Abstract
Glucagon-like peptide-1 (GLP-1), released from gut endocrine L cells in response to glucose, regulates appetite, insulin secretion, and gut motility. How glucose given orally, but not systemically, induces GLP-1 secretion is unknown. We show that human duodenal L cells express sweet taste receptors, the taste G protein gustducin, and several other taste transduction elements. Mouse intestinal L cells also express α-gustducin. Ingestion of glucose by α-gustducin null mice revealed deficiencies in secretion of GLP-1 and the regulation of plasma insulin and glucose. Isolated small bowel and intestinal villi from α-gustducin null mice showed markedly defective GLP-1 secretion in response to glucose. The human L cell line NCI-H716 expresses α-gustducin, taste receptors, and several other taste signaling elements. GLP-1 release from NCI-H716 cells was promoted by sugars and the noncaloric sweetener sucralose, and blocked by the sweet receptor antagonist lactisole or siRNA for α-gustducin. We conclude that L cells of the gut “taste” glucose through the same mechanisms used by taste cells of the tongue. Modulating GLP-1 secretion in gut “taste cells” may provide an important treatment for obesity, diabetes and abnormal gut motility.
Keywords: enteroendocrine cells, gastrointestinal chemosensation, glucose sensor, incretin
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are incretins, peptide hormones secreted from enteroendocrine L and K cells, respectively, that augment insulin secretion after oral intake of glucose (1). How carbohydrates in the gut lumen elicit the release of GLP-1 from L cells and GIP from K cells is unknown (2). Because i.v. glucose administration does not induce secretion of GLP-1 (3) it appears that glucose within the lumen of the gut acts on the luminal surface to stimulate secretion. Thus, we sought to determine what glucose-sensing mechanism in the gut lumen might underlie this L cell response.
One mechanism for sensing glucose is by sweet taste receptors in taste receptor cells of the lingual epithelium (4). Sweet compounds bind to and activate specific G protein coupled receptors that couple through the G protein gustducin (5) to specific second messenger cascades (4, 6). Two type 1 taste G protein coupled receptors (T1Rs) heterodimerize to form the T1R2+T1R3 sweet taste receptor (7–11). Key elements of the taste transduction pathways are the α, β, and γ subunits of gustducin (α-gustducin, Gβ3, and Gγ13) (5, 12), phospholipase Cβ2 (PLCβ2) (13), and transient receptor potential channel type M5 (14), a Ca2+-activated cation channel (15–17). α-Gustducin has been detected in brush cells of the stomach, duodenum, and pancreatic ducts in rat (18, 19), T1R2 and T1R3 are present in rodent gut and the enteroendocrine STC-1 cell line (20), and α-gustducin and GLP-1 are present in enteroendocrine cells of the human colon (21). However, the functional significance of expression of taste signaling elements in cells of the gastrointestinal (GI) tract had been unclear. Here, we present data that indicate that T1R3 and gustducin have a role in glucose-mediated incretin release and may serve as the previously unknown gut lumen glucose sensor.
Results
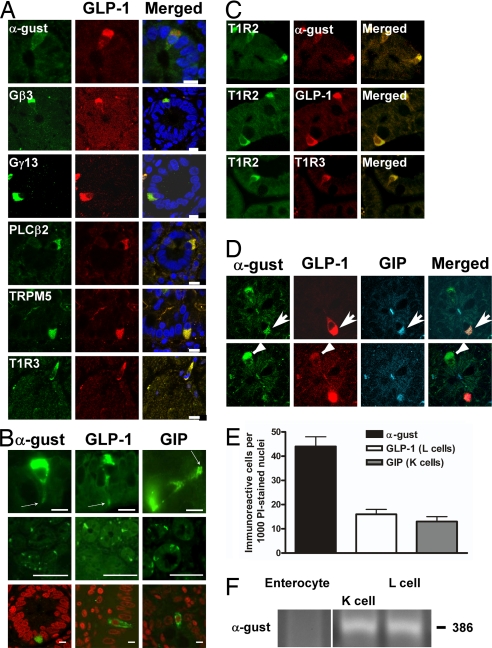
We examined L cells of the gut for the presence of taste receptors and elements of taste transduction pathways. In human duodenal biopsy sections α-gustducin was detected by immunofluorescence (IF) at the apical (luminal) projections and/or cytoplasm of cells with shapes characteristic of enteroendocrine cells [Fig. 1 A–D; see also supporting information (SI) Fig. 5A for negative controls]. Several other taste signaling elements were also detected by IF in human duodenum: T1R2, T1R3, Gβ3, Gγ13, PLCβ2, and transient receptor potential channel type M5 (Fig. 1 A and C).
Fig. 1.
Presence of taste signaling elements in L cells of human duodenum. (A) Indirect immunofluorescent imaging showing coexpression of taste signaling elements (Left) with GLP-1 (Center). Nuclei in the merged images (Right) are stained blue. (Scale bars, 15 μm.) (B) (Top) Cells showing α-gustducin cytosolic expression and dense apical immunostaining (arrows) projecting into the gut lumen. (Scale bars, 5 μm.) (Middle) Low-magnification fields showing immunostaining of α-gustducin, GLP-1, and GIP. (Bottom) Solitary gustducin-expressing, L (GLP-1), and K (GIP) cells amongst the more numerous enterocytes are shown: nuclei are stained red. (Scale bars, 50 μm.) (C) Coexpression of T1R2 sweet taste receptor subunit with α-gustducin (α-gust), GLP-1, and T1R3 in duodenal enteroendocrine cells. (Scale bars, 15 μm.) (D) Triple staining, showing expression of both GLP-1 and GIP in an α-gustducin-expressing cell (Upper, arrow). The same image, taken at a different depth, shows a cell that expresses GLP-1 and α-gustducin but not GIP (Lower, arrowhead). (Scale bars, 15 μm.) (E) Quantitation of cells expressing α-gustducin, GLP-1, or GIP. Statistically significant results determined by Student's t test; values are means ± SEM. (F) RT-PCR amplification of α-gustducin mRNA in the indicated subpopulations of laser-captured cells.
The numbers of cells per thousand nucleated epithelial cells (Fig. 1B Bottom) immunopositive for α-gustducin, 44 ± 5, was higher than that of the entire GLP-1-expressing L cell population (15 ± 2) (Fig. 1E); in addition, there were some α-gustducin-containing cells that did not stain for other components characteristic of L cells (data not shown). GIP was detected in 13 ± 1 cells per 1,000 in the gut biopsies (Fig. 1E), including some of the α-gustducin-containing cells (Fig. 1D). More than 90% of human duodenal L cells contained α-gustducin, but <50% of K cells did so. Five to 10% of incretin cells in human duodenum contain both GIP and GLP-1 (“K/L” cells) (22), and those cells also contained α-gustducin (Fig. 1D). However, some α-gustducin-containing cells did not appear to contain either peptide, consistent with their number per 1,000 nucleated epithelial cells being greater than the sum of L cells and K cells and suggesting that α-gustducin is present in at least one nonenterocyte intestinal cell subpopulation besides L and K cells.
To independently confirm the presence of α-gustducin in human enteroendocrine cells, human gut cells immunostained for GLP-1 or GIP were laser-captured and their RNA subjected to RT-PCR. Captured cells containing GIP or GLP-1 also expressed α-gustducin, whereas captured enterocytes were devoid of α-gustducin (Fig. 1F).
Mouse duodenal GLP-1-containing cells also frequently expressed α-gustducin and α-gustducin promoter-driven GFP (12, 23), along with GLP-2 and peptide YY typically present in L cells (SI Fig. 5 B and C). This was also true for L cells of the mouse jejunum and ileum (SI Fig. 5 D and E). In contrast to our results with human duodenum, mouse K cells rarely expressed α-gustducin.
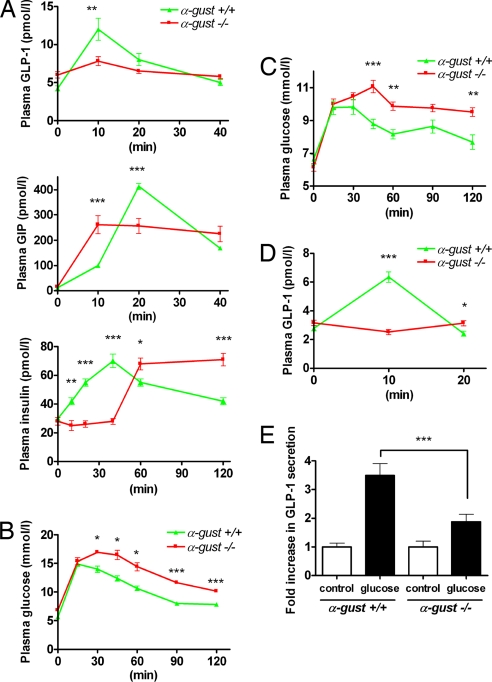
To test whether gut-expressed α-gustducin plays a role in regulating postprandial secretion of GLP-1 and other gut hormones we gavage-administered glucose by feeding needles directly into the stomachs of homozygous mice lacking the gene for α-gustducin (α-gust−/−) (6) and of their wild-type (α-gust+/+) littermates, then compared their plasma concentrations of GLP-1, GIP, and insulin. In α-gust−/− mice, the αβγ-gustducin heterotrimer does not form and therefore any signals mediated by gustducin or its coupled receptors are lost (24). Plasma concentrations of GLP-1 in α-gust−/− mice did not rise in response to glucose (Fig. 2A). Rapid insulin secretion in response to glucose displayed by α-gust+/+ mice was absent from α-gust−/− mice, although they eventually achieved peak insulin concentrations higher than those of α-gust+/+ mice (Fig. 2A). Ten minutes after gavage-administration of glucose, GIP concentrations in α-gust−/− mice were greater than those in wild-type animals (Fig. 2A). For the next 30 min, the α-gust−/− mice maintained their GIP concentrations at a near constant level. In contrast, the wild-type mice showed a higher and more transient rise in GIP concentrations, peaking at 20 min after glucose administration. Glucose homeostasis was also altered in the α-gust−/− mice: plasma glucose concentrations after gavage-administration of glucose (Fig. 2B) or after eating lab chow following an 18h fast (Fig. 2C) were higher in the α-gust−/− mice than in their wild-type littermates, and were maintained at this elevated level for >2 h.
Fig. 2.
Altered secretion of GLP-1, GIP, and insulin in response to gavage-administered glucose in α-gustducin null (α-gust−/−) vs. wild-type (α-gust+/+) mice. (A) Plasma GLP-1 (Top), GIP (Middle) and insulin (Lower) levels after glucose gavage (5 g/kg body weight). (B) Plasma glucose after glucose gavage (2 g/kg body weight). (C) Plasma glucose after postfasting feeding on chow. (D) Plasma GLP-1 responses from surgically isolated duodenum in vivo: the duodenum was ligated away from the stomach and rest of the intestines, and circulatory contact maintained. Ten percent glucose was infused directly into the isolated duodenum. (E) GLP-1 secretory responses to 10% glucose from minced proximal duodenum. For in vivo experiments, n = 6–12 animals per genotype; in vitro experiments were carried out in triplicate and replicated at least twice. Statistical significance determined by ANOVA, values are means ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Thus, insulin secretion was deficient in α-gust−/− mice in response to glucose in the gut lumen; this deficit could be due to failure of either the glucose-sensing arm or the effector arm of the insulin secretory pathway. The α-gust−/− mice did increase insulin secretion in response to the GLP-1 receptor agonist exendin-4 or to i.p. administration of glucose (SI Fig. 6 A and B), indicating that GLP-1 receptors and glucose sensing in beta cells of the pancreas functioned normally in the absence of α-gustducin. We examined both L and K cell numbers and GLP-1 and GIP content in duodenum, jejunum, ileum, and colon of both sets of mice and found no significant differences in this regard between α-gust−/− and wild-type mice (data not shown). However, we did observe that the pancreatic islets from α-gust−/− mice were smaller than those from wild-type mice (SI Fig. 7), consistent with diminished trophic effects from one or more gut hormones.
Gavage-administration of glucose might stimulate α-gustducin-expressing brush cells of the stomach, depending on what receptors are expressed in these cells. Conceivably, these brush cells might contribute to duodenal release of GLP-1. To bypass such effects we examined GLP-1 secretion in α-gust−/− and α-gust+/+ mice in which glucose was infused directly into the duodenum that had been isolated from the stomach and the rest of the small intestine, but remained in circulatory contact. In α-gust+/+ mice plasma concentrations of GLP-1 peaked 10 min after duodenal infusion of 10% glucose, and then returned to baseline within 20 min (Fig. 2D). In contrast, in α-gust−/− mice, plasma concentrations of GLP-1 did not increase. To further eliminate any other external effects that might affect release of GLP-1 from duodenal cells we harvested the proximal duodena of mice and then examined glucose-dependent release of GLP-1 from minced tissues and isolated villi. Culture medium with 10% glucose led to a greater increase in release of GLP-1 from duodenal tissue from α-gust+/+ mice (≈3.5-fold baseline) than it did in tissue from α-gust−/− mice (≈2-fold baseline) (Fig. 2E). Unstimulated amounts of GLP-1 secreted per gram of tissue ranged from 127 pg in α-gust+/+ to 172 pg in α-gust−/− mice; glucose-stimulated amounts ranged from 445 pg in α-gust+/+ to 322 pg in α-gust−/− mice. Similar differences were observed with release of GLP-1 from isolated villi in response to 5% glucose (see SI Fig. 6C and SI Fig. 8 for histology of the villi). Thus, the gustducin-dependent increase in GLP-1 release in vivo in response to luminal glucose appears to be intrinsic to the gustducin-expressing duodenal cells themselves. That glucose stimulated GLP-1 release from the tissue and villi of α-gust−/− mice indicates that one or more gustducin-independent mechanisms also contribute to this effect: possibilities include effects on L cell channels (e.g., closure of KATP channels or transporter associated electrogenic currents), enhanced L cell metabolism, or sweet receptor coupling to other L cell-expressed G proteins.
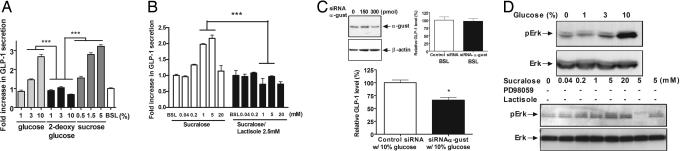
By using IF microscopy and RT-PCR, we confirmed the expression of GLP-1 and peptide YY in NCI-H716 cells, a human enteroendocrine L cell line, and detected the presence in this cell line of several taste signaling elements: α-gustducin, Gβ3; Gγ13; PLCβ2; transient receptor potential channel type M5; T1R1; T1R2; T1R3; IP3 type III receptor (IP3R-3); numerous phosphodiesterases (Pdes), including Pde1a, which has been described in taste cells (4); the fatty acid receptor Gpr40; and the bile acid receptor Tgr5 (25) (SI Fig. 9 A and B). Stimulation of NCI-H716 cells with sucrose, glucose, or sucralose (a high potency noncaloric sweetener) led to a concentration-dependent release of GLP-1 into the medium (Fig. 3 A and B). However, 2-deoxy-glucose, a nonmetabolizable nonsweet sugar used as a control for osmotic effects, did not increase GLP-1 release. Lactisole, a sweetness-antagonizing inhibitor of T1R3 (26), inhibited the sucralose-responsive release of GLP-1 (Fig. 3B). We used siRNA to decrease expression of α-gustducin in NCI-H716 cells, and found diminished glucose-mediated GLP-1 secretion whereas basal secretion was unaffected (Fig. 3C). Thus, T1R3 and gustducin are both implicated in mediating sweetener-induced GLP-1 secretion from NCI-H716 cells.
Fig. 3.
Secretion of GLP-1 in response to glucose, sucrose, and sucralose in NCI-H716 cells. (A and B) Glucose-, sucrose-, and sucralose-mediated GLP-1 secretion from NCI-H716 cells. The sweet receptor inhibitor lactisole inhibited sucralose-mediated GLP-1 secretion. (C) siRNA-mediated diminution of both α-gustducin protein levels (by immunoblotting) and glucose-induced [but not basal (BSL)] GLP-1 secretion from NCI-H716 cells. (D) Immunoblotting of ERK and pERK phosphorylated from NCI-H716 cells in response to increasing concentrations of glucose and sucralose. The inhibitor of Erk phosphorylation, PD98059, inhibited sucralose-mediated Erk phosphorylation. The sweet receptor inhibitor lactisole diminished Erk phosphorylation. BSL, basal. Experiments were carried out in triplicate and replicated at least twice. Statistical significance determined by ANOVA; values are means ± SEM; *, P < 0.05; ***, P < 0.001.
Release of GLP-1 from the mouse enteroendocrine cell line STC-1 in response to stimulation by free fatty acids correlates with increased phosphorylation of ERK (2). We found that phosphorylation of ERK in NCI-H716 cells was activated by glucose and sucralose, and inhibited by the sweet receptor antagonist lactisole or the ERK kinase inhibitor PD98059 (Fig. 3D). Additionally, glucose-mediated GLP-1 secretion from NCI-H716 cells was inhibited by the phospholipase C inhibitor U73122 (SI Fig. 10).
Many taste stimuli elicit Ca2+ release from internal stores or promote Ca2+ entry or both (27, 28). We therefore used single-cell imaging to monitor changes in the intracellular Ca2+ concentration ([Ca2+]i) in NCI-H716 cells loaded with a Ca2+ indicator dye. Addition of glucose to the cells maintained in PBS led to an increase in [Ca2+]i, whereas 2-deoxy-glucose had no such effect (SI Fig. 11 A–C). The phospholipase C inhibitor U73122 inhibited glucose-stimulated Ca2+ mobilization in NCI-H716 cells (SI Fig. 11 D).
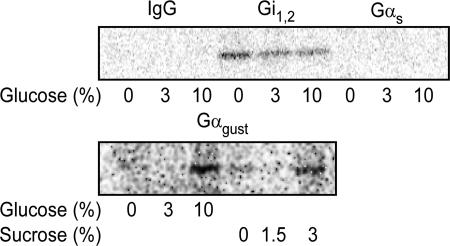
To examine functional coupling of taste receptors to G proteins in membranes from NCI-H716 cells we used the radioactive nonhydrolyzable GTP analog [32P] GTP-azidoanilide. In untreated control membranes there was constitutive GTP-azidoanilide incorporation into Gαi1,2 but not into α-gustducin or Gαs (Fig. 4). Treatment with glucose and sucrose led to a consistent increase in association of GTP-azidoanilide with α-gustducin and a decreased association with Gαi1,2 (Fig. 4).
Fig. 4.
Coupling of taste receptors to G protein α-subunits in NCI-H716 cells. Membranes from NCI-H716 cells were preincubated with the indicated concentrations of glucose and sucrose for 10min at 25°C in the presence of 5–10μCi [32P] GTP-azidoanilide, then irradiated to cross link the GTP analog to G proteins. G protein-specific immune complexes (anti-Gα-gustducin, anti-Gαi1,2, anti-Gαs, and control IgG) were separated by SDS/PAGE, transferred to membranes, and autoradiographically imaged. A positive control for G protein activation is shown in SI Fig. 12.
Discussion
The presence in enteroendocrine cells of functional gustducin-coupled sweet taste receptors responsive to glucose identifies a new signaling mechanism to regulate intestinal hormone secretion. Our data indicate that glucose leads to secretion from L cells via a signaling pathway quite similar to that used by taste cells in the tongue, i.e., glucose activation of gustducin-coupled sweet receptors (T1R2+T1R3).
In vivo, α-gustducin null mice are defective for secretion of GLP-1 in response to luminal glucose. In vitro, isolated duodenum and duodenal villi from α-gustducin null mice likewise show deficient release of GLP-1 in response to glucose. In NCI-H716 enteroendocrine L cells, we observed G protein coupled receptor-mediated activation of α-gustducin by glucose and sucrose. Decreasing α-gustducin expression in NCI-H716 cells by siRNA resulted in decreased glucose-mediated GLP-1 secretion. In addition, the high potency sweetener sucralose induced GLP-1 secretion from NCI-H716 cells in a concentration-dependent manner, and this secretion of GLP-1 was inhibited by the sweet receptor inhibitor lactisole. These results confirm the involvement of α-gustducin coupled sweet receptors in sugar- and sweetener-stimulated secretion of GLP-1 from L cells.
The impression that there are many more L cells in the distal (ileum and colon) vs. proximal (duodenum and proximal jejunum) gut led to the suggestion that the early rapid rise of plasma GLP-1 after a glucose load was not from direct stimulation of the L cells. “Proximal-to-distal” models proposed that indirect neurally mediated signaling, initiated by glucose-sensing K cells or some other non-L cell of the proximal gut, leads to release of GLP-1 from L cells of the distal gut. However, contrary to this indirect neural model there is considerable evidence that luminal glucose directly leads to GLP-1 secretion from the proximal gut. First, although in humans there are more L cells in the gut distally than proximally, there are still many L cells in the duodenum and jejunum (22). Second, the time course (onset, peak, and duration) of glucose-elicited release of GLP-1 in humans is consistent with the time course of glucose reaching the proximal intestine (29–32). Third, instilling small amounts of glucose solution by catheter into the duodenum in humans leads to increased plasma GLP-1 levels (33). Fourth, Roux-en-Y gastric bypass surgery leads to peak plasma levels of GLP-1 that are both higher and reached earlier than before bypass because of dumping of glucose directly into the jejunum (34). Fifth, many animal studies show that glucose directly stimulates GLP-1 secretion even in neurally isolated ileum preparations (35). Finally, our studies with isolated tissues from wild-type and α-gust−/− mice strongly support a model in which direct sensing of glucose by taste signaling elements expressed in proximal L cells leads to GLP-1 release from these same proximal L cells.
GLP-1 receptor agonists and GLP-1 analogs are under intense investigation as treatments for type 2 diabetes and obesity. Exendin-4, an agonist of the GLP-1 receptor, has recently been approved for human use in type 2 diabetes because of its insulinotropic and weight-reducing properties. An alternative mode of modulating signaling through GLP-1 receptors would be to develop secretagogues to increase plasma levels of endogenous ligands, analogous to the use of sulfonylureas in increasing insulin secretion for the treatment of type 2 diabetes. It is now thought that the beneficial effects of Roux-en-Y bypass surgery on postprandial satiety, weight loss and diabetes are due, at least in part, to the increased GLP-1 and peptide YY secretion seen after such surgery (36). If endogenous hormone levels of these peptides could be modulated, without serious anatomic and irreversible interventions such as Roux-en-Y bypass, it would be a major break-through in diabetes and obesity treatment. Sweeteners and secretagogues that increase plasma concentrations of endogenous GLP-1 may provide such an alternative means to promote GLP-1 receptor activity as a treatment for type 2 diabetes and obesity.
Materials and Methods
IF and Confocal Microscopy.
The human paraffin-embedded duodenal sections (n = 3) were from anonymous postmortem samples. Tissue processing and IF were performed as described in ref. 22. For double immunofluorescent staining, sections were incubated overnight at 4°C with both primary antibodies (SI Table 1), washed, and incubated with secondary antibodies (Alexa 568 donkey anti-goat antibody for GLP-1, Alexa 488 donkey anti-rabbit antibody for others, and Cy3-conjugated goat anti-rabbit antibody for GLP-1 and GLP-2 in the gustducin-GFP mice) for 1 h, incubated with TOPRO-3 (Molecular Probes, Eugene, OR) for nuclear staining, washed, and mounted with fluorescence mounting medium, Vectashield (Vector Laboratories, Burlingame, CA). Images were acquired by using an inverted confocal microscope LSM-410 (Carl Zeiss MicroImaging, Thornwood, NY) with a 63× oil-immersed objective and processed by using the MetaMorph software (Universal Imaging, Downingtown, PA). No fluorescent staining was observed when the primary antibody was omitted (SI Fig. 5). The NCI-H716 cells grown on coverslips were fixed in fresh 4% PFA in PBS for 10 min and permeabilized in 0.1% Triton X-100 in PBS for 10 min at room temperature. The cells were treated with blocking buffer (8% BSA in PBS) for 20 min, washed in PBS supplemented with 0.5% BSA and 0.05% Tween 20, and incubated with antibodies (SI Table 1) for 16 h at 4°C. After washing, cells were stained with Alexa Fluor secondary antibody as described above. Pancreata were fixed in 4% paraformaldehyde and embedded in paraffin, sections were incubated with anti-guinea pig insulin antibody and stained with ABC/HRP (DakoCytomation, Carpinteria, CA), and islet diameter was quantified by using MetaMorph software, Version 4.6.3 (Universal Imaging). All methods are as described in ref. 37.
Laser Capture Microdissection of Single Cells and PCR.
Laser capture microdissection and image acquisition were performed as we described in ref. 22. We designed human α-gustducin primers to amplify a 386-bp product (SI Table 2).
Animals.
The design and production of α-gustducin null (α-gust−/−) mice (6), and the mice expressing GFP from the 7.6-kb promoter region upstream from the α-gustducin gene (gustducin-GFP mice) are described in refs. 12, 23, and 24. The genetic background of all mice is C57BL/6. For comparisons of wild-type and α-gustducin null mice, α-gust+/+, α-gust+/−, and α-gust−/− littermate mice were generated from α-gust+/− × α-gust+/− matings.
Gavage Administration of Glucose Load in Wild-Type and Transgenic Mice.
We carried out gastric gavages of glucose (2–5g/kg body weight) on fasted 8-week-old, wild-type, and α-gustducin null mice (n = 7–12 animals per group). Gavage administration of glucose was by syringe attached to feeding needles (20 gauge × 1 ½; Popper and Sons, New Hyde Park, NY) inserted through the mouth into the stomach. Blood samples (100 μl) to which was added dipeptidyl peptidase IV inhibitor (Linco Research, Billerica, MA; 10 μl/ml blood), were obtained before gavage (time 0) and 10, 20, and 40 min after gavage for determination of blood glucose by Glucometer Elite (Bayer, Emeryville, CA), plasma insulin by ELISA (Crystal Chem, Downers Grove, IL), and plasma N-terminal GLP-1 and total GIP by ELISA (Linco Research).
Tissue Measurements of Incretin Levels.
GLP-1 and GIP content of duodenum, jejunum, ileum, and colon were measured in extracts of wild-type (n = 5) and α-gustducin null mice (n = 5) as described in refs. 38 and 39.
Blood Glucose in Wild-Type and Transgenic Mice After Alimentation.
Wild-type and α-gustducin null mice (n = 6 animals per group) were fasted for 18 h and then allowed access to their regular food (Picolab rodent diet 20; Purina Mills, St. Louis, MO; catalog no. 5053) ad libitum. The blood glucose concentration was measured from tail vein samples, using a freestyle glucometer (Therasense, Alameda, CA); measurements were taken immediately before feeding (time 0) and 15, 30, 45, 60, 90, and 120 min after feeding.
Secretion of GLP-1 from Intact Duodenum in Vivo.
Wild-type and α-gustducin null mice (n = 12) were fasted overnight and anesthetized by intraperitioneal injection of avertin (2-2-2 tribromoethanol, 250 mg/kg). Median laparotomy was performed. The duodenum was physically isolated from the stomach and rest of the intestinal tract by clamps placed at the gastroduodenal junction and within the duodenum ≈5 cm distal to the gastroduodenal junction. Care was taken to maintain circulatory contact. Glucose (10%) was injected directly into the lumen of the duodenum. Blood samples were collected from the posterior vena cava before glucose infusion and at 10 and 20 min after glucose infusion. Dipeptidyl peptidase IV inhibitor was added to the blood samples, and plasma GLP-1 was measured.
Secretion of GLP-1 from Minced Duodenum and Duodenal Villi.
To acquire the duodenum for mincing, median laparotomy was done as above, then an incision was made in the antimesenteric side of the duodenum near the gastroduodenal junction, and the duodenum was flushed with 20 ml of HBSS (calcium- and magnesium-free). The proximal 5 cm of duodenum was dissected out, the serosal layer stripped off, then minced pieces of tissue (≈1–2 mm2) were placed in culture medium (40) (DMEM with or without 10% glucose with 10% FBS, 100 units/ml penicillin/100 mg/ml streptomycin/20 μl/ml dipeptidyl peptidase IV inhibitor). Tissues were incubated in 5% CO2 for 1 h at 37°C, the media was collected and GLP-1 levels assayed to determine unstimulated (baseline control) levels and that released into media. At the completion of the experiment cell viability was confirmed by exclusion of trypan blue. Duodenal villi were obtained from the proximal duodenum by scraping with mild pressure from the short edge of a glass slide. The isolated tissue was allowed to settle at the bottom of a tube on ice and then was washed 3 times with HBSS. After the final wash purified villi were resuspended in DMEM as above for minced tissue, aliquoted, and incubated as above but with 5% glucose to determine GLP-1 release.
Culture of NCI-H716 Cells and Secretion Studies.
Human enteroendocrine NCI-H716 cells were maintained in suspension culture as described by the American Type Culture Collection. Two days before experiments, cells were seeded into 24-well culture plates precoated with Matrigel as described in ref. 41. On the day of the experiments, supernatants were replaced by PBS containing 1 mM CaCl2 and dipeptidyl peptidase IV inhibitor. The solutions were adjusted to pH 7.2. Cells were incubated for 1 h at 37°C with or without different test agents and inhibitors. GLP-1 was measured by ELISA and normalized to protein content. Glucose, sucrose, 2-deoxy-glucose, and lactisole were from Sigma (St. Louis, MO) and sucralose was from Toronto Chemical (Toronto, ON, Canada). U73122 and U73343 were from Calbiochem (San Diego, CA).
RNA Isolation and RT-PCR of NCI-H716 Cells.
Total RNA was extracted by using Triazol (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction. RT-PCR was performed by using the Qiagen OneStep RT-PCR kit (Qiagen, Valencia, CA); see SI Table 2 for primer sequences and annealing temperatures. Each target gene was amplified 35 cycles in a Peltier thermal cycler PTC-225 (MJ Research, Hercules, CA).
siRNA Preparation and NCI-H716 Cell Transfection.
The siRNA sequence targeting human α-gustducin (GenBank/European Molecular Biology Laboratory/DNA Data Bank of Japan accession no. XM_294370) was from position 177–195 relative to the start codon. This α-gustducin sequence was reversed and used as the unspecific siRNA control. Twenty-one-nucleotide long RNAs were purchased from Dharmacon (Lafayette, CO) in deprotected and desalted form, and the formation of siRNA duplex (annealing) was performed according to the manufacturer's instructions. Subconfluent differentiated NCI-H716 cells were transiently transfected with siRNAs, using Lipofectamine 2000 according to the manufacturer's protocol (Life Technologies, Carlsbad, CA). The entire mixture was then added to the cells in one dish resulting in a final concentration of 300 nM for the siRNAs. Cells were usually examined 48 h after transfection.
Calcium Imaging.
Levels of [Ca2+]i were quantified by fluorescence imaging, using the calcium indicator dye fura-2 as described in ref. 9. Briefly, cells were incubated for 30min in the presence of 2 μM acetoxymethylester form of fura-2 (Molecular Probes) and then washed twice in PBS (pH 7.4, without Ca2+; Invitrogen) and allowed to incubate an additional 20–30min for complete deesterification of the dye. Cells were imaged on a Zeiss Axiovert microscope (40× oil immersion objective) coupled to an Attofluor imaging system. The average [Ca2+]i in 20–30 cells per microscope field was quantified in four separate cultures per treatment condition.
Immunoblotting.
Western blot analysis was performed as described in ref. 36. Detection of individual proteins was performed by immunoblotting with the primary antibodies, pErk (1:1,000; Cell Signaling, Danvers, MA) and total Erk (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by ECL. PD98059 was from Calbiochem.
Membrane Preparation, Labeling, and Immunoprecipitation of Membrane-Associated G Proteins.
Cell membranes were purified and labeled as described in ref. 36. [32P] GTP-azidoanilide was from ALT Bioscience (Lexington, KY). The clarified supernatants (160 μl) were transferred to tubes containing 5–10 μl of polyclonal rabbit antisera raised against Gα-gustducin, Gαs (Santa Cruz Biotechnology), and Gαi1,2 (Affinity BioReagents, Golden, CO). Immunoprecipitation was performed as described in ref. 42. A 20-μl aliquot of the samples was subjected to SDS/PAGE. Gels were transferred to membrane, and band intensity was quantified by electronic autoradiography, using a Storm (Molecular Dynamics, Sunnyvale, CA). The protein concentration was determined by the Bradford method.
Statistical Analysis.
GLP-1 data represent means ± SEM. Differences between mean values for variables within individual experiments were compared statistically by ANOVA and followed by post hoc testing with Scheffé's test. Comparisons were performed by using Graphpad Prism (GraphPad Software, San Diego, CA). P < 0.05 was viewed as significant.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes on Aging/National Institutes of Health and by National Institute on Deafness and Other Communication Disorders/National Institutes of Health Grants DC003055 and DC003155 (to R.F.M.) and DC007399 (to B.M.).
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- IF
immunofluorescence
- T1R
type 1 taste receptor.
Footnotes
Conflict of interest statement: Dr. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from the Redpoint Bio company, and is an inventor on patents and patent applications which have been licensed to the Redpoint Bio company.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706890104/DC1.
References
- 1.McIntyre N, Holdsworth CD, Turner DS. Lancet. 1964;41:20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 2.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 3.Clocquet AR, Egan JM, Stoffers DA, Muller DC, Wideman L, Chin GA, Clarke WL, Hanks JB, Habener JF, Elahi D. Diabetes. 2000;49:1856–1864. doi: 10.2337/diabetes.49.11.1856. [DOI] [PubMed] [Google Scholar]
- 4.Margolskee RF. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin SK, McKinnon PJ, Margolskee RF. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 6.Wong GT, Gannon KS, Margolskee RF. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 7.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 8.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 9.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 13.Rossler P, Kroner C, Freitag J, Noe J, Breer H. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 14.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann T, Chubanov V, Gudermann T, Montell C. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Liman ER. Proc Natl Acad Sci USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. Proc Natl Acad Sci USA. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer D, Drenckhahn D. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 19.Hofer D, Puschel B, Drenckhahn D. Proc Natl Acad Sci USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 21.Rozengurt N, Wu S, Chen MC, Huang C, Sternini C, Rozengurt E. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 22.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Am J Physiol Endocrinol Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 23.Wong GT, Ruiz-Avila L, Margolskee RF. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. Proc Natl Acad Sci USA. 2001;98:8868–8873. doi: 10.1073/pnas.151235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsuma S, Hirasawa A, Tsujimoto G. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 26.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M. J Biol Chem. 2005;280:15238–15246. doi: 10.1074/jbc.M414287200. [DOI] [PubMed] [Google Scholar]
- 27.Bernhardt SJ, Naim M, Zehavi U, Lindemann B. J Physiol. 1996;490(Pt 2):325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogura T, Margolskee RF, Kinnamon SC. J Neurophysiol. 2002;87:3152–3155. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- 29.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balks HJ, Holst JJ, von zur Muhlen A, Brabant G. J Clin Endocrinol Metab. 1997;82:786–790. doi: 10.1210/jcem.82.3.3816. [DOI] [PubMed] [Google Scholar]
- 31.Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. J Clin Endocrinol Metab. 2005;90:6665–6671. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- 32.Theodorakis MJ, Carlson O, Muller DC, Egan JM. Diabetes Care. 2004;27:1692–1698. doi: 10.2337/diacare.27.7.1692. [DOI] [PubMed] [Google Scholar]
- 33.Vilsboll T, Holst JJ. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 34.Laferrere B. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen L. Regul Pept. 2004;118:11–18. doi: 10.1016/j.regpep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 37.Perfetti R, Zhou J, Doyle ME, Egan JM. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 38.Mortensen K, Christensen LL, Holst JJ, Orskov C. Regul Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt P, Poulsen SS, Rasmussen TN, Bersani M, Holst JJ. Peptides. 1991;12:963–973. doi: 10.1016/0196-9781(91)90045-q. [DOI] [PubMed] [Google Scholar]
- 40.Ferland S, Hugon JS. In Vitro. 1979;15:278–287. doi: 10.1007/BF02618952. [DOI] [PubMed] [Google Scholar]
- 41.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 42.Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. Endocrinology. 1999;140:1132–1140. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.