed affects the activity of SDH in the absence of MT. Nevertheless, GSH strongly influences the capacity of MT-2 to reconstitute apo-SDH in the presence of GSSG (Table 2). Thus, it further modulates the enhancement of zinc transfer from MT to apo-SDH by GSSG (Fig. 4). At a constant concentration of 3.0 mM GSSG, the rate and extent of zinc transfer from MT-2 to apo-SDH increase until a plateau is reached at the GSH concentration of 1.5 mM (Fig. 5A and Table 2). Under these conditions, the rate constant is increased more than 10-fold (from 16 M−1⋅s−1 in the absence of glutathione to about 200 M−1⋅s−1 in its presence) and the amount of zinc transferred is 5-fold greater than that in the absence of GSH and GSSG. When the concentration of GSH was kept constant at 1.5 mM and that of GSSG was varied, the rate and extent of zinc transfer increased linearly with increasing concentrations of GSSG (Fig. 5B and Table 2) but above a concentration of 3 mM GSSG a further increase was not observed.
Table 2.
Modulation of the rate of zinc transfer from MT-2 to apo-SDH by GSH and GSSG
Figure 5.
Dependence of the reactivation of apo-SDH on the concentration of GSH at a fixed concentration of 3 mM GSSG (A) and on the concentration of GSSG at a fixed concentration of 1.5 mM GSH (B). MT (0.24 μM) was incubated with apo-SDH (1.7 μM) in 0.2 M Tris⋅HCl, pH 7.4, for 60 min at the indicated concentrations of GSH and GSSG. Aliquots (10 μl) were then withdrawn from the mixtures and assayed for enzymatic activity.
The measurement of zinc transfer by means of the recovery of SDH activity determines the amount of zinc incorporated into the active site of SDH, but only inferentially that of zinc released from MT. Therefore, 65Zn-MT was employed to determine zinc release and transfer to apo-SDH directly. 65Zn7-MT-2 was incubated with apo-SDH for 1 hr (molar ratio of zinc to apo-SDH of 1.0) in the absence of GSH and GSSG, followed by separation of the mixture by anion-exchange chromatography and analysis. Radioactivity in the MT-2 fractions (fractions 14–17) decreases, whereas it increases in the fractions corresponding to SDH (fractions 1–5) (Fig. 6A). Quantification proves that only one (1.1 ± 0.1, n = 3) of the seven zinc ions is released from MT, confirming the stoichiometry based on the recovery of SDH activity (Fig. 3B). In the presence of GSH/GSSG, more than one zinc is released from MT-2 and transferred to apo-SDH (Fig. 6B). At a GSH/GSSG ratio of 0.5, four of the seven zinc ions are transferred from MT-2 to apo-SDH. Concomitantly, a new radioactive species is formed (fractions 9–11) (Fig. 6B), whose molecular identity is at present unknown. These 65Zn-labeling experiments provide direct evidence that the GSH/GSSG redox couple modulates zinc transfer from MT to another protein and that the extent of zinc transferred depends on the redox ratio.
Figure 6.
Radiochromatograms of 65Zn-MT-2 with apo-SDH in the absence or presence of GSH and GSSG. MT-2 was incubated with apo-SDH (molar ratio between zinc and apo-SDH of 1.0) for 60 min and then the mixture was analyzed by radiochromatography. (A) In the absence of GSH and GSSG. (B) [GSH] = 1.5 mM, [GSSG] = 3 mM. For the determination of the number of zinc atoms transferred, the radiochromatograms were integrated from fractions 14–17. ▪, MT control; □, MT after the reaction with apo-SDH.
Zinc Transfer from MT to PAR in the Presence of GSH.
To determine whether GSH affects zinc transfer in another system, the reaction of MT-2 with PAR, a chromophoric dye with a zinc binding constant of 1012.6 M−1 at pH 7.4 (21), was used. GSH inhibits transfer of one zinc atom in a concentration-dependent manner, yielding 75% inhibition at a concentration of 3 mM GSH (Fig. 7). We attempted to fit the data to a simple model (dotted line) with one GSH binding site and a dissociation constant of 56 μM, which is slightly higher than the value of 15 μM reported for rabbit Cd5Zn2-MT (20). This simple binding model is clearly unsatisfactory due to the complex mechanism of interactions between GSH and MT.
Figure 7.
Inhibition of zinc transfer from MT-2 by GSH. MT (1.3 μM) was incubated with PAR (100 μM) in 0.2 M Tris⋅HCl, pH 7.4, in the presence of increasing amounts of GSH, and the reaction was followed by measuring the increase of absorbance at 500 nm of the zinc-PAR complex for 60 min. The number of zinc atoms released from MT was calculated on the basis of the formation of Zn(PAR)2 (ɛ500 = 61,500 M−1⋅cm−1). Inhibition is expressed as the decrease in the amount of zinc transferred. The dotted line is based on a simple model with one GSH binding site and a dissociation constant of 56 μM.
DISCUSSION
Zinc Transfer in the Absence of Glutathione.
In vitro, MT transfers zinc to apo-SDH and fully restores enzymatic activity. The stoichiometry of the interaction of apo-SDH with MT is most striking (Figs. 3 and 6). One MT molecule is needed to supply each subunit of SDH with one zinc atom, although each MT molecule contains seven zinc atoms. Thus only one of the zinc atoms of MT appears to be available for transfer to apo-SDH.
It has been observed that in Cd5Zn2-MT the two zinc atoms reside at defined positions in the β-domain (22) and that metals in this domain exchange more rapidly than those in the α-domain (5, 23). These findings suggest that the zinc atom transferred from MT-2 to apo-SDH is from the β-domain. An NMR study on Cd7-MT, from which one cadmium atom had been removed from the β-domain with EDTA (24), provides further insight into the structure of MT after one zinc atom has been transferred. In this case, removal of one cadmium atom allows redistribution of the remaining cadmium atoms within the β-domain and yields equal but less than stoichiometric occupancies of all these metal binding sites in this domain. Though no experiments have been performed with zinc, it is conceivable that if there is an exit site for zinc in the β-domain, the empty site will be replenished by inter- and intracluster zinc transfer. A similar situation has been observed for the 3-metal site of ascorbate oxidase. Crystal structure analyses of the enzyme in which “type 2” copper had been removed revealed that the remaining two copper ions scramble among the three available binding sites (25).
Studies with more cellular relevance have led to a concept which postulates that a concerted action between MT, GSH, and GSSG is required to effect zinc transfer and that MT per se cannot be a transfer agent in vivo in the specific sense of the word. Although we found that the binary system MT/apoenzyme functions in vitro, it may not apply in vivo because MT interacts with GSH and zinc transfer from the MT/GSH complex is inhibited.
Zinc Transfer in the Presence of Glutathione.
GSSG, which releases zinc from MT (7), accelerates the rate and dramatically increases the extent of reactivation of apo-SDH by MT (Fig. 5). Moreover, GSH stimulates the rate of zinc transfer in the apo-SDH/MT/GSSG system, signifying control of the MT molecule by glutathione with important implications regarding modulation of zinc transfer by the GSH/GSSG redox couple in vivo (see below).
In terms of molecular mechanism, GSH and GSSG could (i) bind to SDH and affect its activity, (ii) control the amount of free zinc available once it is released from MT, or (iii) bind to MT and affect its conformation and zinc binding. Our data strongly favor the last mechanism. Molecular modeling studies suggest that GSH binds in a cleft of the β-domain and its thiol sulfur displaces the thiol ligand of Cys-26 of the zinc atom designated as Zn-2 in the crystal structure of MT (20). The exposed MT thiol sulfur could then undergo thiol/disulfide interchange with GSSG, explaining why GSH stimulates the rate of the reaction between MT and GSSG. GSH binding would protect MT from the loss of Zn-2, thereby inhibiting its transfer, while providing a reactive thiol for the reaction with GSSG and resulting in zinc transfer through a process that is strictly proportional to and dependent on the concentration of GSSG (Fig. 5B). This zinc distribution system seems to rely on specific interactions between MT and glutathione and to respond to signals that change the cellular GSH/GSSG redox state (see below) rather than on the mutual molecular recognition of MT and the apoprotein. Hence, a system consisting of MT/GSH/GSSG provides a mechanism to control zinc transfer among a large variety of acceptors. Thus regulation at the protein level can now be added to the already known extensive and intricate regulation of MT gene expression (26). Both types of regulation support key functions of MT in cellular zinc traffic.
MT Controls Cellular Free Zinc.
The reactivation of apo-SDH with Zn-MT occurs at a rate that is slower than that with free zinc ions (Fig. 1). Specifically, the rate constant for zinc transfer from MT to apo-SDH is three orders of magnitude less than that of about 20,000 M−1⋅s−1 estimated for apo-SDH and free zinc ions. In the few other enzyme systems studied (27), MT also failed to accelerate the reconstitution of the apoenzyme, as might have been expected if MT had insertase-like enzymatic activity such as, e.g., ferrochelatase, the enzyme that inserts iron into protoporphyrin IX. At best, the rate of zinc transfer is as rapid as that of free zinc ions in the case of apo-carbonic anhydrase (17). At first glance, such a lack of any kinetic advantage might argue against a physiological function of MT as a universal zinc donor for the apoforms of zinc proteins. However, a comparison between free zinc ions and MT as zinc donors is not appropriate, because there is very little free zinc in the cell to serve the purpose. Intracellular zinc concentrations are exceedingly low—i.e., <100 pM (28–30). One of the roles of MT, therefore, seems to be to ensure that zinc concentrations are maintained at such low levels. MT can achieve this by binding zinc very tightly. If metals were supplied to apoproteins in the form of free ions, zinc would compete with other metal ions for the binding site. This is apparently not the case, because purified zinc enzymes, at least those from animal sources, invariably contain only zinc despite the fact that other metal ions can bind to the same site in vitro, sometimes even with partial or full conservation of catalytic or other function. Thus, the choice of a particular metal ion must be “directed by its cellular availability and mobilization processes rather than by its chemical nature” (31) or by the coordination environment provided by the protein. It would seem more likely that specificity of metal incorporation is controlled by proteins and that protein-bound zinc is the source of zinc for apoproteins. MT can serve such a function and can make zinc available in a controlled manner. As the above experiments show, zinc is available from MT despite its relatively high thermodynamic stability, and this property distinguishes it from other zinc proteins where zinc does not exchange on a similar time scale (5). The kinetic lability of zinc in MT becomes strikingly apparent when compared with carbonic anhydrase, in which zinc has a similar thermodynamic stability but is kinetically much less capable of zinc transfer (Fig. 2).
Implications for Zinc Transfer in Vivo.
MT keeps the cellular concentration of free zinc remarkably low. Quite the same it provides zinc to appropriate acceptors in reactions that are modulated by GSH/GSSG. Most interestingly, the rate and amount of zinc released from MT depend on the relative concentrations of both GSH and GSSG, suggesting that zinc distribution from MT is controlled dynamically by the cellular GSH/GSSG state. What do these in vitro studies imply about zinc transfer in vivo? GSH alone inhibits zinc transfer (Figs. 4 and 7). Thus, under the prevailing, reducing conditions in the presence of almost millimolar concentrations of GSH, MT may not transfer zinc to apoproteins in the normal cellular environment, where the GSH/GSSG redox ratio is between 30:1 and 100:1 (32). It is noteworthy that these are steady-state conditions under which there may not be any need for zinc in the cell, and where the role of MT is to sequester zinc, not to distribute it. Hence, this condition must be perturbed to change the role of MT from that of an acceptor to that of a donor when zinc is needed in events such as cell proliferation, for example. We show here that this can be achieved by lowering the GSH concentration or by changing the GSH/GSSG redox state. The cellular concentration of GSH can vary over almost two orders of magnitude (33). Therefore, transfer of but one zinc atom might have physiological significance at low concentrations of GSH and under a regime of demand for zinc that is quite different from that at high concentrations of GSH. It was shown earlier that high concentrations of GSSG release zinc from MT (7), but the effect of GSH was not examined. We demonstrate here that the presence of GSH actually enhances the effect of GSSG. A role of both GSH and GSSG in the process of zinc release creates conditions in vitro that approach those in vivo. The rate of zinc transfer depends linearly on the amount of GSSG (Fig. 5)—i.e., the more oxidative the redox state becomes, the more efficiently zinc is transferred to a suitable acceptor (Figs. 4–6). These observations seem to link the cellular GSH/GSSG redox state and its control circuitry to the biochemistry of MT.
At present we do not know the precise range in which the cellular GSH/GSSG redox state changes, the magnitude of changes that lead to zinc distribution in vivo, where the process takes place, and whether only some or all apoproteins receive zinc from MT. However, it is clear that zinc transfer from MT to other proteins actually occurs in vivo (34). It is noteworthy that substantial deviations from the cytoplasmic GSH/GSSG redox ratio (30:1 to 100:1) control events crucial in signal transduction and gene transcription (35–37). This deviation may be achieved by enzymatically generating high local concentrations of GSSG, or by compartmentalization of the process. Thus, in some compartments of the cell such as the endoplasmic reticulum the GSH/GSSG redox ratio is between 3:1 and 1:1 (32), conditions that are quite similar to those under which we find efficient zinc transfer in vitro. The modulation of the reaction between MT and GSSG by the amount of GSH (Fig. 5A) also occurs in the range where the concentration of GSH changes in the cell. For example, cellular GSH concentrations are relatively high and vary in the range 0.1–10 mM (33). Finally, even if the concentrations of GSSG in our experiments appear to be relatively high compared with what they might be maximally in the cell, they certainly need not be as high as 3 mM to exert an appreciable effect (Fig. 5B). It is also important to note that we have observed higher efficiency in zinc release with disulfides other than GSSG (38). Thus, in the cell, a more reactive disulfide or other compounds might assume the in vitro role of GSSG.
A discussion of a role of MT in the distribution of zinc in the cell would not be complete without addressing its concentration relative to that of proteins to which it donates zinc. In spite of the relatively poor antigenicity of MT, analytical data have been obtained largely by means of antibodies against MT for want of better analytical methods. Even these immunological methods are not ideal, owing to the remarkable composition of MT and of its isoforms, all of which lack strong epitopes as well as aromatic amino acids or any other characteristic spectroscopic property that could be employed for the direct analysis of the protein. Therefore, information on the amount and distribution of the MT isoforms in different tissues remains sparse and is documented incompletely; work in this area is in great need of extension. Given these circumstances it is actually a remarkable achievement that considerable variations of the basal level of MT have been detected. Antibody screening of a panel of over 50 tumor cell lines did reveal a more than 400-fold difference in the concentrations of MT isoforms (39) ranging from 0.009 to 3.839 μg/mg of protein and including a more than 50-fold variation in the same type of tissue. Thus far the sensitivity of immunological methods is not high enough to detect small but perhaps significant cellular fluctuations that could be due to physiological changes but might also arise from the effect of inducers. Quite the same, we have indeed detected fluctuations of MT during the cell cycle (40). Zinc in MT reflects a significant pool of the total amount of cellular zinc present. For example, the amount of zinc in MT in a biosynthetic organ such as the liver represents about 5–10% of the total zinc in this organ (41). This and the fact that MT is responsive to dietary zinc (42) would all seem to underscore its role in zinc distribution.
Acknowledgments
We thank Drs. J. F. Riordan and G. F. Hu for helpful discussions. This work was supported by the Endowment for Research in Human Biology, Inc.
ABBREVIATIONS
- MT
metallothionein
- Tris
tris(hydroxymethyl)aminomethane
- SDH
sorbitol dehydrogenase
- apo-SDH
zinc-depleted SDH
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- PAR
4-(2-pyridylazo)resorcinol
Footnotes
A commentary on this article begins on page 3333.
References
- 1.Vallee B L. Experientia Suppl. 1979;34:19–40. doi: 10.1007/978-3-0348-6493-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Vallee B L. Experientia Suppl. 1987;52:5–16. doi: 10.1007/978-3-0348-6784-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Vallee B L, Maret W. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 1–27. [Google Scholar]
- 4.Kägi J H R. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 29–55. [Google Scholar]
- 5.Maret W, Larsen K S, Vallee B L. Proc Natl Acad Sci USA. 1997;94:2233–2237. doi: 10.1073/pnas.94.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallee B L. Methods Enzymol. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- 7.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vašák M. Methods Enzymol. 1991;205:41–44. doi: 10.1016/0076-6879(91)05082-7. [DOI] [PubMed] [Google Scholar]
- 9.Scopes R K. Protein Purification. New York: Springer; 1982. p. 266. [Google Scholar]
- 10.Maret W. Biochemistry. 1989;28:9944–9949. doi: 10.1021/bi00452a011. [DOI] [PubMed] [Google Scholar]
- 11.Jeffery J, Chesters J, Mills C, Sadler P J, Jörnvall H. EMBO J. 1984;3:357–360. doi: 10.1002/j.1460-2075.1984.tb01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlach U. In: Methods of Enzymatic Analysis. Bergmeyer H U, editor. New York: Academic; 1965. pp. 761–764. [Google Scholar]
- 13.Hunt J B, Neece S H, Ginsburg A. Anal Biochem. 1985;146:150–157. doi: 10.1016/0003-2697(85)90409-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaw C F, III, Laib J E, Savas M M, Petering D H. Inorg Chem. 1990;29:403–408. [Google Scholar]
- 15.Jeffery J, Commins L, Carlquist M, Jörnvall H. Eur J Biochem. 1981;120:229–234. doi: 10.1111/j.1432-1033.1981.tb05693.x. [DOI] [PubMed] [Google Scholar]
- 16.Maret W, Auld D S. Biochemistry. 1988;27:1622–1628. doi: 10.1021/bi00405a035. [DOI] [PubMed] [Google Scholar]
- 17.Li T Y, Kraker A J, Shaw C F, III, Petering D H. Proc Natl Acad Sci USA. 1980;77:6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winge D R, Miklossy K A. Arch Biochem Biophys. 1982;214:80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 19.Lindskog S, Malmström B G. J Biol Chem. 1962;237:1129–1136. [PubMed] [Google Scholar]
- 20.Brouwer M, Brouwer T H, Cashon R E. Biochem J. 1993;294:219–225. doi: 10.1042/bj2940219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw C F, III, Savas M M, Petering D H. Methods Enzymol. 1991;205:401–414. doi: 10.1016/0076-6879(91)05122-c. [DOI] [PubMed] [Google Scholar]
- 22.Robbins A H, McRee D E, Williamson M, Collett S A, Xuong N H, Furey W F, Wang B C, Stout C D. J Mol Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 23.Nettesheim D G, Engeseth H R, Otvos J D. Biochemistry. 1985;24:6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez F, Vašák M. Biochem J. 1988;253:611–614. doi: 10.1042/bj2530611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messerschmidt A, Steigemann W, Huber R, Lang G, Kroneck P M H. Eur J Biochem. 1992;209:597–602. doi: 10.1111/j.1432-1033.1992.tb17325.x. [DOI] [PubMed] [Google Scholar]
- 26.Andrews G K. Prog Food Nutr Sci. 1990;14:193–258. [PubMed] [Google Scholar]
- 27.Udom A O, Brady F O. Biochem J. 1980;187:329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peck E J, Ray W J. J Biol Chem. 1971;246:1160–1167. [PubMed] [Google Scholar]
- 29.Simons T J B. J Membr Biol. 1991;3:63–71. doi: 10.1007/BF01993964. [DOI] [PubMed] [Google Scholar]
- 30.Atar D, Backx P H, Appel M M, Gao W D, Marban E. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 31.Dauter Z, Wilson K S, Sieker L C, Moulis J M, Meyer J. Proc Natl Acad Sci USA. 1996;93:8836–8840. doi: 10.1073/pnas.93.17.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang C, Sinskey A J, Lodish H F. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 33.Meister A. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 34.Cherian M G. J Nutr. 1977;107:965–972. doi: 10.1093/jn/107.6.965. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro H P, Stern A. Free Radical Biol Med. 1996;21:323–333. doi: 10.1016/0891-5849(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 36.Sen C K, Packer L. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Oberley L W. Free Radical Biol Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 38.Maret W. Neurochem Int. 1995;27:111–117. doi: 10.1016/0197-0186(94)00173-r. [DOI] [PubMed] [Google Scholar]
- 39.Woo E S, Monks A, Watkins S C, Wang A S, Lazo J S. Cancer Chemother Pharmacol. 1997;41:61–68. doi: 10.1007/s002800050708. [DOI] [PubMed] [Google Scholar]
- 40.Nagel W W, Vallee B L. Proc Natl Acad Sci USA. 1995;92:579–583. doi: 10.1073/pnas.92.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bühler R H O, Kägi J H R. FEBS Lett. 1974;39:229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- 42.Cousins R J, Lee-Ambrose L M. J Nutr. 1992;122:56–64. doi: 10.1093/jn/122.1.56. [DOI] [PubMed] [Google Scholar]