Abstract
The factors involved in the differentiation of memory CD4 T cells from naïve precursors are poorly understood. We developed a system to examine the effect of increased competition for antigen by CD4 T cells on the generation of memory in response to infection with a recombinant vesicular stomatitis virus. Competition was initially regulated by increasing the precursor frequency of adoptively transferred naïve T cell antigen receptor transgenic CD4 T cells. Despite robust proliferation at high precursor frequencies, memory CD4 T cells did not develop, whereas decreasing the input number of naïve CD4 T cells promoted memory development after infection. The lack of memory development was linked to reduced blastogenesis and poor effector cell induction, but not to initial recruitment or proliferation of antigen-specific CD4 T cells. To prove that availability of antigen alone could regulate memory CD4 T cell development, we used treatment with an mAb specific for the epitope recognized by the transferred CD4 T cells. At high doses, this mAb effectively inhibited the antigen-specific CD4 T cell response. However, at a very low dose of mAb, primary CD4 T cell expansion was unaffected, although memory development was dramatically reduced. Moreover, the induction of effector function was concomitantly inhibited. Thus, competition for antigen during CD4 T cell priming is a major contributing factor to the development of the memory CD4 T cell pool.
Keywords: infection, effector, differentiation, precursor frequency
Although many of the requisites for T cell activation and expansion are known, the factors that contribute to the development of CD4 T cell memory after infection remain largely undefined. After exposure to a pathogen, antigen-presenting cells (APCs) initiate the activation of T cells by providing access to antigen and costimulatory molecules. Naïve T cells must integrate signals initiated by ligation of the T cell antigen receptor (TCR) by MHC–antigen complexes for the programming of proliferation and differentiation to occur. However, the precise mechanism by which an activated CD4 T cell differentiates to a memory cell remains unknown. For CD8 T cells, a brief period of stimulation has been shown to trigger activation and proliferation of the cells, whereas longer periods of stimulation are required to generate optimal effector function (1–4). In apparent contrast to CD8 T cells, naive CD4 T cells are believed to require longer periods of stimulation to initiate proliferation (5–7) and differentiation into effector cells (8, 9). Additionally, in vivo studies indicate that shortening the length of Listeria monocytogenes infection by antibiotic treatment can affect CD4 T cell activation and development of memory T cells, but this effect is dependent on infectious dose, suggesting that overall antigen levels may play a role (10, 11). Thus, divergent mechanisms seem to regulate the differentiation of CD4 and CD8 T cells.
After initial activation, the transition from naïve to memory CD4 T cell may be due in part to a stochastic process whereby activated T cells must compete for additional signals or other extrinsic factors, such as cytokines, to be rescued from deletion. In vitro, CD4 T cells require an early antigen-dependent phase for activation and proliferation followed by an antigen-independent phase dependent on IL-2 for continued expansion and differentiation to occur (12). In vivo studies also support a role for IL-2 in driving optimal CD8 T cell expansion, but not in initiation of T cell cycling (13). Additionally, IL-7 is important in the generation and survival of both memory CD4 and CD8 T cells (14–17). Similarly, IL-15 plays a role in the maintenance of memory CD8 (18) and CD4 (19) T cells. Thus, although the initial events driving CD4 versus CD8 T cell activation may exhibit distinct characteristics, similarities in the development and maintenance of memory CD4 and CD8 T cells are also evident.
Efforts to investigate T cell function and differentiation in response to antigen, particularly for CD4 T cells, have involved the use of the adoptive transfer of antigen-specific TCR transgenic T cells. This is due in large part to the lack of an extensive panel of MHC class II tetramers to monitor endogenous CD4 T cell responses directly ex vivo. However, adoptive transfer increases naïve precursor frequencies, which can result in reduced T cell division as well as decreased cytokine production, as compared with responses obtained after the transfer of lower numbers of T cells (20, 21). A recent report also found that the survival of transferred naïve CD4 T cells was inversely related to precursor frequency (22). Additionally, our group has demonstrated that CD8 T cell memory phenotypes and the proliferative capacity of memory cells are altered with respect to the differentiation of effector versus central memory lineages when different numbers of TCR transgenic CD8 T cells are transferred (23). Therefore, it is now evident that precursor frequency must be taken into account when investigating T cell responses in vivo, and it suggests that competition at some level is responsible for these differences. Indeed, several studies have demonstrated that competition plays a role during the immune response in establishing immunodominance as well as T cell function (21, 24–27). After immunization, responding T cells of multiple specificities must compete for access to MHC–antigen complexes on antigen-bearing APCs (24, 25). T cells can compete with other T cells of either the same (25, 26) or differing (24, 27) specificities, resulting in the dampening of responses to other epitopes. In the case of CD8 T cells, competitive events for dendritic cell (DC) access occur surprisingly early, within a few hours after immunization (27). Moreover, when the amount of antigen is limiting, CD4 T cells can inhibit the CD8 T cell response, indicating that competition between subsets of T cells may also alter the outcome of the response (28). Increasing the amount of antigen (20, 25) or the number of peptide-loaded APCs (26, 27, 29) can overcome the effects of competition, suggesting that a key factor responsible for T cell competition is the availability of antigen and/or APCs. A recent and elegant study using real-time imaging demonstrated that increasing CD4 T cell precursor frequencies resulted in a decrease in stable conjugate formation with DCs, based on competition for a limited number of MHC–peptide complexes (29). Thus, the characteristics of primary CD4 and CD8 T cell activation are influenced by initial precursor frequency.
Although it is clear that clonal competition can affect T cell priming and CD8 memory development, little is known about the relationship between competition for antigen during CD4 T cell priming and the development of memory cells after infection. This led us to question how antigen availability during the priming of CD4 T cells would affect downstream cell differentiation events. To experimentally determine whether intraclonal competition for antigen during infection influences the development of memory CD4 T cells, we altered initial precursor frequency and also used an Ab blocking system to limit antigen availability in vivo. Our results indicated that clonal competition and antigen levels during early T cell priming events were critical for the successful downstream generation of memory CD4+ T cells.
Results
Precursor Frequency Controls Memory Development.
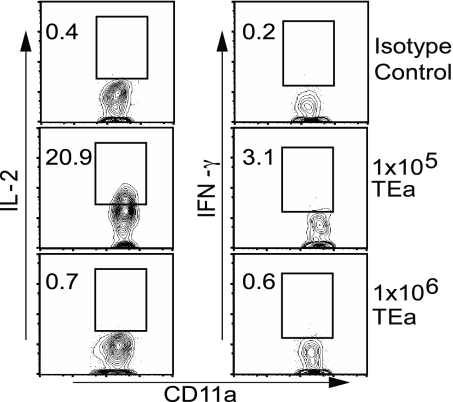
To address the role of precursor frequency in memory CD4 T cell development, we used an adoptive transfer system in conjunction with virus infection. TCR transgenic TEa CD4 T cells are specific for an Eα-derived peptide (amino acid residues 52–68) in the context of MHC class II molecule I-Ab (30). To allow activation of TEa cells in response to infection, we produced a recombinant vesicular stomatitis virus (VSV) containing a gene cassette encoding the ovalbumin-derived peptide SIINFEKL, the Eα peptide (amino acid residues 52–68), and DsRed (VSV-SED). Varying numbers of CD45.2 TEa cells were transferred into congenic CD45.1 C57BL/6 mice 1 day before infection with VSV-SED, and the TEa response was measured at various time points. Analysis of blood revealed that, whether 1 × 105 or 1 × 106 TEa cells were transferred, donor CD4 TEa T cells expanded to the same extent by ≈6 days after infection (Fig. 1 A and B). The peak of the response occurred on days 5–6 with both transfer frequencies (Fig. 1B). Surprisingly, after the contraction phase, memory cells were barely detectable at day 16 and not detectable at day 30 in the blood after infection (Fig. 1 A and B) if 1 × 106 TEa cells had been transferred. In contrast, when either 1 × 105 or 1 × 104 TEa cells were transferred, a population of memory cells was detectable in the blood (Fig. 1 A and B) and tissues (data not shown) at 30 days after infection. In addition, ≈2 months after infection, TEa memory populations were present in the spleen, lung, liver, and intestinal lamina propria (LP) of mice that had received 1 × 105 or 1 × 104 TEa cells (Fig. 1C). Furthermore, splenic memory TEa cells produced IL-2 and IFN-γ when restimulated with the Eα peptide in vitro (Fig. 1D), indicating that the memory cells were functional. Thus, a high initial precursor frequency resulted in a lack of memory cell development after activation, despite robust expansion.
Fig. 1.
CD4 T cell precursor frequency affects memory T cell development in response to virus infection. CD45.2 TEa CD4 T cells (1 × 106, 1 × 105, or 1 × 104 cells) were transferred into CD45.1 mice before infection with VSV-SED. (A) The TEa response was assessed at days 7, 16, and 30 after infection. (The asterisk indicates that mice from the 1 × 106 transfers were bled at day 28 after infection.) Numbers indicate the percentage of donor CD4 T cells (CD45.2+) relative to the total CD4 population in the blood. (B) Comparison of the percentage of donor cells within the entire CD4 population in response to VSV-SED when either 1 × 106 or 1 × 105 TEa cells were initially transferred. Days 3, 4, and 5 after infection represent the lymphocyte population from the spleen, whereas days 7, 16, and 30 represent donor CD4 T cells from the blood. (C) The presence of TEa memory cells was determined in the indicated tissues 58 days after infection. (D) The production of IL-2 and IFN-γ by TEa cells (1 × 105 cells transferred) isolated from the spleen 56 days after infection was determined by intracellular cytokine staining after in vitro stimulation with the Eα peptide. The values in the upper right quadrants represent the percentages of positive CD4 cells.
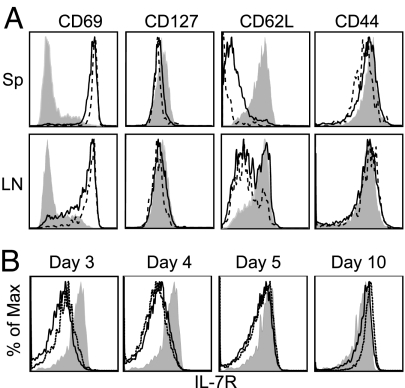
To begin to address the reasons for the disparity in memory development based on CD4 T cell input number, we analyzed the activation status of transferred T cells at 24 h after infection. Donor TEa cells from the spleen and lymph nodes (LNs) of mice that received either 1 × 106 or 1 × 105 TEa cells were assessed for cell surface expression of CD69, CD127, CD62L, and CD44. CD69 (an early activation marker) was up-regulated on nearly all donor CD4 T cells in the spleens and LNs of both groups, indicating that regardless of frequency TEa cells were antigen-experienced by 24 h after infection (Fig. 2A). CD127 (IL-7Rα) expression was slightly reduced in both groups at 24 h after infection. Interestingly, splenic and LN TEa cells from the low precursor frequency transfers had down-regulated CD62L to a much greater extent than TEa cells derived from high precursor frequencies (Fig. 2A). CD44 expression had not changed from naïve CD4 T cell levels at this time point.
Fig. 2.
Effect of initial precursor frequency on early activation phenotype and IL7R expression during the primary response. (A) Either 1 × 106 (solid line) or 1 × 105 (dashed line) CD45.2 TEa cells were transferred to CD45.1 mice that were infected 1 day later with VSV-SED. Cells were isolated 24 h later from spleen (Upper) and peripheral lymph nodes (LNs) (Lower), and donor cells were analyzed for expression of the indicated proteins. Shaded histograms represent naïve transferred TEa cells. (B) Either 1 × 106 (solid line) or 1 × 105 (dashed line) CD45.2 TEa cells were transferred as above. Mice were infected with 1 × 105 pfu of VSV-SED, and spleens were harvested on days 3, 4, 5, and 10 after infection. CD4+ CD45.2+ TEa cells were analyzed for IL-7Rα expression by flow cytometry. Shaded histograms represent naïve TEa cells.
We also examined the kinetics of IL-7Rα expression by responding TEa cells, because IL-7 has been shown to be important for CD4 memory T cell development or survival (15–17, 31). Therefore, we determined whether the lack of memory generation after high precursor frequency transfers was linked to differences in IL-7R expression. In both the high and low precursor frequency transfers, IL-7R expression was down-regulated by most cells by day 3 after infection (Fig. 2B). By day 4 and particularly by day 5 after infection, IL-7R expression by cells from both groups had increased as compared with day 3. By day 10, all remaining cells expressed levels of IL-7R that were increased over that of naïve TEa cells, as has been previously shown for cells transiting to memory CD8 T cell development (14, 32, 33). Therefore, regulation of IL-7R expression was not linked to initial precursor frequency and did not correlate with memory cell development.
Precursor Frequency Affects Cell Division and Blastogenesis.
Because only cells from the low precursor frequency transfers differentiated to CD4 memory cells despite robust proliferation in both the low and high precursor frequency transfers, the fate of the activated T cells may be programmed early during the response. The input numbers differed by 10-fold, but the responses were similar in magnitude: This suggested that the cells from the low precursor frequency underwent more rounds of division. To test this possibility, the division history and blastogenesis of carboxyfluoroscein succinimidyl ester (CFSE)-labeled TEa cells were investigated. By day 3 after infection, TEa cells had divided equally regardless of initial frequency. However, from then on, the proliferation of TEa cells derived from a high input frequency consistently lagged behind that of the TEa cells derived from a low input number. The latter had essentially lost CFSE fluorescence by day 5 (Fig. 3A).
Fig. 3.
Increased precursor frequency limits blastogenesis but not early division in response to infection. Naïve CD45.2 TEa cells were CFSE-labeled and transferred into CD45.1 mice that were then infected with VSV-SED 1 day later. Spleen cells were analyzed for the presence of CD45.2 TEa cells on the indicated days, and donor cells were analyzed for CFSE dilution (A) and forward light scatter (FSC) properties (B) by flow cytometry. Solid line, 1 × 106 TEa cells transferred; dashed line, 1 × 105 TEa cells transferred. Shaded histograms represent naïve CFSE-labeled TEa cells.
When blastogenesis was examined, a striking difference was observed between responding CD4 T cells derived from the high versus low precursor transfers (Fig. 3B). After low frequency transfer, TEa cells underwent sustained blastogenesis through day 4 and subsequently underwent a major size reduction. However, when higher numbers of TEa cells were transferred, blastogenesis occurred but was not sustained, and the blasting cells were smaller than those found after the transfer of lower cell numbers. At day 10 after infection, the remaining cells from both groups were slightly larger than naïve cells (Fig. 3B). Thus, increasing the initial TEa precursor frequency resulted in diminished proliferation as an apparent result of transient blastogenesis.
Effect of Precursor Frequency on Induction of Effector Function.
To determine whether the defects in cell division and blastogenesis correlated with effector function, cytokine production by the donor CD4 T cells was measured. Intracellular cytokine staining for IL-2 and IFN-γ at day 5 after infection in response to Eα peptide stimulation in vitro revealed that, in the high precursor frequency transfers, the TEa cells produced much less IL-2 and IFN-γ when compared with the cells responding in a low precursor transfer, which produced substantial amounts of IL-2 and IFN-γ (Fig. 4). However, this finding did not rule out the possibility that the donor cells from the high precursor frequency transfer group did not produce similar levels of cytokine at an earlier time point after infection. To address this issue, cytokine production was measured at 24 h after infection directly ex vivo following the in vivo administration of brefeldin A (BFA) (34). Mice were infected with VSV-SED 1 day after adoptive transfer and then treated with BFA 18 h later; tissues were harvested 6 h after BFA treatment. IL-2 was produced by ≈20% of the TEa cells in mice that had received the low number of naïve cells, although much of the population had shifted upward as compared with controls, suggesting that more cells were producing lower amounts of IL-2. In contrast, TEa cells in mice that had received a higher cell number did not produce detectable IL-2 (Fig. 5). IFN-γ was not produced at this time point by cells from either group. These results suggested that early events were regulating T cell function and memory development.
Fig. 4.
Naïve CD4 T cell precursor frequency modulates induction of effector function. Splenocytes from mice receiving 1 × 106 or 1 × 105 TEa cells were analyzed 5 days after VSV-SED infection for the production of IL-2 and IFN-γ by intracellular cytokine staining after Eα peptide stimulation in vitro. Numbers indicate the percentage of TEa cells producing IL-2 (Left) or IFN-γ (Right).
Fig. 5.
IL-2 production early after infection is regulated by CD4 T cell precursor frequency. The indicated number of CD45.2 TEa cells was transferred to CD45.1 mice that were infected 24 h later with VSV-SED. The mice were injected i.v. with 250 μg of BFA 18 h later. TEa cells in the spleen were analyzed 6 h later by intracellular cytokine staining directly ex vivo. Numbers represent the percentage of TEa CD4 T cells producing IL-2 (Left) or IFN-γ (Right).
Competition for Antigen During CD4 T Cell Priming Does Not Affect Expansion but Inhibits Memory T Cell Generation.
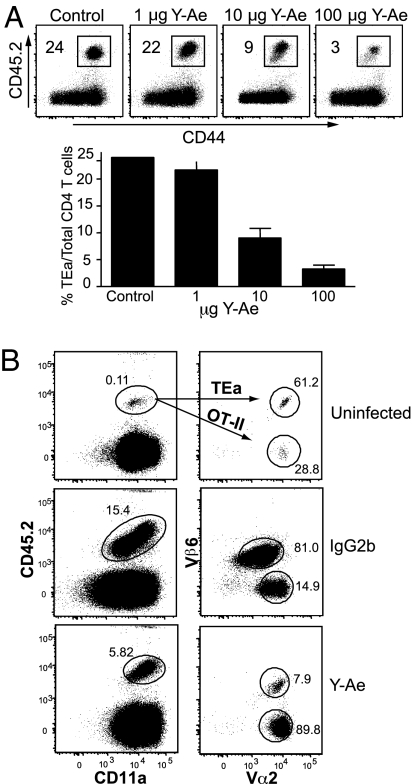
Based on the above data and previous reports (21, 24–27) demonstrating that T cells compete for antigen during the immune response, we hypothesized that competition for access to antigen during the priming of T cells is important in programming the development of memory T cells. To dissociate competition for antigen from other events, we used the Y-Ae mAb specific for MHC class II Eα peptide complexes (35, 36). The Y-Ae mAb blocks antigen presentation in vitro (37–39) and interferes with negative selection in the thymus in transgenic systems (38). Therefore, we tested whether Y-Ae treatment interfered with Eα presentation to TEa cells during infection. To test the efficacy of Y-Ae mAb treatment, 1 × 105 TEa cells were transferred, and 1 day later the recipients were injected i.p. with 100 μg of Y-Ae mAb and infected with VSV-SED. TEa T cell expansion 6 days after infection was inhibited by ≈90% in mice treated with 100 μg of Y-Ae mAb; this inhibition diminished with decreasing mAb doses such that 1 μg of Y-Ae had no effect on TEa expansion (Fig. 6A). We excluded the possibility that Y-Ae was depleting antigen-bearing APCs or T cells by treating mice that had received Eα peptide-loaded splenocytes with Y-Ae and showing that mAb treatment did not result in loss of the cells (data not shown). Moreover, Y-Ae treatment had no effect on the CD8 T cell response to ovalbumin or VSV nucleoprotein (data not shown). To further substantiate the possibility that Y-Ae affected “bystander” CD4 T cell responses, we used a double adoptive transfer system. Equal numbers of OT-II TCR transgenic CD4 T cells (40), specific for the albumin peptide (amino acid residues 323–339) in the context of MHC class II molecule I-Ab, and TEa TCR transgenic CD4 T cells were cotransferred into congenic mice. Mice were treated 1 day later with either 250 μg of the Y-Ae mAb or isotype mouse IgG2b and coinfected with 1 × 105 pfu of VSV-SED and VSV-OVA to provide the specific epitopes to both the TEa and OT-II CD4 T cells. In the isotype-treated group, donor OT-II and TEa cells proliferated extensively by day 6 after infection compared with uninfected mice (Fig. 6B). In contrast, Y-Ae mAb treatment inhibited expansion of TEa cells, whereas OT-II cell expansion remained robust (Fig. 6B). In fact, the OT-II response was somewhat greater when the TEa response was inhibited, indicating interclonal competition. These data demonstrated that the mAb did not exert global effects on antigen presentation but selectively inhibited the antigen-specific TEa response.
Fig. 6.
Y-Ae mAb limits CD4 T cell expansion in vivo in a dose-dependent and antigen-specific manner. (A Upper) Mice that received 1 × 105 TEa cells were treated with 100 μg of control mouse IgG2b or 1, 10, or 100 μg of the Y-Ae mAb immediately before VSV-SED infection. Donor T cell expansion in the spleen was assessed 6 days after infection. Numbers indicate the percentage of donor T cells of the total CD4 population. (Lower) Graphical representation of the flow cytometry data shown in Upper. Error bars indicate the standard error. (B) Y-Ae specifically blocks antigen presentation to Eα-specific CD4 T cells. TEa cells (1 × 105) and OT-II CD4 T cells (1 × 105) were cotransferred into CD45.1 C57BL/6 mice. Mice were then treated with 250 μg of the Y-Ae or control mouse IgG2b Ab before coinfection with VSV-SED and VSV-OVA (1 × 105 pfu). At day 6 after infection, expansion of TEa and OT-II cells was assessed via flow cytometry. Differentiation of TEa (CD45.2+ Vα2+Vβ6+) from OT-II (CD45.2+ Vα2+ Vβ6−) cells was attained by differential expression of the Vα and Vβ TCR chains.
Taking advantage of the inhibitory effects of the Y-Ae Ab on Eα antigen presentation, we sought to determine the effect of competition for antigen by T cells on priming and memory development. To this end, 1 × 105 TEa T cells were adoptively transferred into congenic recipients, and 1 μg of Y-Ae Ab was administered to the recipient mice before VSV-SED infection. This dosage of Ab treatment was chosen because it did not effect the expansion of the TEa cells relative to control Ab treatment (Fig. 6A). During the primary and memory phases of the immune response, donor cell populations were measured in both lymphoid and nonlymphoid tissues. With or without Y-Ae treatment, donor cells expanded equally well in both lymphoid and nonlymphoid compartments (Fig. 7 A and B Upper). Remarkably, treatment with only 1 μg of Y-Ae resulted in dramatic effects on the ability of responding TEa cells to produce a memory population, such that ≈10-fold fewer memory cells were produced (Fig. 7 A and B Lower). Therefore, although reducing the level of antigenic stimulation had no effect on clonal expansion, memory differentiation was altered, demonstrating that competition for antigen during the primary immune response is critical for the differentiation of memory cells.
Fig. 7.
Y-Ae treatment at the time of infection inhibits generation of memory TEa cells. (A) Recipient mice that received 1 × 105 TEa cells were treated i.p. with 1 μg of Y-Ae or 1 μg of mouse IgG2b before VSV-SED infection. Cells from the spleen and lung were analyzed for the presence of TEa cells at day 5 (primary) or day 23–24 (memory) after infection. Numbers indicate the percentage of the donor TEa population of the total CD4 population (values represent the mean of three mice for primary and eight mice for memory experiments). (B) Graphical representation of data presented in A. *, P < 0.05. (C) Cytokine production by TEa cells in Y-Ae-treated mice. TEa T cells (1 × 105) were transferred into congenic recipients. Before infection with VSV-SED, mice were treated with 1–2 μg of Y-Ae mAb (n = 6) or IgG2b control Ab (n = 3). TEa cells were analyzed 5 days later for intracellular cytokine production after in vitro peptide stimulation.
Because we had observed a defect in cytokine production after the transfer of high numbers of TEa cells, we also wished to determine whether a defect in cytokine production occurred after Y-Ae mAb treatment. As shown in Fig. 7C, responding TEa cells in mice treated with Y-Ae exhibited reduced IL-2 and IFN-γ production as compared with isotype control mAb-treated mice. These results correlated with the results from high precursor frequency transfers where the TEa cells also had a muted cytokine response compared with cells derived from a lower precursor frequency (Fig. 4). These data further substantiated the role of competition for antigen early during the response to virus infection as a key component in the programming of memory CD4 T cell differentiation.
Discussion
The work presented here sought to determine the role of competition for antigen in the development of memory CD4 T cells in response to virus infection. The presence of fewer naïve antigen-specific CD4 T cells at the time of infection favored the development of memory T cells. A memory population developed over a 100-fold range of input precursors (1 × 103 to 1 × 105) (Fig. 1 and data not shown). However, a threshold was reached at an input of 1 × 106 TEa TCR transgenic CD4 T cells such that memory development was essentially blocked. This result was somewhat surprising, given the robust expansion that occurred in response to virus infection after the transfer of 1 × 106 cells. In addition, IL-7R regulation was independent of initial precursor frequency and was therefore not directly linked to memory development. It remains possible that IL-7R is necessary but not solely sufficient for memory CD4 T cell development, as has been suggested for memory CD8 T cell development in some cases (41, 42).
Recent studies have shown that, for CD4 and CD8 T cells, competition during the primary immune response can occur at multiple levels (27, 29). In these systems, immunization was accomplished by the adoptive transfer of peptide-coated DCs activated in vitro. CD8 T cells competed for access to the same DCs during the first few hours after immunization in an antigen-nonspecific manner. For CD4 T cells, competition for antigen-bearing DCs occurred at increased T cell precursor frequency, resulting in abbreviated T cell–DC interactions. In these cases, T cells gained access to the DCs but competed for MHC–peptide complexes (29). Whether these competitive events altered memory CD4 or CD8 T cell development was not examined. With respect to memory CD4 T cell development, a recent, elegant study using adoptive transfer of TCR transgenic T cells and peptide/LPS immunization revealed that increased clonal abundance, even beyond the primary response, resulted in decreased survival (22). It is unknown which factors are limiting in this case, although a likely candidate is ongoing TCR–MHC interactions (22). In our system, antigen presentation, inflammation, and the kinetics of the overall response are dictated by the infectious cycle of the virus and the input number of naïve CD4 T cells. Moreover, the use of the Y-Ae mAb to specifically block a single MHC–peptide–TCR interaction provided a highly direct method to test the role of antigen levels in memory CD4 T cell development. Y-Ae treatment had no effect on the concomitant CD8 T cell response to virus-encoded antigens, nor did it hinder antigen presentation to other TCR transgenic CD4 T cells, indicating that the mAb was not disrupting generalized APC function.
In our studies, high initial CD4 T cell precursor frequency led to an intrinsic defect in the T cells and resulted in reduced division and cytokine production and in the lack of a detectable memory population. These data corroborated the results of other studies showing that changes in T cell precursor frequency can result in differences in T cell division and induction of effector function (20, 21) and that T cell competition could be overcome with the addition of increased amounts of antigen (20, 25) or antigen-bearing DCs (27). In those studies, a direct demonstration of competition for antigen was not possible, because competition for access to DCs, as well as for other factors, could be involved. We hypothesized that the advantage resulting in enhanced function and survival at low precursor frequencies was afforded by decreased competition for antigen during early phases of the immune response. Remarkably, at a precursor frequency that normally resulted in memory generation, the CD4 T cell response to virus infection in the face of administration of a small amount (1 μg) of Y-Ae mAb mimicked the response obtained at a high input frequency. Thus, we recapitulated the phenomenon observed in the high precursor frequency transfers by limiting Eα presentation to the TEa cells. The fact that limiting antigen availability did not affect proliferation, yet hindered further differentiation of T cells, suggested that the signaling threshold for proliferation of naïve T cells was lower than the threshold necessary to drive the differentiation of memory cells. Thus, although high precursor frequencies may result in competition for factors other than antigen, limiting only antigen availability altered memory T cell development. When interpreting results obtained with other TCR transgenic T cells or polyclonal T cells, it should be considered that each T cell clone will exhibit a distinct TCR avidity that will likely result in distinct competitive characteristics in vivo. Competition will also be dictated by the stability of MHC–peptide complexes as well as the quantity of each antigenic moiety available. Therefore, we predict that modification of these variables, either singly or in combination, will determine the efficacy of memory T cell development. Our results also suggested that effector development was inexorably linked to memory development. Other work has suggested that, for CD4 T cells, cells producing effector cytokines do not produce memory cells (43), although other studies do not support this concept (44). Although we cannot conclude that effector cells are the direct antecedents of memory CD4 T cells, our findings indicated effector development at the population level was requisite for efficient memory CD4 T cell development.
There are at least two scenarios by which limited availability of antigen to T cells could result in incomplete differentiation of the activated T cells. Limited antigen presentation to naïve T cells may result in insufficient signaling through the TCR, because all of the cells are competing for a finite amount of antigen. In this case, the Y-Ae mAb treatment may decrease the antigen density per APC, further reducing the quality of signaling through the TCR. Alternatively, limiting antigen availability may also effect the T cells by limiting the number of subsequent interactions that may occur between activated T cells and antigen-bearing APCs. These subsequent interactions early during the immune response have been recently shown to be important with regard to T cell differentiation (9). Thus, the integration of signals mediated by sustained initial TCR–APC interactions and potentially by multiple T cell–APC interactions will be modulated by limiting the antigen display, thereby affecting T cell differentiation toward the memory lineage. Understanding the factors responsible for efficient memory T cell development is essential to the development of efficacious vaccines. In addition, the possibility of specifically modifying single variables of the immune response may hold implications for immunotherapy.
Materials and Methods
Mice.
C57BL/6J (CD45.1) mice were purchased from Charles River Laboratories/National Cancer Institute (Wilmington, MA). TEa TCR transgenic mice (30) specific for Eα peptide (amino acid residues 52–68) from the I-Eα MHC class II molecule in the context of I-Ab were provided by R. Noelle (Dartmouth Medical School, Lebanon, NH) and bred onto a recombinase-activating gene (RAG)-deficient background. OT-II TCR transgenic mice (40), specific for chicken ovalbumin peptide (amino acid residues 323–339) in the context of MHC class II, were bred onto a RAG-deficient background. Animal protocols were approved by the University of Connecticut Health Center Animal Care Committee.
Sample Preparation and Flow Cytometry.
To isolate lymphocytes from lung, liver, and intestinal lamina propria (LP), tissues were collected from perfused mice, minced, and collagenase-digested for one hour at 37°C. Liver and LP cells were then subjected to Percoll gradient separation. For staining, cells were resuspended in 0.2% BSA and 0.01% NaN3 in PBS at a concentration of ≈1 × 107 cells per milliliter. mAbs were obtained from BD PharMingen (San Diego, CA), eBioscience (San Diego, CA), and Caltag (Burlingame, CA), and cytometry data were gathered with a FACSCalibur or LSR II (BD Biosciences, San Jose, CA). Data were analyzed by using FlowJo software (Tree Star, Ashland, OR). CFSE labeling of TEa cells was performed as previously described (45).
Infection, Adoptive Transfer, and mAb Blocking.
To produce recombinant VSV, PCR was used to generate a product encoding SIINFEKL and Eα upstream of DsRed2 with flanking XhoI and NheI restriction sites; this product was then cloned into pVSVXN2. The resulting pVSV-SED was then used to generate recombinant VSV as previously described (46). For adoptive transfer, TEa-RAG−/− cells were injected i.v. followed 24 h later by i.v. infection with 1 × 105 VSV-SED. Purified Y-Ae mAb (35) was purchased from the National Cell Culture Center (Minneapolis, MN) and diluted in PBS. Y-Ae mAb was administered i.p. in 500 μl of PBS before infection. A mouse IgG2b Ab (eBioscience) was used as isotype control. Similarly, in the cotransfer experiment, equal numbers (1 × 105) of TEa and OT-II cells were cotransferred into recipient mice. Mice received the Y-Ae mAb or isotype control 1 day later and were infected with 1 × 105 pfu of VSV-SED and VSV-OVA (47).
Intracellular Cytokine Staining.
Lymphocytes from indicated tissues were isolated and restimulated in vitro with or without 10 μg/ml of Eα peptide and GolgiStop (BD PharMingen) for 5 h at 37°C. Cells were then stained for surface markers, fixed, permeabilized in Cytofix/Cytoperm (BD PharMingen), and stained with anti-IL-2, anti-IFN-γ, or isotype control mAb (BD PharMingen). Alternatively, mice were treated with BFA (Sigma, St. Louis, MO) i.v. 6 h before killing to directly assess cytokine production as previously described (34).
Acknowledgments
We thank Quynh-Mai Pham for expert technical assistance. This work was supported by National Institutes of Health Grants AI41576, DK45260, and T32 AI07080 (to D.A.B.).
Abbreviations
- APC
antigen-presenting cell
- TCR
T cell antigen receptor
- DC
dendritic cell
- VSV
vesicular stomatitis virus
- CFSE
carboxyfluoroscein succinimidyl ester
- BFA
brefeldin A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Stipdonk MJ, Lemmens EE, Schoenberger SP. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 4.Van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 5.Iezzi G, Karjalainen K, Lanzavecchia A. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 6.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 7.Obst R, van Santen HM, Mathis D, Benoist C. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajenoff M, Wurtz O, Guerder S. J Immunol. 2002;168:1723–1729. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 9.Celli S, Garcia Z, Bousso P. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbin GA, Harty JT. J Immunol. 2004;173:5679–5687. doi: 10.4049/jimmunol.173.9.5679. [DOI] [PubMed] [Google Scholar]
- 11.Williams MA, Bevan MJ. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 12.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. J Immunol. 2000;165:5017–5026. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 13.D'Souza WN, Lefrancois L. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Huston G, Swain SL. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddon B, Tomlinson P, Zamoyska R. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 18.Schluns KS, Lefrancois L. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 19.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foulds KE, Shen H. J Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 22.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 23.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butz EA, Bevan MJ. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AL, Wikstrom ME, Fazekas de St Groth B. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 26.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis RA, Kappler JW, Marrack PC. Proc Natl Acad Sci USA. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cose S, Brammer C, Zammit DJ, Blair DA, Lefrancois L. Int Immunol. 2006;18:1285–1293. doi: 10.1093/intimm/dxl061. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Proc Natl Acad Sci USA. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubin CE, Kovats S, deRoos P, Rudensky AY. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 31.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 33.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Whitton JL. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 35.Rudensky AY, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DB, Rath S, Pizzo E, Rudensky AY, George A, Larson JK, Janeway CA., Jr J Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- 37.Chervonsky AV, Medzhitov RM, Denzin LK, Barlow AK, Rudensky AY, Janeway CA., Jr Proc Natl Acad Sci USA. 1998;95:10094–10099. doi: 10.1073/pnas.95.17.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viret C, Janeway CA., Jr J Immunol. 2000;164:4627–4634. doi: 10.4049/jimmunol.164.9.4627. [DOI] [PubMed] [Google Scholar]
- 39.Viret C, Wong FS, Janeway CA., Jr Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 40.Barnden M, Allison J, Heath WR, Carbone FR. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 41.Lacombe MH, Hardy MP, Rooney J, Labrecque N. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 42.Sun JC, Lehar SM, Bevan MJ. J Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 44.Swain SL. Microbes Infect. 2003;5:213–219. doi: 10.1016/s1286-4579(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 45.Lyons AB, Parish CR. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 46.Lawson ND, Stillman EA, Whitt MA, Rose JK. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrançois L. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]