Abstract
Naturally occurring Foxp3+CD4+CD25+ T cells (nTregs) isolated from lungs of naive mice regulate allergic airway hyperresponsiveness (AHR) and inflammation. Here, we demonstrate the critical requirement for engagement of MHC class I on CD4+CD25+ T cells by CD8 for the functional activation of these nTregs. Suppression of allergen-induced AHR and inflammation by nTregs was abolished in mice treated with anti-CD8. Correspondingly, decreased levels of IL-10 and TGF-β and increased levels of Th2 cytokines in bronchoalveolar lavage were detected in these treated mice. Similarly, nTregs isolated from β2m−/− mice or from mice treated with anti-MHC I antibody in vitro before intratracheal transfer failed to modulate AHR or inflammation. Coculture of nTregs with CD8+ T cells increased IL-10 and TGF-β. Addition of anti-MHC I or anti-CD8 reduced IL-10 and TGF-β. These results demonstrate that functional activation of nTregs requires the interaction between MHC I on CD4+CD25+ T cells and CD8.
Keywords: IL-10, TGF-β, airway reactivity
CD4+ T cells can be subdivided on the basis of their ability to modulate inflammatory responses through production and release of certain cytokines. One subset, with constitutive expression of the low-affinity IL-2 receptor α-chain, CD25 (1), has been shown to play prominent roles in dictating the outcome of several diseases (2–11). Th2-like CD4+ T cells producing IL-4, IL-5, and IL-13 play a central role in the pathogenesis of asthma (12–14). Increased airway hyperresponsiveness (AHR) and inflammation, Th2 cytokines, goblet cell metaplasia, excessive mucus production, elevated antigen-specific IgE, and structural changes in the airways are characteristic of allergic asthma. These changes can be prevented by depletion of CD4+ cells (15, 16) or by inhibition and/or alteration of their activities (17, 18). There is increasing evidence for the pivotal role of a subset of CD4+CD25+ T cells in regulating the development and outcome of atopic allergic diseases in animals (9, 19) and humans (20).
The regulatory T cells (Tregs) encompass different subsets that are capable of suppressing cellular immune functions (1, 21–23). CD4+CD25+ T cells, in both humans and mice, comprise a small fraction (5–10%) (1) of CD4+ T cells produced in the thymus (24–26). They are anergic (27, 28) and express CTLA-4 (CD152) (29), glucocorticoid-induced TNF receptor (30), and the transcription factor Foxp3, which appears to be specific for CD4+CD25+ regulatory T cells (25, 26). They have been shown to suppress allergen-driven T cell activation (9, 31, 32) and regulate Th2 immune responses in humans (33) and animals (34, 35) and modulate both T cell activation and Th2 cytokine skewing (9). Their suppressive activity both in vitro and in vivo appears to be mediated by several mechanisms depending on the model used and includes cell-to-cell contact (27, 36, 37) and the release of IL-10 (9, 38) and TGF-β (9, 39, 40). A possible mechanism of suppression in humans is the cytolytic activity of CD4+CD25+ regulatory T cells that are granzyme- and perforin-mediated (41).
Although the regulatory profiles of CD4+CD25+ T cells have been described in mouse models of allergen-induced AHR and airway inflammation (9, 34, 35), the mechanisms that direct the functional activation of these regulatory activities have not been well defined. In the present study, we investigated the role of MHC I on naturally occurring CD4+CD25+ regulatory T cells (nTregs) and the requirement for interaction with CD8 in the lung and show that interactions between MHC I and CD8 are essential for the expression of the immunoregulatory properties of nTregs on lung allergic responses.
Results
CD4+CD25+ T Cells Suppress AHR and Inflammation Mediated by Primed CD8+ T Cells.
As shown in ref. 43, after sensitization and airway challenge, CD8−/− mice developed significantly lower AHR (Fig. 1A) and significantly reduced airway eosinophilia (Fig. 1D), bronchoalveolar lavage (BAL) Th2 cytokine levels (Fig. 1E), and goblet cell metaplasia (Fig. 1F) when compared with sensitized and challenged WT mice. Adoptive transfer of allergen-primed CD8+ T cells (but not naive CD8+ T cells; data not shown), isolated by either negative or positive selection, into sensitized CD8−/− recipient mice restored full AHR after airway allergen challenge (Fig. 1B), airway eosinophilia (Fig. 1D), BAL IL-5 and IL-13 cytokine levels (Fig. 1E), and goblet cell metaplasia (Fig. 1F) to a similar extent as seen in sensitized and challenged WT mice.
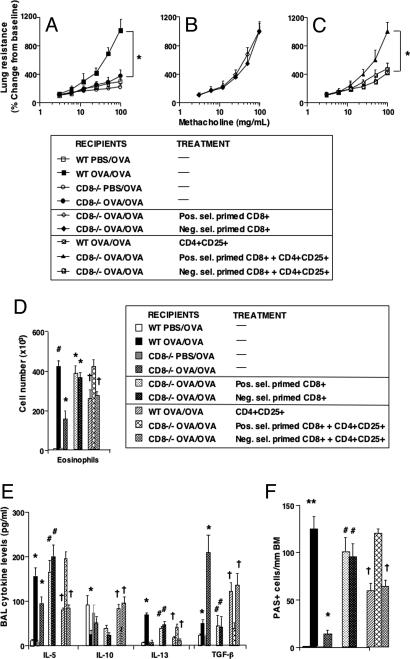
Fig. 1.
Effect of transfer of CD4+CD25+ T cells into sensitized and challenged CD8−/− recipients of positively or negatively selected CD8+ T cells. Sensitized CD8−/− mice received CD8+ T cells and CD4+CD25+ T cells before challenge. (A–C) AHR. (D) BAL eosinophil numbers. (E) BAL cytokine levels. (F) Numbers of PAS+ cells. Shown are the mean ± SEM from three independent experiments (n = 12). (A–C) *, P < 0.05, indicates significant differences between indicated groups. (D) *, P < 0.05; #, P < 0.01, indicates comparison of sensitized and challenged mice with challenged-alone mice and comparison of CD8+-reconstituted recipients with CD8−/− mice; †, indicates comparison of WT and recipients of negatively selected CD8+ T cells that received CD4+CD25+ T cells with those that received positively selected CD8+ T cells. (E) *, P < 0.05 or #, P < 0.01, indicates comparison of sensitized and challenged mice with challenged-alone mice and comparison of CD8+-reconstituted recipients with CD8−/− mice. #, P < 0.05, indicates comparison of recipients of CD8+ T cells with CD8−/− mice. †, P < 0.05, indicates comparison of WT and recipients of negatively selected T cells with recipients of positively selected CD8+ T cells. (F) **, P < 0.01; *, P < 0.05, indicates comparison of sensitized and challenged with challenged-alone mice; #, P < 0.01, indicates comparison of recipients of CD8+ T cells with CD8−/− mice; †, P < 0.05, indicates comparison of recipients of CD4+CD25+ T cells with nontransferred recipients.
Similar to the suppressive effects of nTregs on allergen-induced airway responses in WT mice, intratracheal administration of nTregs into (negatively selected) CD8+ T cell-reconstituted CD8−/− mice before airway allergen challenge also suppressed the development of CD8 T cell-mediated AHR (Fig. 1C) and attenuated airway eosinophilia (Fig. 1D), IL-5 and IL-13 cytokine levels (Fig. 1E), and goblet cell metaplasia (Fig. 1F). Associated with this suppression, BAL levels of IL-10 and TGF-β were significantly increased in these mice (Fig. 1E). The low levels of IL-4 and IFN-γ levels were little changed [supporting information (SI) Fig. 6]. Serum levels of total IgE and allergen-specific IgE, IgG1, IgG2a, and IgG2b antibodies were unchanged in all experiments after intratracheal transfer of nTregs (data not shown). Most surprisingly, the suppression of CD8 T cell-mediated airway responses was only seen in the CD8−/− recipients reconstituted with negatively selected, but not positively selected, CD8+ T cells (Fig. 1 C–F). As determined by flow cytometry, analysis of BAL CD8 T cell numbers after adoptive transfer revealed no CD8+ T cells in the recipients of positively selected CD8 T cells, contrasting with the numbers detected in BAL of WT mice or CD8−/− recipients reconstituted with negatively selected CD8 T cells (SI Fig. 7). These findings suggested that, after challenge of sensitized recipients, transferred CD8+ T cells migrated and accumulated in the lung. On the basis of their ability to reconstitute the CD8−/− mice, we presume that the failure to detect positively selected CD8+ T cells in the lung was because of the masking of CD8 by the anti-CD8-coated microbeads.
Anti-CD8 Attenuates the Regulatory Activity of nTregs.
To confirm the importance of CD8 in nTreg activity, we investigated the consequences of intratracheal administration of anti-CD8α before and immediately after the transfer of nTregs. Intratracheal administration of PBS, control antibody, or anti-CD8α alone to sensitized WT mice before allergen challenge did not alter the development of AHR (Fig. 2A) or airway eosinophilia (SI Fig. 8). However, the suppression of AHR and airway eosinophilia by nTregs was only demonstrated in mice that received control antibody but not the CD8α (or anti-CD8β; data not shown) antibody. In parallel, levels of IL-4, IL-5, and IL-13 were significantly (P < 0.05) increased, and levels of IL-10 and IFN-γ were significantly (P < 0.05) decreased, in the BAL fluids of sensitized and challenged mice given PBS, control antibody, or anti-CD8α (Fig. 2B). Intratracheal transfer of nTregs after administration of control antibody resulted in a significant reduction in levels of IL-4, IL-5, and IL-13 and in increases in levels of IL-10 and TGF-β in the BAL fluid of sensitized and challenged mice. These effects were abolished if the mice were treated with anti-CD8α before transfer of nTregs.
Fig. 2.
Effect of anti-CD8 on CD4+CD25+ regulatory function. Sensitized WT mice were treated with anti-CD8 before and immediately after transfer of CD4+CD25+ nTregs. (A) AHR to inhaled methacholine was monitored. (B) BAL cytokine levels. Results are shown as mean ± SEM from three independent experiments (n = 12). *, P < 0.05; #, P < 0.01, indicates comparison of treatment with control antibody (rat IgG) to treatment with anti-CD8 in recipients of CD4+CD25+ T cells.
Anti-MHC I Inhibits the Regulatory Activity of nTregs.
On the basis of the demonstration of the role of CD8 in the induction of nTreg activities, we investigated the effects of in vitro treatment of lung nTregs with anti-MHC I before adoptive transfer into sensitized and challenged WT recipient mice. To control for the ability of host natural killer (NK) cells to eliminate cells lacking expression of MHC class I molecules (43, 44), we first depleted NK cells (to <0.1% in spleens) in recipient mice. After NK cell depletion, sensitized and challenged WT mice retained the ability to develop significant increases in AHR (Fig. 3A), airway eosinophilia (SI Fig. 9), and BAL Th2 cytokine levels (IL-5, IL-13) (Fig. 3B), similar to mice treated with control antibody.
Fig. 3.
Effect of anti-MHC treatment on nTreg activity. Isolated lung CD4+CD25+ T cells were treated with anti-MHC before transfer into WT recipients, and AHR to methacholine (A) and BAL cytokine levels (B) were determined. In some cases, recipient mice were first treated with anti-NK to deplete NK cells. Results are shown as mean ± SEM from three independent experiments (n = 12). *, P < 0.05; #, P < 0.01, indicates comparison of results in WT mice receiving CD4+CD25+ T cells and treated with anti-MHC, anti-NK, or control antibody.
A large proportion (>90%) of CD4+CD25+ nTregs stained positively with anti-MHC I antibody and were Foxp3+ (SI Fig. 10). In vitro, treatment of isolated nTregs with anti-MHC I before intratracheal transfer into sensitized and challenged recipient mice depleted of NK cells prevented suppression of AHR (Fig. 3A) or airway eosinophilia (SI Fig. 9). Similar results were obtained after transfer of nTregs pretreated with anti-MHC I into recipients that were not treated with the NK antibody. In contrast, untreated nTregs significantly suppressed AHR and eosinophilia in sensitized and challenged WT recipient mice.
Unlike recipients of untreated nTregs, anti-MHC I-treated nTregs failed to reduce the levels of IL-5 or IL-13 or increase the levels of IL-10 and TGF-β in BAL fluid (Fig. 3B). IFN-γ levels were little changed. The results of anti-MHC I treatment were similar in NK-depleted and nondepleted recipients.
MHC I-Deficient nTregs Fail to Regulate Allergic AHR and Airway Inflammation.
To confirm the results observed with anti-MHC I antibody, we investigated the activity of nTregs isolated from mice lacking the β2m gene (β2m−/−) that fail to express MHC I antigens on the cell surface. When compared with lung nTregs from WT mice, lung nTregs from naive β2m−/− mice failed to suppress allergen-induced AHR (Fig. 4A) or airway eosinophilia (Fig. 4B) in sensitized and challenged, NK-depleted (anti-NK) or nondepleted (control antibody) WT recipient mice.
Fig. 4.
CD4+CD25+ T cells isolated from β2m−/− mice fail to regulate lung allergic responses. Isolated lung nTregs from WT or B2m−/− mice were adoptively transferred into sensitized WT recipient mice before challenge. In some cases, WT recipient mice were also depleted of NK cells. (A) AHR. (B) BAL cell composition. (C) IL and TGF-β levels. Results represent mean ± SEM from three independent experiments (n = 12). *, P < 0.05; #, P < 0.01, indicates comparison of results of transfer of WT or β2m−/− mice depleted or not depleted of NK cells.
Transfer of WT nTregs reduced the levels of IL-4, IL-5, and IL-13 in the BAL fluid of sensitized and challenged WT mice; this was also associated with significant increases in IL-10 and TGF-β levels (Fig. 4C). By contrast, transfer of nTregs from β2m−/− mice failed to reduce the levels of IL-4, IL-5, or IL-13 or to increase the levels of IL-10 or TGF-β in NK-depleted or nondepleted WT mice.
Blockade of CD8 or MHC I Inhibits IL-10 and TGF-β Production in Cocultures of nTregs with CD8+ T Cells.
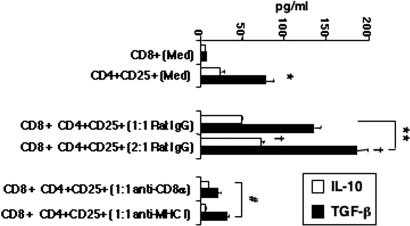
When cultured individually, lung nTregs, but not naive CD8+ T cells, produced significant amounts of IL-10 and TGF-β (Fig. 5). Coculture of nTregs with naive CD8+ T cells increased the levels of IL-10 and TGF-β compared with cultures of nTregs alone. The addition of CD8−/− T cells to cultures of CD4+CD25+ T cells did not alter cytokine levels (data not shown). When nTregs were separated by a cell-impermeable membrane, the presence of CD8+ T cells on the other side of the membrane did not influence the production of IL-10 or TGF-β by nTregs (data not shown), suggesting that cell-cell contact is critical to the effect of CD8 on nTregs. When the numbers of naive CD8+ T cells were increased while keeping the number of CD4+CD25+ cells constant, the levels of IL-10 and TGF-β further increased. The addition of anti-CD8α (or anti-CD8β; data not shown) or anti-MHC I antibody to the cocultures prevented the increases in IL-10 and TGF-β production.
Fig. 5.
Effect of anti-CD8 or anti-MHC on nTreg cytokine production in vitro. WT nTregs and CD8+ T cells were isolated as described in Methods. CD8+ T cells (0.5–1.0 × 106) and nTregs (0.5 × 106) were cultured alone or together at different ratios (1:1, 2:1) and in the presence of rat IgG, anti-MHC, or anti-CD8. After 24 h, culture supernates were collected and assayed for IL-10 and TGF-β content. Results of three experiments (mean ± SEM) carried out in triplicate are shown. *, P < 0.05, indicates comparison of cultures of nTregs to CD8+ T cells; **, P < 0.01, indicates comparison of cocultures of nTregs and CD8+ T cells; †, P < 0.05, indicates comparison of cultures at different ratios; #, P < 0.01, indicates comparison of cultures containing anti-MHC or anti-CD8 to rat IgG.
Discussion
The suppressive role of naturally occurring lung CD4+CD25+ T cells (nTregs) on lung allergic responses is complex, as indicated by somewhat inconsistent and oftentimes contradictory results reported by various investigators (9, 32, 35). In this study, we demonstrate the requirements for activation of naturally occurring lung Foxp3+CD4+CD25+ T cells resulting in the suppression of allergen-induced AHR and inflammation. Both in vitro and in vivo, inhibition or interference with the interaction/engagement of MHC class I on naturally occurring CD4+CD25+ T cells with CD8 was shown to effectively prevent the expression of Treg activity.
Despite sensitization and challenge with allergen, CD8−/− mice, unlike WT (C57BL/6) mice, failed to develop significant AHR and inflammation. Consistent with our previous report (42), adoptive transfer of either negatively or positively selected allergen-primed (but not naive) CD8+ T cells fully restored AHR and inflammation. When BAL cells were analyzed by FACS, significant numbers of CD8+ T cells were detected in the lungs of sensitized and challenged WT and CD8−/− mice given allergen-primed, but not naive CD8+ T cells (data not shown), suggesting that only primed CD8+ T cells were being recruited into the airways. However, few positively selected, primed CD8+ T cells could be identified in the airways, presumably because the microbead antibody remained bound on the CD8 cells blocking staining, but not their function, because positively selected CD8+ T cells could restore AHR and inflammation in the CD8−/− recipients to the same degree as the negatively selected cells.
Previously, we demonstrated that intratracheal transfer of naturally occurring Foxp3+CD4+CD25+ lung T cells (nTregs) suppressed AHR and inflammation by increasing levels of IL-10 and TGF-β and concomitantly decreasing levels of IL-4, IL-5, and IL-13 in sensitized and challenged BALB/c or C57BL/6 mice (9). Indeed, inhibition of TGF-β attenuated the suppressive activities, and IL-10 was deemed essential to the nTreg production of TGF-β (9). Similar to the effects in WT recipient mice, transfer of nTregs decreased AHR, inflammation, and levels of IL-4, IL-5, and IL-13 and increased levels of IL-10 and TGF-β in sensitized and challenged CD8−/− mice reconstituted with negatively selected, primed CD8+ T cells. In contrast, in the mice reconstituted with positively selected, primed CD8+ T cells, nTregs exerted little regulatory activity. Coupled with the absence of regulation of airway function and lung inflammation, low levels of IL-10 and TGF-β and high levels of IL-4, IL-5, and IL-13 were detected in the BAL of recipients of positively selected CD8 cells. Together, these results suggest a critical requirement for the interaction of nTregs with CD8 in the induction of nTreg activities.
To confirm this presumed importance for CD8 expression or accessibility in the functional activation of nTregs, we investigated the effects of anti-CD8 delivered intratracheally before and immediately after the transfer of nTregs in sensitized and challenged WT mice. Instillation of control antibody or anti-CD8 alone did little to alter responses in sensitized and challenged mice. Significant down-regulation of AHR and inflammation, associated with increases in BAL levels of IL-10 and TGF-β and decreases in IL-4, IL-5, and IL-13 levels, was observed in recipient mice given the control antibody before transfer of nTregs. However, adoptive transfer of nTregs into sensitized and challenged mice that received anti-CD8 failed to suppress AHR and inflammation; there were few to no increases in BAL levels of IL-10 and TGF-β or decreases in IL-4, IL-5, and IL-13 levels. These data further demonstrate the importance of CD8 in triggering nTreg activity.
Interactions between CD8 and MHC I are well characterized (45, 46), and the expression of MHC I on cell surfaces has been shown to be essential for the development and survival of CD4+ and CD8+ T cells (47–49). Based on the data with CD8 blockade, we posit that expression of MHC I on the Tregs may also be necessary for the induction of regulatory activities. More than 90% of CD4+CD25+ T cells expressed MHC I and Foxp3. Because NK cells are reported to efficiently remove any cells lacking MHC I on the cell surface (43, 44), sensitized recipient mice were first depleted of NK cells before the intratracheal transfer of nTregs treated with the MHC I antibody. In sensitized and challenged mice shown to be depleted of NK cells, transfer of anti-MHC I-treated Tregs failed to down-regulate AHR or inflammation or increase levels of IL-10 or TGF-β, presumably because the interaction between MHC I on nTregs and CD8 in the airways of recipient mice was prevented. This effect was independent of the depletion of NK cells because sensitized and challenged mice given control antibody before intratracheal transfer of anti-MHC I-treated Tregs also exhibited little suppressive activity.
The role of MHC I in the suppressive activity of Tregs was further examined in β2-microglobulin-deficient (β2m−/−) mice. Similar to the described effects of the in vitro treatment of Tregs with anti-MHC I, nTregs isolated from naive β2m−/− mice failed to reduce AHR or airway inflammation in both sensitized and challenged WT recipient mice whether or not they were depleted of NK cells. As with all other experiments, the absence of Treg activity from β2m−/− nTregs was associated with low levels of BAL IL-10 and TGF-β and increased levels of Th2 cytokines. These data identify a direct association between the regulatory activities of nTregs and the requirements (interaction between) for MHC I and CD8 expression. Any disruption of this interaction by either blocking or eliminating expression of MHC I on the Tregs or eliminating or blocking expression/accessibility of CD8 resulted in abrogation of suppressive activities by lung nTregs. Collectively, the data identify an in vivo mechanism for activation of nTregs, triggering the suppression of allergen-induced AHR and inflammation in the lung through the up-regulation of BAL levels of IL-10 and TGF-β and down-regulating release of IL-4, IL-5, and IL-13.
This apparent critical requirement for interaction between CD8 and MHC I on nTregs in the induction of regulatory activity was further defined by analyzing the effect of coculture of isolated CD8+ T cells (with >99% purity) and nTregs (with >95% purity) in both single-chamber and two-chamber experiments in the presence of anti-CD8 or anti-MHC I in vitro. Isolated nTregs alone, unlike naive CD8+ T cells, produced and released significant amounts of IL-10 and TGF-β in medium alone. When CD8+ T cells were cocultured together in close proximity in the same wells with nTregs, the levels of IL-10 and TGF-β increased significantly above the basal levels, and these levels were further enhanced by increasing (doubling) the number of CD8+ T cells. If the CD8 T cells were separated from the Tregs by a cell-impermeable membrane, no increases were seen, confirming the need for direct cell–cell contact. However, when either anti-CD8 or anti-MHC I was added to the chamber containing CD8+ T cells and nTregs, this resulted in significantly lower levels of IL-10 and TGF-β, consistent with the in vivo attenuation of suppression by nTregs when interactions between MHC I and CD8 were interrupted by blocking antibody or genetic manipulation of the mice.
These in vitro and in vivo data demonstrate a mechanism for activating nTreg activity, one which involves the interaction of MHC I on lung nTregs and CD8 on T cells (or possibly other cells in the lung, e.g., dendritic cells). Activation of these naturally occurring CD4+CD25+ lung T cells effectively reduces AHR, eosinophilic lung inflammation, Th2 cytokine production, and goblet cell metaplasia, likely through the up-regulation of IL-10 and TGF-β (9). Controlling the activation of this subset of Tregs offers a previously unrecognized therapeutic approach to the treatment of lung allergic diseases.
Methods
Animals.
Pathogen-free, 8- to 10-week-old female C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and β2m−/− and CD8α−/− mice were provided by Philippa Marrack. All mice were maintained on an ovalbumin (OVA)-free diet. All protocols were approved by the Institutional Animal Care and Use Committee at the National Jewish Medical and Research Center.
Sensitization.
Sensitization was carried out by i.p injection of 20 μg of OVA (Grade V; Sigma Aldrich, St. Louis, MO) emulsified in 2.25 mg of alum hydroxide (AlumImject; Pierce, Rockford, IL) in a total volume of 100 μl on days 1 and 14. Sensitized and naive littermates received aerosol challenges for 20 min each day on 3 consecutive days (days 26, 27, and 28) with 1% OVA in PBS by using an ultrasonic nebulizer (Omron, Vernon Hills, IL) (9).
Cell Preparation and Culture.
CD4+CD25+ Tregs from naive donors were isolated by collagenase digestion from lungs and enriched using nylon wool columns as described in ref. 9. Lymphocytes were further purified by CD4+CD25+ Treg magnetic-activated cell sorting beads (Miltenyi Biotec, Bergisch-Gladbach, Germany), resulting in a purity of >95% CD4+CD25+ T cells.
Naive- and OVA-primed CD8+ T cells were isolated from spleens with magnetic-activated cell sorting beads by either negative or positive selection, providing 97% and 99% CD8+ T cells, respectively. For negative selection, CD8+ T cells were enriched from isolated spleen mononuclear cells by using the mouse CD8+ T cell isolation kit, and for positive selection of CD8+ T cells, CD8α (Ly-2) microbeads were used (Miltenyi Biotec).
In some adoptive transfer experiments, CD4+CD25+ T cells were treated with anti-MHC I (200 mg/ml, 28–8-6; BD Biosciences Pharmingen, San Diego, CA) in vitro for 1 h and washed twice with PBS before transfer.
In Vitro Analysis and Treatment.
Isolated CD4+CD25+ T cells were cultured alone or in combination with naive CD8+ T cells at different cell ratios in complete medium, containing control antibody, anti-CD8α that reacts with the 38-kDa and 34-kDa a chains of the CD8 antigen (Ly-2 or Lyt-2) of all mouse strains, or anti-CD8β that reacts with the b chain (Ly-3.2 or Lyt-3.2) of most mouse strains (200 μg/ml) (53–6.7 or 53–5.8; American Type Culture Collection, Manassas, VA), or anti-MHC I. There were no differences observed when either anti-CD8α or anti-CD8β was used. Transwell permeable inserts were obtained from Corning (Corning, NY). Supernatants were collected 24 h later, and levels of IL-10 and TGF-β were quantitated by ELISA.
Adoptive Transfer.
Recipient mice received 5 × 105 isolated lung CD4+CD25+ T cells intratracheally in 50 μl of PBS before allergen challenge. Naive or primed CD8+ T cells (5 × 106 in 200 ml of PBS) were injected intravenously before the intratracheal instillation of CD4+CD25+ T cells.
Antibodies.
mAb from the culture supernates of the IgG-producing hybridomas PK136 (anti-mouse NK), 53–6.7 (anti-CD8α), and 53–5.8 (anti-CD8β) were purified by protein G chromatography. Anti-mouse NK (600 μg) was injected i.v. before allergen challenge, and anti-mouse CD8 (50 μg) was administered by microspray intratracheally using a microsprayer (Penn-Century, Philadelphia, PA) before and immediately after intratracheal transfer of CD4+CD25+ T cells. There were no differences observed when either anti-CD8α or anti-CD8β was used.
Measurement of Airway Responsiveness.
Airway responsiveness (lung resistance), 48 h after the last challenge, was assessed as a change in airway function to increasing concentrations of aerosolized methacholine (9).
BAL.
Immediately after measurement of AHR, lungs were lavaged (1 × 1 ml, 37°C). Total leukocyte numbers were counted, and differential cell counts were performed (Coulter Counter; Coulter, Hialeah, FL).
Determination of Serum Antibody Titers by ELISA.
Serum levels of total IgE, OVA-specific IgE, IgG1, IgG2a, and IgG2b were measured by ELISA as described in ref. 9. Total IgE levels were calculated by comparison with known mouse IgE standards (BD Biosciences Pharmingen).
Measurement of Cytokine Levels.
Cytokine levels in the BAL fluid and supernatants of in vitro cultured lung cells were measured by ELISA [IL-4, IL-5, IL-10, IFN-γ, and TGF-β (BD Biosciences Pharmingen); IL-13 kits (R & D Systems, Minneapolis, MN)]. ELISAs were performed according to the manufacturers' directions. The limits of detection were 4 pg/ml for IL-4 and IL-5, 10 pg/ml for IL-10 and IFN-γ, 8 pg/ml for IL-13, and 6 pg/ml for TGF-β.
FACS Analysis.
Enriched lung and BAL cells, after preincubation with naive mouse serum in staining buffer (PBS/2% FCS/ 0.2% sodium azide), were labeled with the following conjugated antibodies purchased from BD Biosciences Pharmingen: anti-CD3 FITC, PE, PerCP, APC (17A2); anti-CD4 FITC, PE, PerCP, APC (L3T4); anti-CD25 FITC (7D4), PE (PC61); anti-CD8α FITC, PE, PerCP (53–6.7); anti-CD122 PE (TM-β1); anti-panNK FITC (DX5), and anti-H-2kb FITC, PE. For intracellular staining, cells were stimulated with phorbol 12-myristate 13-acetate (100 ng/ml) and ionomycin (2 mg/ml; Sigma-Aldrich, St. Louis, MO) in complete medium overnight and for 6 h in the presence of brefeldin A (10 mg/ml; Sigma-Aldrich). Cells were fixed with 4% formaldehyde in PBS, permeabilized in 0.5% saponin, and stained with anti-IL-10 PE, APC (JES5–16E3); IFN-γ PE, APC (XMG1.2); Foxp3 PE and TGF-β (A75–3.1) (eBioscience, San Diego, CA). Fluorochrome (FITC, PE, PerCPAPC)-labeled, isotype-matched control antibodies were used for background fluorescence staining. Staining was analyzed on a FACSCalibur flow cytometry system (BD Pharmingen, San Jose, CA) by using CellQuest Pro software. Fluorescence intensity was compared with cells stained with corresponding labeled isotype-matched controls.
Histochemistry.
Lungs were fixed by inflation (1 ml) and immersion in 10% formalin. Cells containing eosinophilic major basic protein were identified by immunohistochemical staining using a rabbit anti–mouse major basic protein (provided by J. J. Lee, Mayo Clinic, Scottsdale, AZ). The slides were examined with an Olympus (Melville, NY) BX40 light microscope. Images were captured with a QColor3 digital camera (QImaging, Burnaby, Canada) and transferred to a computer for analysis. Numbers of peribronchial tissue eosinophils were counted in six to eight different fields per animal in a blinded fashion. Mucus-containing goblet cells were detected by periodic acid/Schiff reagent staining and quantitated as described in ref. 43.
Statistical Analysis.
ANOVA was used to determine statistical significance. Comparisons for all pairs were performed by the Tukey–Kramer highest significant difference test. The P values for significance were set to 0.05. Values for all measurements were expressed as the mean ± SEM.
Supplementary Material
Acknowledgments
We thank Ms. Diana Nabighian for assistance in the preparation of this manuscript. This work was supported by National Institutes of Health Grants HL-36577 and HL-61005 and by Environmental Protection Agency Grant R825702.
Abbreviations
- AHR
airway hyperresponsiveness
- Treg
regulatory T cell
- nTreg
naturally occurring regulatory T cell
- BAL
bronchoalveolar lavage
- NK
natural killer
- β2m−/−
β2-microglobulin-deficient
- OVA
ovalbumin.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706765104/DC1.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Lui C, Nelson DR. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Carvalho TL, Demengeot J. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 6.Graca M, Cobbold SP, Waldman H. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujit T, Nakayama E. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 8.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 9.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 10.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 13.Hogan SP, Koskinen A, Matthaei KI, Young IG, Foster PS. Am J Respir Crit Care Med. 1998;157:210–218. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- 14.Walker C, Kaegi MK, Braun P, Blaser K. J Allergy Clin Immunol. 1991;88:935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 15.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 16.Lin RY, Lazarus TS. Ann Allergy Asthma Immunol. 1995;74:510–515. [PubMed] [Google Scholar]
- 17.Tang C, Rolland JM, Ward C, Li X, Bish R, Thien F, Walters EH. Eur Respir J. 1999;14:106–112. doi: 10.1034/j.1399-3003.1999.14a18.x. [DOI] [PubMed] [Google Scholar]
- 18.Varghese J, Gerblich A, Salik H, Scheyler M. Lung. 1990;168:69–78. doi: 10.1007/BF02719677. [DOI] [PubMed] [Google Scholar]
- 19.Brunkow ME, Jeffrey EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PF, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 21.Groux H, O'Gara A, Bigler M, Rouleau M, Antonenko S, deVrie JE, Roncarolo MG. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 23.Heller F, Fuss I, Nieuwwenhuis E, Blumberg R, Strober W. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 24.Itoh M, Takahashi T, Sakaguchi N, Kuriyasu Y, Shiminu J, Otsuka F, Sakaguchi S. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 25.Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 26.Fontenot JD, Gavin MA, Rudensky AY. Nat Immunol. 2003;4:330–336. [PubMed] [Google Scholar]
- 27.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 28.Gavin MA, Clark SR, Negrou E, Gallegos A, Rudensky A. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Tagami T, Yamcozaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 31.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Lancet. 2004;362:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 32.Kearley J, Barker J, Robinson D, Lloyd C. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grindebacke M, Wing K, Anderson AC, Suri-Payer E, Rak S, Rudin A. Clin Exp Allergy. 2004;34:1364–1372. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 34.Suto A, Nakajima H, Kagami S, Szuki K, Saito Y, Iwamoto I. Am J Respir Crit Care Med. 2001;164:680–687. doi: 10.1164/ajrccm.164.4.2010170. [DOI] [PubMed] [Google Scholar]
- 35.Hadeiba H, Locksley R. J Immunol. 2003;170:5502–5510. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- 36.Read S, Mauze S, Asseman C, Bean A, Coffman R, Powrie F. J Immunol. 1998;164:183–190. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Thornton AM, Shevach EM. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asano M, Toda M, Sakaguchi N, Sakaguchi S. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Nature Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 43.Ljunggen HG, Karre K. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 44.Ryan JC, Naper C, Hayashi S, Daws MR. Immunol Rev. 2001;181:126–137. doi: 10.1034/j.1600-065x.2001.1810110.x. [DOI] [PubMed] [Google Scholar]
- 45.Cantor H, Boyse EA. J Exp Med. 1975;141:1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinherz EL, Schlossman SF. Cell. 1980;19:821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- 47.Jameson SC, Hogquist K, Bevan M. Annu Rev Immunol. 1995;19:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 48.Marrack P, Kappler J. Curr Opin Immunol. 1997;9:250–255. doi: 10.1016/s0952-7915(97)80144-6. [DOI] [PubMed] [Google Scholar]
- 49.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Nature. 1998;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.