Abstract
Dietary sugars are transported from the intestinal lumen into absorptive enterocytes by the sodium-dependent glucose transporter isoform 1 (SGLT1). Regulation of this protein is important for the provision of glucose to the body and avoidance of intestinal malabsorption. Although expression of SGLT1 is regulated by luminal monosaccharides, the luminal glucose sensor mediating this process was unknown. Here, we show that the sweet taste receptor subunit T1R3 and the taste G protein gustducin, expressed in enteroendocrine cells, underlie intestinal sugar sensing and regulation of SGLT1 mRNA and protein. Dietary sugar and artificial sweeteners increased SGLT1 mRNA and protein expression, and glucose absorptive capacity in wild-type mice, but not in knockout mice lacking T1R3 or α-gustducin. Artificial sweeteners, acting on sweet taste receptors expressed on enteroendocrine GLUTag cells, stimulated secretion of gut hormones implicated in SGLT1 up-regulation. Gut-expressed taste signaling elements involved in regulating SGLT1 expression could provide novel therapeutic targets for modulating the gut's capacity to absorb sugars, with implications for the prevention and/or treatment of malabsorption syndromes and diet-related disorders including diabetes and obesity.
Keywords: carbohydrate absorption, gastrointestinal chemosensation, glucose sensor, glucose uptake
To date, the only identified sugar sensors in the mammalian gastrointestinal tract are those involved in taste transduction (1). Although the gut epithelium senses luminal sugars and modulates its glucose absorptive capacity accordingly, the nature of the sugar-sensing molecule(s) and downstream events remain unknown. Several studies have shown that in many species expression of the intestinal sodium-dependent glucose transporter 1 (SGLT1) is directly regulated by monosaccharides in the lumen of the gut independently of metabolism and appears to involve a G protein-linked second messenger pathway (2–6). Furthermore, the addition of membrane-impermeable glucose analogues to the lumen of the intestine stimulates SGLT1 expression, implying that a glucose sensor on luminal membranes is involved (6).
In taste cells, the detection of sugars and sweeteners depends on T1R2+T1R3, a heterodimer of type 1 taste receptor subunits (T1Rs) (7, 8). The taste receptor cells of the anterior tongue that express T1R2+T1R3 typically also express gustducin, a transducin-like heterotrimeric G protein (9). Gustducin's α-subunit (Gαgust) has been detected in brush cells of the rat stomach, duodenum, and pancreatic ducts (10). Gαgust and bitter-responsive type 2 taste receptors (T2Rs) are expressed in mouse intestinal endocrine cells and in the murine enteroendocrine cell line STC-1 (11).
We reported previously that T1R taste receptors and Gαgust are expressed in the mouse small intestinal epithelium and proposed that they function as luminal sugar sensors to control SGLT1 expression in response to dietary sugar (12). Here, we provide three lines of evidence in favor of T1R taste receptors and Gαgust acting as luminal sugar/sweetener sensors. First, dietary sugars and artificial sweeteners increase SGLT1 mRNA and protein expression and glucose-absorptive capacity in wild-type mice, but not in T1R3 or Gαgust knockout mice. Second, T1R taste receptors and Gαgust are expressed in human and mouse enteroendocrine cells. Third, artificial sweeteners, acting on sweet taste receptors expressed on enteroendocrine GLUTag cells stimulate secretion of gut hormones implicated in SGLT1 up-regulation.
Results
Regulation of Intestinal SGLT1 Expression in Wild-Type and Knockout Mice in Response to Dietary Carbohydrate.
We maintained wild-type, Gαgust knockout (13) and T1R3 knockout (14) mice on diets of varied carbohydrate content and then measured intestinal expression of the SGLT1 gene. In wild-type mice kept on a high-carbohydrate (70% sucrose) diet for 2 weeks, the amount of intestinal SGLT1 mRNA was 1.6-fold higher (P = 0.003) than that in mice fed a low-carbohydrate (1.9% sucrose) diet (Fig. 1A). However, the Gαgust and T1R3 knockout mice showed no change in SGLT1 mRNA expression: on either diet, the amount of SGLT1 mRNA in both types of knockout mice was identical to that of wild-type animals on the low-carbohydrate diet. This implies that there is a constitutive pathway, independent of luminal sugar sensing by T1R3 and/or Gαgust, that maintains the basal expression of SGLT1, and an inducible pathway dependent on T1R3 and Gαgust.
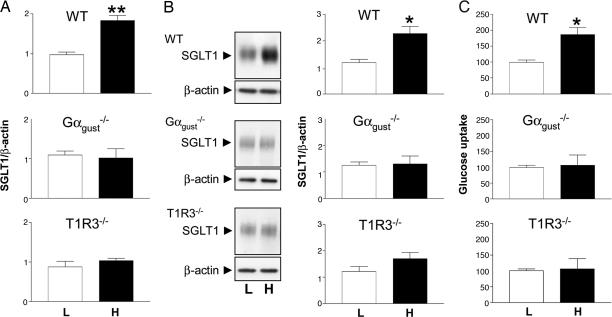
Fig. 1.
Increased SGLT1 expression in response to dietary carbohydrate in wild-type, but not in Gαgust or T1R3 knockout, mice. Wild-type (WT), Gαgust, and T1R3 knockout mice were given low (L) or high (H) carbohydrate diets for two weeks. (A) Steady-state levels of SGLT1 mRNA determined by QPCR were normalized to β-actin mRNA. (B) SGLT1 protein from brush-border membrane vesicles (BBMV) isolated from mid small intestine was detected in Western blots (Left). Densitometric analysis (Right) of Western blots normalized SGLT1 protein expression to that of β-actin. (C) SGLT1-mediated glucose uptake was measured by Na+-dependent d-[U14C] glucose uptake into BBMV. Mean uptake rates are presented as arbitrary units relative to rates measured in BBMV of wild-type mice maintained on the low-carbohydrate diet (defined as 100). All values are expressed relative to SGLT1 in wild-type mice on low-carbohydrate diets as means ± SD. Data were generated in triplicate, with n = 4 animals in each group. Statistically significant results determined by Student's unpaired two-tailed t test are indicated by *, P < 0.05; **, P < 0.005.
The abundance of SGLT1 protein in brush-border membrane vesicles (BBMV) from mid small intestine was assessed by Western blotting (Fig. 1B). In BBMV from wild-type mice on the high-carbohydrate diet, there was a 1.9-fold increase (P = 0.006) in SGLT1 protein abundance, which correlated with a 1.9-fold increase (P = 0.0076) in the initial rate of Na+-dependent glucose transport into the isolated BBMV from mid small intestine (Fig. 1C); a similar increase in glucose transport was observed with BBMV isolated from duodenum and ileum (data not shown). Gαgust and T1R3 knockout mice had similar amounts of intestinal SGLT1 protein and Na+-dependent glucose transport when maintained on low- or high-carbohydrate diets. Thus, whereas wild-type animals are known to respond to increased dietary carbohydrate with enhanced SGLT1 expression (4, 15), neither Gαgust nor T1R3 knockouts responded in this way. Morphometric analysis indicated that neither crypt depths nor villus heights differed in the intestines of mice maintained on either a low- or a high-carbohydrate diet, ruling out a trophic effect [see supporting information (SI) Fig. 5 A and B]. Furthermore, Western blots showed no differences in the abundance of villin and β-actin structural proteins in the intestines of wild-type vs. knockout mice and that there were no changes in the abundance of these proteins as a function of dietary carbohydrate (SI Fig. 5C). It appears, therefore, that knocking out either Gαgust or T1R3 abolishes the ability of mouse intestine to increase SGLT1 expression in response to increased dietary carbohydrate. This supports our hypothesis that Gαgust and T1R3 are part of the sugar-sensing regulatory machinery within the small intestinal mucosa that regulates SGLT1 expression in response to dietary sugars.
Regulation of Intestinal SGLT1 Expression in Wild-Type and Knockout Mice in Response to Dietary Sweeteners.
If the T1R2+T1R3 sweet receptor is involved in intestinal sugar sensing, then artificial sweeteners that activate this receptor in taste cells of the tongue might increase intestinal expression of SGLT1. We kept wild-type and knockout mice for 2 weeks on a low-carbohydrate diet with or without supplementation with water containing the artificial sweetener sucralose. In wild-type mice consuming sucralose-sweetened water, there was a 2.1-fold increase (P = 0.0015) in SGLT1 mRNA, a 2.2-fold increase (P = 0.0037) in SGLT1 protein, and a 1.9-fold increase (P = 0.0354) in the initial rate of Na+-dependent glucose transport as compared with the wild-type controls (Fig. 2 A–C). The magnitude of these changes was similar to those produced by the high-carbohydrate diet containing natural sugars (see Fig. 1). In contrast, in response to supplementation with sucralose, neither the Gαgust nor the T1R3 knockouts showed an increase in SGLT1 mRNA (Fig. 2A) or protein (Fig. 2B), similar to the effects of the high-carbohydrate diets on SGLT1 expression in these knockout mice (see Fig. 1). This suggests that T1R2+T1R3 and Gαgust are involved in sensing the presence of sugars and artificial sweeteners in the intestinal lumen.
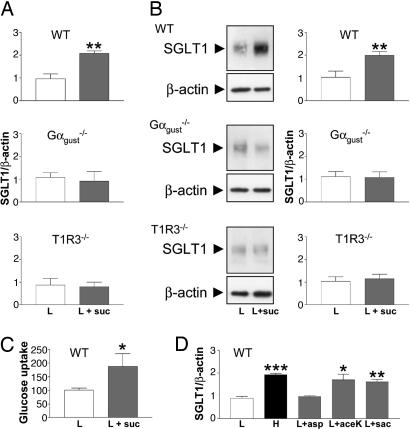
Fig. 2.
Increased SGLT1 expression in response to dietary supplementation with artificial sweeteners in wild-type, but not in Gαgust or T1R3 knockout, mice. Wild-type (WT), Gαgust, and T1R3 knockout mice were given a low-carbohydrate diet without (L), or with 2 mM sucralose (L+suc) for 2 weeks. (A–C) Steady-state levels of SGLT1 mRNA (A), SGLT1 protein (B), and SGLT1-mediated glucose transport rates (C), were measured (see Fig. 1). (D) Steady-state levels of SGLT1 mRNA were measured in wild-type (WT) mice that had been maintained for 2 weeks on a high-carbohydrate diet (H) or low-carbohydrate diet without (L) or with the indicated artificial sweeteners aspartame (L+asp), acesulfame K (L+ace-K), or saccharin (L+sac). All data are expressed relative to SGLT1 in wild-type mice on low-carbohydrate diets as means ± SD. Data were generated in triplicate, with n = 3 animals in each group. Statistically significant results determined by Student's unpaired two-tailed t test are indicated by *, P < 0.05; **, P < 0.005; or ***, P < 0.001.
The responsiveness of the intestinal sugar/sweetener sensor in wild-type mice to various artificial sweeteners was similar to that of the T1R2+T1R3 sweet receptor in taste cells of the tongue. In wild-type mice consuming the low-carbohydrate diet plus artificial sweetener-containing water, SGLT1 mRNA expression increased 1.8-fold in response to saccharin (P = 0.002) and 1.9-fold in response to acesulfame K (P = 0.01) but did not increase in response to aspartame (Fig. 2D). The lack of response to aspartame is expected because this compound is not sweet to mice and does not stimulate expressed mouse T1R2+T1R3 (1, 7–9). The increased levels of SGLT1 mRNA in response to saccharin or acesulfame K were similar to those of wild-type mice on the high-carbohydrate diet, 2.2-fold (P = 0.0001) (see Fig. 1).
Expression of T1Rs and Gαgust in Enteroendocrine Cells.
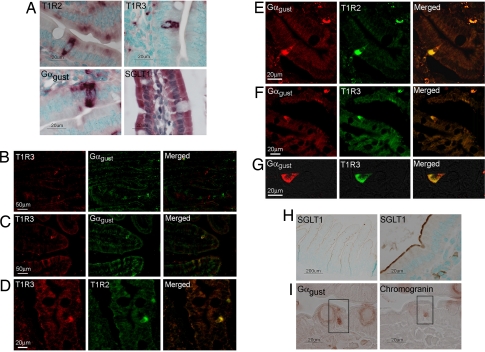
Having implicated Gαgust and T1R3 in regulating SGLT1 expression in vivo in response to dietary sugars, we assessed which cells along the crypt–villus axis express the T1R taste receptors and Gαgust. In situ hybridization showed that mRNAs for T1R2, T1R3, and Gαgust were expressed only in a subpopulation of epithelial cells along the crypt–villus axis in mouse intestine (Fig. 3A). In contrast, SGLT1 mRNA was expressed in all enterocytes along the villus (Fig. 3A). There was negligible expression of T1R2, T1R3, Gαgust, and SGLT1 in the crypts. None of the sense probe controls hybridized to cells of the small intestine (SI Fig. 6). Immunodetection methods demonstrated that T1R and Gαgust proteins were expressed in a subset of cells along the crypt–villus axis of both mouse (Fig. 3 B and C) and human (Fig. 3 D–G) small intestine. SGLT1 protein, however, was present on the luminal membrane of all of the villus enterocytes in mouse intestine (Fig. 3H) (6, 16). To investigate the colocalization of T1R taste receptor subunits and Gαgust, we used serial sections of wax-embedded mouse (Fig. 3 B and C) and human (Fig. 3 D–G) proximal intestines. In mouse villi, most T1R3-expressing cells expressed Gαgust (Fig. 3B) or were in close proximity to such cells (Fig. 3C). In human duodenum, virtually all T1R3-expressing cells also expressed Gαgust (Fig. 3 F and G). Omission of the primary antibodies for gustducin or T1R3 showed no nonspecific immunoreactivity in small intestine (SI Fig. 7). The cells that contain T1Rs and Gαgust appear to be either triangular or flask-like in shape, suggesting they are of enteroendocrine type (Fig. 3G), and immunohistochemistry of serial sections of mouse intestine showed that the enteroendocrine cell marker chromogranin was indeed coexpressed with Gαgust (Fig. 3I).
Fig. 3.
Detection and localization of T1R receptors and Gαgust along the crypt–villus axis of small intestine. (A) In situ hybridization with antisense riboprobes to T1R2, T1R3, and Gαgust. Complementary sense probes to all targets did not hybridize to any transcripts within the tissue sections (see SI Fig. 6). (B and C) Immunofluorescent detection of the T1R3 taste receptor subunit (red) and Gαgust (green) in serial wax sections of mouse duodenum. (D) Immunofluorescent detection of the T1R3 (red) and T1R2 (green) taste receptor subunits in a single wax section of human duodenum. (E–G) Immunofluorescent detection of the T1R2 and T1R3 taste receptor subunits (green), and Gαgust (red) in a single wax section of human duodenum. (H) Chromogenic detection of SGLT1 in wax sections of mouse small intestine. (I) Chromogenic detection of Gαgust and chromogranin in serial wax sections of mouse proximal intestine. The boxed cell expresses both Gαgust and chromogranin.
Sucralose Activates Sweet Taste Receptors on Enteroendocrine Cells to Elicit Hormone Secretion.
Having determined that T1Rs and Gαgust are expressed in enteroendocrine cells, we next examined the contribution of these cells to regulation of SGLT1 expression. Because of practical difficulties in harvesting adequate numbers of viable enteroendocrine cells of specific subtypes, we turned to intestinal endocrine cell lines. GLUTag and STC-1 cells, two mouse enteroendocrine cell lines, have been shown to secrete hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotrophic peptide (GIP), in response to glucose (17, 18). GLUTag cells also respond to fructose and nonmetabolizable glucose analogs (18, 19). STC-1 cells have been shown to express T1Rs, T2Rs, and taste G proteins (11, 12). We have examined GLUTag cells and found that they express T1Rs and Gαgust (SI Fig. 8).
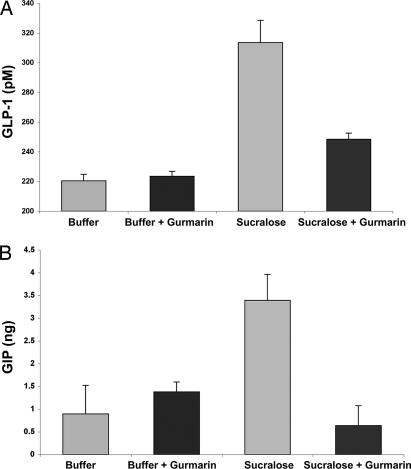
Using GLUTag cells, we sought to determine whether enteroendocrine cells, through their T1R2+T1R3 sweet taste receptor, respond to sweeteners with the release of hormone effectors; in particular, those hormones implicated in SGLT1 up-regulation (20). GLUTag cells tonically release GLP-1 and GIP into the culture medium; however, the addition of sucralose elicited increased release of GLP-1 (Fig. 4A) and GIP (Fig. 4B) and an elevation of intracellular calcium (SI Fig. 9). Gurmarin, a specific inhibitor of sweet taste in mice (21) that acts on the T1R2+T1R3 sweet taste receptor (SI Fig. 10), blocked both the sucralose-stimulated release from GLUTag cells of GLP-1 (Fig. 4A) and GIP (Fig. 4B) and the sucralose-dependent mobilization of calcium in GLUTag cells (SI Fig. 9). We conclude that sweetener-dependent release of GLP-1 and GIP from GLUTag cells depends on stimulation of sweet taste receptors.
Fig. 4.
Sucralose stimulation of endogenously expressed sweet taste receptors in GLUTag cells leads to GLP-1 and GIP release. (A) GLP-1 release into the culture medium of GLUTag cells was monitored after treatment of cells with buffer alone or buffer containing sucralose (50 mM final concentration). Addition of sucralose led to increased GLP-1 release from GLUTag cells (P = 0.00098). Preincubation (15 min) of the GLUTag cells with gurmarin (3 μg/ml) blocked most of the sucralose-dependent increase in released GLP-1 (P = 0.00574 vs. sucralose alone). n = 4 samples per group; the experiment was carried out in triplicate; a representative experiment is shown, and levels are expressed ± SEM. (B) GIP release into the culture medium of GLUTag cells was monitored after treatment of cells with buffer alone or buffer containing sucralose (50 mM final concentration). The addition of sucralose led to a large increase over baseline in GIP release from GLUTag cells (P = 0.047). Preincubation (15 min) of the GLUTag cells with the sweet taste receptor inhibitor gurmarin (3 μg/ml) blocked most of the sucralose-dependent increase in released GIP (P = 0.00260 vs. sucralose alone). n = 2–4 samples per group; the experiment was carried out in duplicate; a representative experiment is shown, and levels are expressed ± SEM.
Discussion
The Na+/glucose cotransporter SGLT1 is the major route for the transport of dietary sugars from the lumen of the intestine into enterocytes. Regulation of this protein is essential for the provision of glucose to the body and, thus, is important for maintenance of glucose homeostasis. In this article, we show that T1R3 and Gαgust are expressed in enteroendocrine cells and are required for the enhanced expression of SGLT1 shown by enterocytes in vivo in response to luminal sugars or sweeteners. The finding that Gαgust and T1Rs are expressed in enteroendocrine cells, whereas SGLT1 is expressed in enterocytes, implies that a chemical signaling event takes place between the chemosensory enteroendocrine cells and absorptive enterocytes. Enteroendocrine cells, in response to luminal nutrients, secrete endocrine hormones including cholecystokinin (CCK), peptide tyrosine tyrosine (PYY), neurotensin, GLP-1, GLP-2, and GIP (18, 19, 22–24). GLP-1 and GIP, known as incretins, are secreted in response to dietary sugars, and influence glucose transport, metabolism, and homeostasis (25). Infusion of the intestinal lumen with glucose, galactose, fructose, 3-O-methyl-d-glucose and α-methyl-d-glucose causes GIP and GLP-1 secretion in rats, pigs, and humans (22–24). These sugars also increase SGLT1 expression when infused into the intestinal lumen (4, 6, 26). The addition of GIP to the serosal side of intestinal tissue in vitro results in increased expression of SGLT1 (20). Collectively, these reports imply a strong correlation among intestinal glucose sensing, subsequent endocrine secretion, and modulation of SGLT1 expression. Our observation that endogenous sweet taste receptors in GLUTag cells mediate sweetener-dependent release of GLP-1 and GIP, coupled with the finding that the presence of GIP on the serosal aspect of intestinal tissue results in increased expression of SGLT1 (20), suggests that one or both of these hormones could serve in vivo as the signal between the sensory enteroendocrine cells and absorptive enterocytes.
We propose that the T1R2+T1R3 sweet taste receptor, expressed on the luminal membrane of villus enteroendocrine cells, senses the luminal glucose concentration. Luminal glucose above a threshold level may activate in enteroendocrine sensor cells a signaling pathway involving T1R2+T1R3, Gαgust, and other taste signaling elements, resulting in the secretion of GLP-1, GIP, and/or other endocrine products. We infer that one or more of these hormones bind to receptors on their target cells and through a paracrine mechanism enhance SGLT1 expression. We speculate that glucose transport across the basolateral membrane facilitated by GLUT 2 may also be subject to this form of regulation.
Materials and Methods
Mice, Diets, and Tissue Collection.
The generation of Gαgust−/− and T1R3−/− mice has been described (13, 14). Six- to 8-week-old C57BL/6 Gαgust−/− and T1R3−/− mice and their wild-type littermates of the same origin were used. Animals were placed individually in standard tub cages in a room with automatically controlled temperature, humidity, and 12-h light/12-h dark cycle. Mice were divided into three groups, with equal number of wild-type, Gαgust−/−, and T1R3−/− animals of both genders. Group one was fed a high-carbohydrate diet (High-carbohydrate Diet (70%), Testdiet #5810; Purina Mills, Richmond, IN), group two was fed a low-carbohydrate diet (Low-carbohydrate Diet (1.9%) Testdiet #590N; Purina Mills). The carbohydrate content of both diets consisted of sucrose. The two diets are equicaloric; being 3.73 and 3.86 (Kcal/g) (3) for the high- and low-carbohydrate diets, respectively. Group three was maintained on the low-carbohydrate diet, and 2 mM sucralose solution was supplied instead of water. A 2 mM concentration of sucralose was used because C57BL/6 wild-type mice displayed markedly increased behavioral responses to this concentration (14). The sucralose solution was changed every second day. All diets were provided ad libitum. An additional three groups of wild-type mice were fed the low-carbohydrate diet and given the following artificial sweetener solutions instead of water: 10 mM acesulfame K, 20 mM saccharin, and 1 mM aspartame. Saccharin and acesulfame K at these concentrations taste sweet to mice and activate expressed mouse T1R2+T1R3 (1, 7, 8, 14). Aspartame tastes sweet to humans but not to rodents; neither 1 mM nor 10 mM concentrations of aspartame activate the mouse sweet receptor (7–9). After 2 weeks, animals were killed by cervical dislocation, and intestines were immediately excised, flushed with ice-cold saline, immersed in liquid nitrogen, and stored at −80°C until use.
Quantitative PCR.
Total RNA isolated from intestinal tissue by using the RNeasy Mini Kit with on-column DNase 1 digestion (Qiagen, Crawley, U.K.) was used for cDNA synthesis using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT)12–18 primers. cDNA was cleaned up by using the Machery–Nagel Nucleospin extract kit (AB Gene, Epsom, U.K.), and 50 ng of cDNA was used per reaction. PCR primers and probes (FAM/TAMRA-labeled) for the amplification of T1R1, T1R2, T1R3, Gαgust, and the Na+/glucose cotransporter, SGLT1, (FAM/TAMRA), along with β-actin (JOE/TAMRA-labeled) were designed by using Primer Express (Applied Biosystems, Warrington, U.K.), and purchased from Eurogentec (Seraing, Belgium) (SI Table 1). For real-time PCRs, the enzyme was activated by heating at 95°C for 2 min. A two-step PCR procedure was used, 15 s at 95°C and 60 s at 60°C for 45 cycles in a PCR mix containing 5 μl of cDNA template, 1× Jumpstart qPCR master mix (Sigma–Aldrich, Poole, U.K.), 900 nM concentrations of each primer, and 250 nM probe in a total volume of 25 μl. Where multiplex reactions were performed, the β-actin primers were primer-limiting and used at 600 nM. All reactions were performed in a Rotor-Gene 3000 (Corbett Research, Cambridge, U.K.). Relative amounts of mRNA were normalized to β-actin mRNA within each sample.
Western Blotting and Glucose Transport.
BBMV were isolated from mouse small intestine by the method described (27) in the presence of a mixture of protease inhibitors (Roche Diagnostics, Indianapolis, IN). Western blot analysis was performed as described (5) with antisera to SGLT1 (5, 6), villin (clone 1D2C3; Abcam, Cambridge, U.K.), and β-actin (clone AC-15; Sigma–Aldrich). Immunoreactive bands were visualized by using horseradish peroxidase-conjugated secondary antibodies (DAKO, Carpenteria, CA) and enhanced chemiluminescence (Amersham Biosciences). Scanning densitometry was performed by using Phoretix 1D (Nonlinear Dynamics, Newcastle upon Tyne, U.K.). Relative levels of SGLT1 protein were normalized to β-actin levels within each sample. Glucose transport assays were performed as described (5, 27). d-glucose uptake was initiated by the addition of 100 μl of incubation medium containing 100 mM NaSCN (or KSCN), 100 mM mannitol, 20 mM Hepes/Tris (pH 7.4), 0.1 mM MgSO4, 0.02% (wt/vol) NaN3, and 0.1 mM d-[U14C]glucose to BBMV (100 μg of protein). The reaction was stopped after 3 sec by addition of 1 ml of ice-cold stop buffer, containing 150 mM KSCN, 20 mM Hepes/Tris (pH 7.4), 0.1 mM MgSO4, 0.02% (wt/vol) NaN3, and 0.1 mM phlorizin (5, 27). A 0.9-ml portion of the reaction mixture was removed and filtered under vacuum through a 0.22-μm pore cellulose acetate/nitrate filter (GSTF02500; Millipore, Bedford, MA). The filter was washed five times with 1 ml of stop buffer, and the radioactivity retained on the filter was measured by liquid scintillation counting. All uptakes were measured in triplicate.
Morphometry.
Morphometry was performed on 5-μm sagittal sections prepared from paraffin-embedded intestinal samples and stained with hematoxylin and eosin. Digital images were captured with an Eclipse microscope and DXM 1200 digital camera (Nikon, East Rutherford, NJ) and analyzed by using Image J software (National Institutes of Health, Bethesda, MD). Crypt depth was measured as the distance from crypt base to crypt–villus junction on an average of 17 well oriented crypts from 10 different sagittal sections of intestinal tissues from mice maintained on different diets and sweetener supplements. Villus height was measured as villus base to villus tip from an average of 18 well oriented villi.
In Situ Hybridization Histochemistry.
Tissue sections (fixed for 4 h in 4% paraformaldehyde in PBS) were paraffin wax embedded and sectioned at 5- to 7-μm thickness. After dewaxing and hydration, sections were permeabilized by a 20-min incubation in 0.2 M HCl, followed by two 3-min washes in 2× SSC, 1 h of incubation at 37°C in 50 mM Tris·HCl (pH 7.4) containing 2 μg/ml proteinase K (Sigma), and two 3-min washes in 0.2% glycine/PBS. Slides were then treated with two 10-min washes in 0.1 M triethanolamine (pH 8.0), containing 0.25% (vol/vol) acetic anhydride; postfixation in 4% paraformaldehyde/PBS; and 1-min block of endogenous alkaline phosphatase in 20% acetic acid to reduce background. Sections were prehybridized in hybridization buffer [50% deionized formamide/300 mM NaCl/20 mM Tris·HCl (pH 8.0)/5 mM EDTA/1× Denhardt's/1× RNA Protect (Sigma)/100 mg/ml dextran sulfate] for 1 h at 60°C and hybridized overnight at 25°C in hybridization buffer containing 100 μg/ml tRNA and 100 ng/ml DIG-labeled probe (SI Table 2). After hybridization, slides were washed for 1 h in 2× SSC, followed by 4 h at 25°C in 300 mM NaCl, 200 mM Tris·HCl (pH 8.0), 10 mM EDTA, 50% formamide, 1× Denhardt's and then 30 min in 2× SSC and 30 min in 0.2× SSC. Bound DIG-labeled probes were detected by 5-min equilibration in digoxigenin–alkaline phosphatase (DIG-AP) buffer [100 mM Tris·HCl (pH 7.5)/150 mM NaCl], followed by 30-min block in DIG-AP buffer containing 1% (wt/vol) DIG blocking reagent (Roche), overnight incubation in DIG-AP buffer containing 1% (wt/vol) DIG blocking reagent and anti-DIG AP-conjugated antibody diluted 1:1,000, 5-min wash in DIG-AP buffer, 5-min equilibration in NBT/BCIP buffer [100 mM Tris·HCl (pH 9.5)/100 mM NaCl/50 mM MgCl2], 1-h incubation in the dark in NBT/BCIP buffer containing 10% polyvinyl alcohol and NBT/BCIP mixture (Roche) diluted 1:50, and 5-min wash in 10 mM Tris·HCl (pH 8.0), 1 mM EDTA. Slides were then rinsed in tap water, counterstained in chloroform-extracted 1% methyl green for 5 min, and mounted in Vectamount (Vector Laboratories, Burlingame, CA).
Immunohistochemistry.
Sections of paraffin-embedded tissue (6-μm thick) were dewaxed in xylene and rehydrated; antigen retrieval was performed by incubation of tissue sections with 0.05% pronase (Roche) solution for 15 min at 37°C in a humidified chamber. Sections were washed in PBS and blocked for 30 min in 3% BSA, 0.3% Triton X-100, 2% goat serum, and 0.1% Na azide. Serial sections were incubated with primary antibody against Gαgust (Santa Cruz Biotechnology, Santa Cruz, CA), 1:1,000; T1R2 (Santa Cruz Biotechnology), 1:500; or T1R3 (14) 1:1,000, washed in PBS three times for 5min, and incubated with Cy3-conjugated goat anti-rabbit secondary antibody, (Jackson ImmunoResearch, West Grove, PA), and FITC-conjugated goat anti-rabbit secondary antibody (Molecular Probes, Eugene, OR). Images from serial sections were merged by using Volocity imaging software for confocal microscope (Improvision, Coventry, U.K.). For double-staining with Gαgust/T1R2/T1R3, the Zenon Alexa Fluor Rabbit IgG Labeling kit (Molecular Probes) was used: in brief, antibodies were preincubated with Alexa Fluor 488-labeled Fab fragments (green) or Alexa Fluor 594-labeled Fab fragments (red) for 10 min after 10-min incubation with blocking reagent (nonspecific IgG) to absorb excess labeling reagent. Directly labeled rabbit anti-Gαgust/T1R2/T1R3 antibodies were applied for 1 h. The sections were washed in PBS three times for 5 min after final incubation and mounted. Negative controls omitting the primary antibodies for gustducin and T1R3 were done with mouse and human tissues and were uniformly negative.
For chromogenic detection, after postfixation, antigen retrieval was performed by autoclaving in 10 mM Tris buffer (pH 10) for 22 min, and slides were pretreated for peroxidase blocking by incubation in 3% H2O2/PBS for 15 min. Sections were blocked for 1 h at room temperature in 5% BSA/PBS. The slides were then incubated at room temperature overnight with primary antibodies to SGLT1 or chromogranin A+B (Abcam), diluted 1:100 in 1% BSA/TBS, washed, and incubated with horseradish peroxidase-conjugated swine anti-rabbit secondary antibody (DAKO) diluted 1:200 in 1% BSA/TBS for 2 h at room temperature. Another three 5-min washes were performed, and then the slides were developed in 0.05% DAB/0.03% H2O2/50 mM Tris·HCl (pH 7.6) for 10 min at room temperature in the dark. The slides were then counterstained in 1% chloroform-extracted methyl green and mounted by using DPX (Raymond Lamb, Eastbourne, East Sussex, U.K.).
GLUTag Cells, GLP-I, GIP, and Ca2+ Imaging Assays.
For GLP-1 and GIP release assays, GLUTag cells (28) were incubated in PBS with or without gurmarin (3 μg/ml) for 15 min, followed by incubation (1 h) with sucralose (50 mM final concentration). The concentrations of GLP-1 and GIP (total) (active form) in the culture medium were determined by using GLP-1 and GIP ELISA kits (Linco Research, St. Charles, MO). For the Ca2+ imaging experiments GLUTag cells were transiently transfected by using Lipofectamine 2000 (Invitrogen) with the following plasmids: Gα16/gust44 [a plasmid encoding a Gα16-Gαgust chimeric G protein α-subunit that couples to sweet taste receptors (29)], YC3.60 (a ratiometric fluorescent indicator of free Ca2+) (30), and REEP-EI (a plasmid encoding a receptor-enhancing protein that promotes function of olfactory (30) and gustatory receptors (E.I. and R.F.M., unpublished data). Calcium responses of GLUTag cells to 20 mM sucralose were monitored 60 h after transfection with a Zeiss LSM510 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany). Time-lapse fluorescence imaging of YC3.60 was achieved by appropriate filter sets using a multitracking mode. Inhibition of T1R2+T1R3 sweet taste receptors endogenous to GLUTag cells was obtained by preincubation of cells (15 min) with the sweet taste inhibitor gurmarin (1 μg/ml) (25), which acts on the mouse T1R2+T1R3 sweet taste receptor (SI Fig. 10).
Statistical Analysis.
Results are expressed as means ± SD or means ± SEM, as indicated. Data were analyzed, as appropriate for the data set, by ANOVA with Dunnett's post test, or by unpaired two-tailed Student's t test (GraphPad Prism). P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. A. Ellis (Consultant Gastroenterologist, Royal Liverpool and Broadgreen Hospitals, Liverpool, U.K.) for the provision of human intestinal biopsies, A. Miyawaki (Brain Science Institute, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan) for the YC3.60 plasmid, D. J. Drucker (Department of Medicine and the Banting and Best Diabetes Centre, Toronto General Hospital, University of Toronto, Toronto, ON, Canada) for GLUTag cells and Tate and Lyle (London, U.K.) for the provision of sucralose. This work was supported by grants from the Wellcome Trust and the University of Liverpool (to S.P.S.-B.) and by National Institutes of Health(NIH)/National Institute on Deafness and Other Communication Disorders Grants DC003055 and DC003155 (to R.F.M.) and DC007399 (to B.M.). Confocal laser scanning microscopy was performed at the Mount Sinai School of Medicine-Microscopy Shared Resource Facility, supported by NIH and National Science Foundation Grants 5R24 CA095823-04, 1 S10 RR0 9145-01, and DBI-9724504.
Abbreviations
- BBMV
brush-border membrane vesicles
- GIP
glucose-dependent insulinotrophic peptide
- GLP-1
glucagon like peptide-1
- SGLT1
sodium-dependent glucose transporter isoform 1
- T1R
type 1 taste receptor.
Footnotes
Conflict of interest statement: R.F.M. has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from the Redpoint Bio company, and is an inventor on patents and patent applications which have been licensed to the Redpoint Bio company. S.P.S.-B. is the inventor on the subject matter of this paper “intestinal glucose sensor,” which is protected by a patent filed with the European Patent Office, EP04077610.6 and the U.S. Patent and Trademark Office, PCT/EP2005/054760.
See Commentary on page 14887.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706678104/DC1.
References
- 1.Margolskee RF. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 2.Solberg DH, Diamond JM. Am J Physiol. 1987;252:G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- 3.Ferraris RP, Diamond JM. Proc Natl Acad Sci USA. 1993;90:5868–5872. doi: 10.1073/pnas.90.12.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirazi-Beechey SP, Hirayama BA, Wang Y, Scott D, Smith MW, Wright EM. J Physiol (London) 1991;437:699–708. doi: 10.1113/jphysiol.1991.sp018620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer J, Hosie KB, Shirazi-Beechey SP. Gut. 1997;41:56–59. doi: 10.1136/gut.41.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer J, Vayro S, King TP, Shirazi-Beechey SP. Eur J Biochem. 2003;270:3377–3388. doi: 10.1046/j.1432-1033.2003.03721.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 8.Li XD, Staszewski L, Hu H, Durick K, Zoller M, Adler E. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin SK, McKinnon PJ, Margolskee RF. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 10.Höfer D, Asan E, Drenckhahn D. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Proc Natl Acad Sci USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer J, Salmon KSH, Zibrik L, Shirazi-Beechey SP. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 13.Wong GT, Gannon KS, Margolskee RF. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 14.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 15.Shirazi-Beechey SP, Smith MW, Wang Y, James PS. J Physiol. 1991;437:691–698. doi: 10.1113/jphysiol.1991.sp018619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Cell Tissue Res. 1992;267:3–9. doi: 10.1007/BF00319362. [DOI] [PubMed] [Google Scholar]
- 17.Kieffer TJ, Huang Z, McIntosh CH, Buchan AM, Brown JC, Pederson RA. Am J Physiol. 1995;269:E316–E322. doi: 10.1152/ajpendo.1995.269.2.E316. [DOI] [PubMed] [Google Scholar]
- 18.Reimann F, Gribble FM. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 19.Hansen L, Hartmann B, Mineo H, Holst JJ. Regul Pept. 2004;118:11–18. doi: 10.1016/j.regpep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan S, Wolfe MM, Schwartz JH, Singh SK. Gastroenterol. 2006;130(Suppl 2):A67–A68. [Google Scholar]
- 21.Ninomiya Y, Imoto T. Am J Physiol. 1995;268:R1019–R1025. doi: 10.1152/ajpregu.1995.268.4.R1019. [DOI] [PubMed] [Google Scholar]
- 22.Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Endocrinol. 1998;139:3780–3786. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- 23.Orskov C, Holst JJ, Khuhtsen K, Baldissera FGA, Poulsen SS, Nielsen OV. Endocrinol. 1986;119:1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- 24.Layer P, Holst JJ, Grandt D, Goebell H. Digest Dis Sci. 1995;40:1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 25.Rehfeld JF. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 26.Lescale-Matys L, Dyer J, Scott D, Freeman TC, Wright EM, Shirazi-Beechey SP. Biochem J. 1993;291:435–440. doi: 10.1042/bj2910435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirazi-Beechey SP, Davies AG, Tebbutt K, Dyer J, Ellis A, Taylor CJ, Fairclough P, Beechey RB. Gastroenterol. 1990;98:676–685. doi: 10.1016/0016-5085(90)90288-c. [DOI] [PubMed] [Google Scholar]
- 28.Drucker DJ. Am J Physiol. 1994;267:E629–E635. doi: 10.1152/ajpendo.1994.267.5.E629. [DOI] [PubMed] [Google Scholar]
- 29.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LMJ, Osman R, Margolskee RF, Max M. J Biol Chem. 2005;280:34296–34305. doi: 10.1074/jbc.M505255200. [DOI] [PubMed] [Google Scholar]
- 30.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.