Abstract
We report 10 heterozygous mutations in the human insulin gene in 16 probands with neonatal diabetes. A combination of linkage and a candidate gene approach in a family with four diabetic members led to the identification of the initial INS gene mutation. The mutations are inherited in an autosomal dominant manner in this and two other small families whereas the mutations in the other 13 patients are de novo. Diabetes presented in probands at a median age of 9 weeks, usually with diabetic ketoacidosis or marked hyperglycemia, was not associated with β cell autoantibodies, and was treated from diagnosis with insulin. The mutations are in critical regions of the preproinsulin molecule, and we predict that they prevent normal folding and progression of proinsulin in the insulin secretory pathway. The abnormally folded proinsulin molecule may induce the unfolded protein response and undergo degradation in the endoplasmic reticulum, leading to severe endoplasmic reticulum stress and potentially β cell death by apoptosis. This process has been described in both the Akita and Munich mouse models that have dominant-acting missense mutations in the Ins2 gene, leading to loss of β cell function and mass. One of the human mutations we report here is identical to that in the Akita mouse. The identification of insulin mutations as a cause of neonatal diabetes will facilitate the diagnosis and possibly, in time, treatment of this disorder.
Keywords: endoplasmic reticulum stress, insulin biosynthesis, disulfide bonds, unfolded protein response
Diabetes mellitus is a heterogeneous group of metabolic diseases characterized by high blood glucose levels that can present at any age, from birth to old age. Genetic factors play an important role in the development of diabetes with some forms resulting from mutations in a single gene. Although monogenic forms of diabetes are collectively relatively uncommon, the identification of the underlying genetic defects has provided important insights into pancreatic development and the control of pancreatic β cell function and improvements in diagnosis and treatment (1–3). Moreover, common variation in the same genes that can cause monogenic forms of diabetes can affect the risk of developing the common polygenic forms (4, 5). Neonatal diabetes (ND), or diabetes developing within the first few weeks or months of life, is a very rare condition with an incidence of 1 in 400,000 to 500,000 live births (6). Recent studies have shown that ND can result from abnormalities of an imprinted region of chromosome 6q24 and mutations in the genes encoding the glycolytic enzyme, glucokinase, and the two protein subunits (Kir6.2 and SUR1) of the ATP-sensitive potassium channel of the pancreatic β cell (2, 7). In addition, mutations in other genes (IPF1, PTF1A, FOXP3, GLIS3, TCF2, EIF2AK3) can lead to multisystem diseases that include ND (2). As a result of genetic studies, the view has emerged that ND is primarily a genetic disorder and not a congenital form of type 1 diabetes with a cutoff at around 6 months (8, 9). Spontaneous mutations are common in ND with 80% of the mutations in KCNJ11 (the gene encoding Kir6.2) occurring de novo (10). Here, we show that missense mutations in insulin and its precursors, preproinsulin and proinsulin, affecting insulin structure and biosynthesis are a cause of ND. Moreover in 80% of the cases studied to date, they arose de novo, implicating spontaneous mutations in key pancreatic β cell proteins (Kir6.2, SUR1, and insulin) as major causes of ND.
Results
Identification of Insulin Gene Mutations in ND.
During the course of our studies of ND, we identified a family (UC41; Fig. 1) in which ND appeared to segregate as a dominant trait. The proband and other affected family members were screened by direct sequencing for mutations in KCNJ11 and ABCC8, and none were found. We were able to obtain DNA samples from several other family members and carried out a linkage study even though the maximum expected logarithm of odds (LOD) score was <3. However, we felt that a search under the peaks could reveal candidate gene(s).
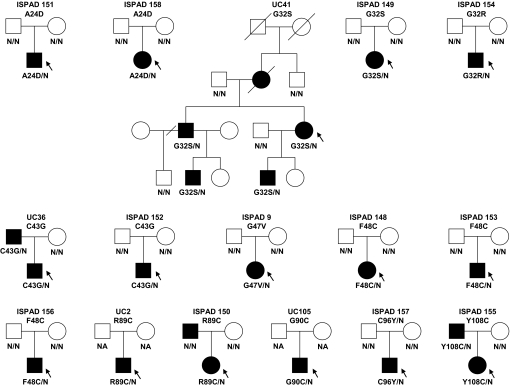
Fig. 1.
Segregation of INS mutations in 16 families. Squares represent male family members, and circles represent female members. Black circles and squares represent persons with diabetes. A slash mark through the square or circle indicates deceased. A slash mark through the branch indicates the couple has divorced. The allele status is indicated under the symbols: N/N, two normal alleles; amino acid change/N, one mutated and one normal allele; N/A, not available for testing. The arrow indicates the proband. Family relationship was confirmed in all families by microsatellite analysis, except in UC2 (parents not available for testing) and UC105.
We identified four regions of the genome that had parametric LOD scores at or approaching 1.50, the maximum possible LOD score for the family, assuming autosomal dominant transmission [supporting information (SI) Fig. 4]. Chromosome 2 had two adjacent regions of maximum LOD scores at 2q31.2–2q31.3 and 2q32.3–2.33.1: 184.6–187.1 and 191.8–198.1 cM, respectively. Chromosome 3 had a single region of maximum LOD score at 3q29: 216.5–219.3 cM. Chromosome 6 had three adjacent regions of maximum LOD scores at 6q21, 6q22.1–6q22.31, and 6q22.31: 109.6–113.6, 118.4–119.8, and 123.7–125.3 cM, respectively. Lastly, chromosome 11 had a single region of maximum LOD score at 11p15.4: 5.8–8.7 cM. Cataloguing the genes under each of these linkage peaks led to the identification of NEUROD1 at chromosome 2q31.3, which previously had been implicated as a maturity-onset diabetes of the young (MODY6) gene (11, 12), and INS at chromosome 11p15 as possible candidate genes. There were no mutations in the coding region of NEUROD1. Sequencing of the INS revealed a missense mutation (G32S) in the codon for residue B8 of the insulin molecule in the affected family members. This glycine residue is invariant among insulin sequences and conserved among insulin-like growth factors. Substitution of GlyB8 with other l-amino acid residues affects native disulfide pairing, suggesting that the INS mutation observed in this pedigree was likely to be pathogenic.
We then sequenced INS in other unrelated patients with ND without a known genetic cause, collected in Chicago (11 patients with onset before 6 months of age and 41 patients with onset between 6 months and 12 months) and Exeter (31 patients with onset before 6 months of age), and identified nine additional heterozygous missense mutations in 15 families (Fig. 2 and SI Table 2), including one individual (ISPAD 157) with the same mutation (C96Y) found in the Akita mouse (13). Several of the mutations (A24D, G32S, C43G, F48C, and R89C) were identified in more than one pedigree, indicating that these positions could be hot spots for mutations in the insulin gene. Two (G32S and R89C) of these five recurrent mutations are transitions at a CpG dinucleotide, hot spots for mammalian mutations. The mutations are in critical regions of the preproinsulin molecule, and we predict they prevent normal folding, leading to stimulation of the unfolded protein response (14, 15). All of the mutations in the A and B chains of insulin affect residues that are invariant among insulin sequences and also conserved in the insulin-like growth factors. The mutation R89C affects the site of proteolytic processing of proinsulin and introduces an additional exposed cysteine residue at this invariant site among proinsulin sequences. In the 16 families with an INS mutation identified to date, the mutation arose de novo in 13 and cosegregated with diabetes in the other 3 (Fig. 1).
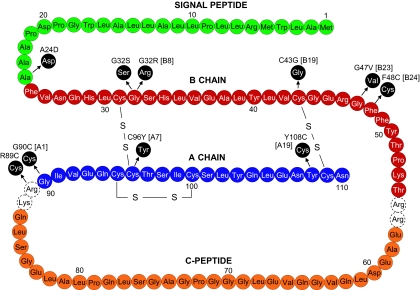
Fig. 2.
Diagram representing the human preproinsulin molecule showing location of mutations causing ND. The amino acid residues in the signal peptide are indicated in green, the B chain in red, the C-peptide in orange, and the A chain in blue. The dashed circles indicate the basic residues that are the cleavage site for conversion from proinsulin to insulin. The mutations are noted in black circles together with location in the B or A chain.
Clinical Characteristics.
We identified 10 missense mutations in the INS gene in 21 patients (20 with ND and one with type 2 diabetes) from 16 families (Fig. 1 and SI Table 2). The median age-at-diagnosis of diabetes in the probands was 9 weeks with 15/16 being diagnosed in the first 6 months. However, three mutation carriers were diagnosed between 6 months and 1 year, and in family UC36 with the C43G mutation, the father of the proband was also a carrier but was surprisingly diagnosed with mild type 2 diabetes at 30 years of age. Diabetes usually presented with diabetic ketoacidosis or marked hyperglycemia (median plasma glucose at diagnosis was 681 mg/dl), was not associated with β cell autoantibodies and was treated from diagnosis with insulin usually in replacement doses. C-peptide values where measured were very low or undetectable, with all values <200 pmol/liter. All of these features suggest most patients are insulin-dependent. The median HbA1c was 7.9 (range 5.0 to 9.8), indicating the difficulty in achieving good glycemic control in insulin-dependent diabetes in infants and young children.
Birth weight was reduced in keeping with reduced insulin secretion in utero. The median birth weight was 2,846 g (range 2,115 to 4,026 g), corresponding to the 20th percentile. Interestingly, the pedigrees with patients with later onset of diabetes (>6 months) also had higher birth weights (median 3,392, range 2,892 to 4,026 g), suggesting they had less severe or less rapid reduction in β cell function in utero and in the first year of life. We note that three of the patients in the UC41 pedigree were born to a diabetic mother (the mother of the proband was diagnosed with diabetes before 2 years of age, she was not available for genetic testing), which is likely to have increased their birth weights.
Discussion
We have identified 10 mutations in the insulin gene causing ND. These mutations differ sharply in their clinical severity from all of the previously described mutations of this gene in humans. The earlier described mutations primarily affected the biological activity of the resultant (pro)insulin molecules but did not impair their biosynthesis significantly, leading to impaired clearance accompanied by hyperinsulinemia and hyperproinsulinemia (16, 17). They only resulted in diabetes in the presence of insulin resistance and in adults. Most carriers were asymptomatic, although they did exhibit high insulin or proinsulin levels. In contrast, the mutations identified in patients with ND are severe enough to cause disruption of the β cells and diabetes early in life.
Insulin mutations are an important cause of ND. In the large Exeter permanent ND series they accounted for 38% of cases in which the genetic etiology was unknown. This finding is ≈20% of all permanent ND cases, similar to ABCC8 mutations (19%), but less than KCNJ11 mutations (30%) (18). KCNJ11 and ABCC8 mutations also cause transient ND for which the major cause is abnormalities of imprinting of the ZAC region on chromosome 6q (19). Clinically, patients with INS mutations are very similar to those with KCNJ11 and ABCC8 mutations except that they are generally older at diagnosis (Table 1). In this regard, some of the patients with INS mutations in this series were diagnosed outside the first 6 months, and further large series are needed to establish the prevalence of INS mutations in patients presenting at a wide variety of ages, especially in those who do not have autoantibodies to islet cell proteins.
Table 1.
Comparison of clinical characteristics of patients with diabetes caused by an INS mutation, KCNJ11 (8) mutation, or ABCC8 (18) mutation
| Characteristic | Molecular genetic etiology |
P | ||
|---|---|---|---|---|
| INS (n = 21) | KCNJ11 (n = 37) | ABCC8 (n = 16) | ||
| Sex, % male | 66 | 62 | 40 | 0.16 |
| Age at diagnosis, weeks | 13 (4–1,560) | 5 (0–26) | 7 (0–26) | 0.001 |
| Gestational age, weeks | 40 (36–41) | 39 (32–42) | 40 (37–40) | 0.3 |
| Birth weight, g | 2,846 (2,115–4,026) | 2,548 (1,440–3,500) | 2,710 (2,200–4,200) | 0.4 |
| Centile for birth weight | 20 (<1–89) | 3 (<1–58) | 12 (<1–95) | 0.3 |
Results given in median range are in parentheses.
Several of the mutations that we report here either disrupt normal disulfide bonds at B19–A20 (C43G) or A7–B7 (C96Y) or add an additional unpaired cysteine residue (R89C; G90C) at the A-chain C-peptide cleavage site, a solvent-exposed position in the molecule (20). Two other added cysteine mutations lie in close proximity to the A20–B19 disulfide bond at A19 (Y108C) and at B24 (F48C) and may cause mispairing of cysteines in this critical region. The formation of the A20–B19 disulfide bridge is believed to occur as the first step during folding of the peptide chain and thus may facilitate subsequent folding steps (21, 22). All of these mutants are very likely to act in a dominant manner analogous to the Akita and/or Munich mouse Ins2 mutations to disrupt insulin biosynthesis and induce endoplasmic reticulum (ER) stress, i.e., all of these mutants have reactive cysteine residues that are unpaired. The exact mechanism by which these unpaired cysteines disrupt ER function remains unclear at this time (23).
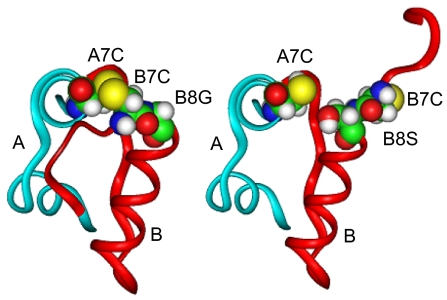
Three other mutations occur at B8 (G32S and G32R) and B23 (G47V) that at first glance do not appear to involve disulfide bridges. However both glycines are invariant in both insulin and the insulin-like-growth-factors and must play an important structural role because this amino acid uniquely lacks a side chain and therefore is unlikely to be directly involved in receptor binding. In the normal insulin T-state conformation, B8 participates in a β turn at the end of the central B chain α-helix that lies adjacent to the A7–B7 disulfide bond (Fig. 3). Because B8 glycine has a positive (Phi) dihedral angle (forbidden for l-amino acids), the presence of l-serine at B8 requires a reorientation of B8 conformation to accommodate its side chain, which results in rotation of B1–B8, turning the B7 cysteine away from its intended partner at A7. This change is reflected in lowered thermodynamic stability of the insulin molecule and significantly impairs yields in chain recombination experiments (24, 25). On the basis of these and other observations, Nakagawa et al. (24) have suggested that “the correct B8 conformation plays a critical role in the mechanism of disulfide pairing” during insulin biosynthesis and when substituted may “kinetically block the formation of the A7–B7 disulfide bridge.” Similar considerations apply to B23 glycine where l-amino acid substitutions would likely disrupt normal folding of the peptide chain near the critical A20–B19 disulfide bridge (26). Therefore, it appears very likely that these glycine mutations also act similarly to impair proinsulin folding and thereby induce ER stress via the unfolded protein response.
Fig. 3.
Predicted effect of the substitution of GlyB8 with Ser on folding of the insulin molecule. (Left) Normal conformation of the mature insulin molecule highlighting residues A7, B7, and B8 (shown as space-filling models in both images). (Right) The effect of substitution at B8 with l-serine, which induces a major conformational change resulting in reorientation of residues B1–B8, so as to prevent B7 cysteine from interacting with its intended partner at A7 during folding (see Discussion).
Two of the patients have identical signal peptide cleavage site mutations that are predicted to inhibit cleavage (Signal P 3.0; www.signalp.com). Defects in signal peptide cleavage are known to cause ER stress (27), and similar kinds of mutations have been described in preprocollagen which decrease expression (28). Likewise certain preproparathyroid hormone (preproPTH) mutations that impair signal peptide cleavage lead to reduced secretion with ER retention and rapid degradation accompanying anchoring of preproPTH to the ER membrane (29). One issue is whether these preproinsulin cleavage mutants have dominant-negative effects, but they likely would if the proinsulin is retained in the ER by signal peptide anchoring and/or it misfolds. The presence of remnants of the signal peptide at its amino terminus may also impair folding if the signal peptide is processed within the ER membrane at another site.
It is important to note that one of the mutations we have found is identical to that of the Akita mouse (13). This finding clearly establishes the connection between this class of insulin gene mutations and activation of the unfolded protein response, leading to inhibition of protein syntheses and eventually β cell death. Both the Akita and the Munich mice (30) displayed a more severe phenotype in male than in female mice. This sex difference is not observed in the patients reported here but should be kept in mind as more cases of insulin gene mutations are described in humans.
Studies remain to directly examine the biosynthesis of these mutant proinsulin molecules to assess their ability to efficiently fold and be secreted. Furthermore, the possibility exists that treatments aimed to reduce ER stress might result in improved β cell survival after differentiation of β cell precursors. This could partially ameliorate the diabetic state if secretion resulting from the normal insulin allele could be better preserved. Glucagon-like peptide-1 receptor agonists and related agents could be useful in this regard (31, 32).
Materials and Methods
Subjects.
Patients from the Chicago group were self-referred or referred by their local endocrinologist for a genetic evaluation of ND [11 cases with a diagnosis <6 months of age and 41 with a diagnosis between 6 and 12 months, negative for KCNJ11 and ABCC8 mutations (ABCC8 only tested in the latter group in patients with a birth weight <3,500 g)]. The patients studied at Peninsula Medical School involved 31 cases with ND diagnosed before 6 months of age. These patients were selected from the International Society for Pediatric and Adolescent Diabetes collection at the Peninsula Medical School on the basis of normal sequencing of the coding region of the KCNJ11 and ABCC8 genes, and the availability of parental DNA. They were used in a previous study of ABCC8 in permanent ND (18). The clinical and biochemical characteristics of these patients were obtained by the patients' local endocrinologists or during a physical examination. Percentiles for birth weights were calculated by using Child Growth Foundation LMS data (33). Age- and sex-specific percentiles for body mass index were calculated by using the Centers for Disease Control and Prevention growth charts (www.cdc.gov).
Linkage Analysis.
Four affected and four unaffected family members from the UC41 pedigree (Fig. 1) were genotyped by using the XbaI chip of the GeneChip Human Mapping 100K Chip Set (Affymetrix, Santa Clara, CA). SNPs were discarded if they did not have genetic/physical map positions, were monomorphic, or were on the X or Y chromosomes. Genetic map distances and allele frequencies were obtained from Affymetrix (www.affymetrix.com/analysis/downloads/na22/genotyping/Mapping50K_Xba240.na22.annot.csv.zip). A total of 41,310 SNPs were available for linkage analysis. The data were checked for genotyping errors and misspecified relationships using by PEDCHECK (34) and PLINK (35); additional 471 SNPs with Mendelian incompatibilities were removed. From the remaining SNPs, we selected for linkage analysis 8,090 SNPs that were at least 0.2 cM apart and had a 100% call rate in the eight individuals. Linkage analysis was conducted by using Allegro 2.0 (36) under a parametric (dominant) genetic model with disease allele frequency of 0.01% and penetrances 0.0001/0.9999/0.9999.
Mutation Screening.
The human INS gene (37) was amplified in two segments by using PCR. We used the following primers for the noncoding exon 1 and the coding exon 2: forward, 5′-GGG TTG AGA GGT AGG GGA GA-3′ and reverse, 5′-ACA GGG AGC TGG TCA CTT TT-3′. For exon 3, we used the forward 5′-AGA GAG CGT GGA GAG AGC TG-3′ and the reverse primer 5′- CCC TGA CTG TGT CCT CCT GT-3′. We used the Elongase Amplification System (Invitrogen, Carlsbad, CA). The PCR conditions were: 94°C 1 min, 35 cycles of 94°C for 30 s, 58°C for 30 s, and 68°C for 90 s; 68°C for 10 min and 10°C for storage. We used identical primers for sequencing, and both strands of the three exons were sequenced by using a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA). The data analysis was done with Mutation Surveyor software, version 3.0 (SoftGenetics, State College, PA). To confirm family relationships we used a panel of seven microsatellites on chromosome 20: D20S107, D20S851, D20S103, D20S477, D20S482, D20S171, and D20S481.
Proinsulin Folding and Models.
Space-filing models of human insulin showing the predicted effect of G32S (GlyB8) mutation on structure were generated by using the Insight graphic environment software (Biosym Technologies, San Diego).
Supplementary Material
Acknowledgments
We thank all of the families and the referring clinicians for their participation in this study; the International Society for Pediatric and Adolescent Diabetes (ISPAD), which set up the ISPAD Rare Diabetes registry; and Dr. S. H. Nakagawa, Dr. C. Ober, Dr. R. Nicolae, Mr. C. Boustred, Mr. A. Parrish, Ms. L. Hampton, Ms. A. Pastore, and Ms. Z. Paz for excellent advice and/or technical assistance. Funding for the work in Exeter was provided by the Wellcome Trust. A.T.H. is a Wellcome Trust Research Leave Fellow. Funding for the work carried out in Chicago was provided by Public Health Service Grants DK-13914, DK-20595, DK-44752, DK-73541, and DK-77489 and by a gift from the Kovler Family Foundation.
Abbreviations
- ND
neonatal diabetes
- LOD
logarithm of odds
- ER
endoplasmic reticulum.
Footnotes
The authors declare no conflict of interest.
aFaculty of Medicine, Pediatrics, Saitama Medical University, Saitama 338, Japan
bTokai University Hachioji Hospital, Tokyo 151-8677, Japan
cJeffrey Kelson Centre, Central Middlesex Hospital, London NW10 7NS, United Kingdom
dDuPont Hospital for Children, Wilmington, DE 19803
eSiriraj Hospital, Mahidol University, Bangkok 10700, Thailand
fQueen Silvia Children's Hospital, 416-85 Gothenburg, Sweden
gBarnet General Hospital, London EN5 3DJ, United Kingdom
hDepartment of Endocrinology and Genetics, 9100, Skopje, Republic of Macedonia
iGuangzhou Children's Hospital, Guangzhou 510120, China
jDepartment of Pediatrics, University of Wisconsin Medical School, Madison, WI 53705
kMother and Child Healthcare Institute of Serbia “Dr. Vukan Cupic,” 11000 Belgrade, Serbia
lKinderkrankenhaus Park Scönfeld, 34121 Kassel, Germany; and
m1300 East Main Street, Danville, IN 46122.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707291104/DC1.
References
- 1.Slingerland AS, Hattersley AT. Ann Med. 2005;37:186–195. doi: 10.1080/07853890510007287. [DOI] [PubMed] [Google Scholar]
- 2.Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K. Pediatr Diabetes. 2006;7:352–360. doi: 10.1111/j.1399-5448.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Shield JP. Horm Res. 2007;67:77–83. doi: 10.1159/000096354. [DOI] [PubMed] [Google Scholar]
- 4.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, Rayner NW, Shields B, Owen KR, Hattersley AT, Frayling TM. PLoS Med. 2006;3:1877–1882. doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen KR, McCarthy MI. Curr Opin Genet Dev. 2007;17:239–244. doi: 10.1016/j.gde.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Shield JP, Gardner RJ, Wadsworth EJ, Whiteford ML, James RS, Robinson DO, Baum JD, Temple IK. Arch Dis Child Fetal Neonatal Ed. 1997;76:F39–F42. doi: 10.1136/fn.76.1.f39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattersley AT, Pearson ER. Endocrinology. 2006;147:2657–2663. doi: 10.1210/en.2006-0152. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Diabetologia. 2006;49:1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 9.Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM. Diabetes. 2006;55:1895–1898. doi: 10.2337/db06-0094. [DOI] [PubMed] [Google Scholar]
- 10.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 11.Malecki MT, Jhala US, Antonellis A, Fields L, Doria A, Orban T, Saad M, Warram JH, Montminy M, Krolewski AS. Nat Genet. 1999;23:323–328. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- 12.Kristinsson SY, Thorolfsdottir ET, Talseth B, Steingrimsson E, Thorsson AV, Helgason T, Hreidarsson AB, Arngrimsson R. Diabetologia. 2001;44:2098–2103. doi: 10.1007/s001250100016. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ron D. J Clin Inves. 2002;109:443–445. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tager H, Given B, Baldwin D, Mako M, Markese J, Rubenstein A, Olefsky J, Kobayashi M, Kolterman O, Poucher R. Nature. 1979;281:122–125. doi: 10.1038/281122a0. [DOI] [PubMed] [Google Scholar]
- 17.Steiner DF, Tager HS, Nanjo K, Chan SJ, Rubenstein AH. In: The Metabolic Basis of Inherited Disease. 7th Ed. Scriver C, Beaudet A, Sly W, Valle D, editors. Vol 1. New York: McGraw–Hill; 1995. pp. 897–904. [Google Scholar]
- 18.Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJG, Proks P, Shimomura K, et al. Am J Hum Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Diabetes. 2007;56:2178–2182. [Google Scholar]
- 20.Lipkind G, Steiner DF. Biochemistry. 1999;38:890–896. doi: 10.1021/bi981556q. [DOI] [PubMed] [Google Scholar]
- 21.Qiao ZS, Min CY, Hua QX, Weiss MA, Feng YM. J Biol Chem. 2003;278:17800–17809. doi: 10.1074/jbc.M300906200. [DOI] [PubMed] [Google Scholar]
- 22.Hua QX, Mayer JP, Jia W, Zhang J, Weiss MA. J Biol Chem. 2006;281:28131–28142. doi: 10.1074/jbc.M602616200. [DOI] [PubMed] [Google Scholar]
- 23.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Diabetes. 2003;52:409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa SH, Zhao M, Hua QX, Hu SQ, Wan ZL, Jia W, Weiss MA. Biochemistry. 2005;44:4984–4999. doi: 10.1021/bi048025o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua QX, Nakagawa S, Hu SQ, Jia W, Wang S, Weiss MA. J Biol Chem. 2006;281:24900–24909. doi: 10.1074/jbc.M602691200. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa SH, Hua QX, Hu SQ, Jia W, Wang S, Katsoyannis PG, Weiss MA. J Biol Chem. 2006;281:22386–22396. doi: 10.1074/jbc.M603547200. [DOI] [PubMed] [Google Scholar]
- 27.Green N, Fang H, Miles S, Lively MO. In: Cotranslational and Posttranslational Proteolysis of Proteins. Dalbey RE, Sigman DS, editors. Vol XXII. San Diego: Academic; 2001. pp. 58–76. [Google Scholar]
- 28.Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, Schmahl W, Quintanilla-Fend L. Genetics. 2007;175:725–736. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. J Clin Invest. 1990;86:1084–1087. doi: 10.1172/JCI114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R. Diabetes. 2007;56:1268–1276. doi: 10.2337/db06-0658. [DOI] [PubMed] [Google Scholar]
- 31.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Wek RC, Anthony TG. Cell Metab. 2006;4:333–334. doi: 10.1016/j.cmet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell JR, Weeks DE. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, de Bakker PIW, Daly MJ, Sham PC. Am J Hum Genet. 2007;81:559–579. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A. Nat Genet. 2000;25:12–13. doi: 10.1038/75514. [DOI] [PubMed] [Google Scholar]
- 37.Bell GI, Pictet RL, Rutter WJ, Cordell B, Tischer E, Goodman HM. Nature. 1980;284:26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.