Abstract
Coronary vasodilation is impaired in the postischemic heart with a loss of endothelial nitric oxide synthase (eNOS) activity, but the mechanisms underlying ischemia-induced eNOS dysfunction are not understood. For nitric oxide (NO) synthesis, eNOS requires the redox-sensitive cofactor tetrahydrobiopterin (BH4); however, the role of BH4 in ischemia-induced endothelial dysfunction remains unknown. Therefore, isolated rat hearts were subjected to varying durations of ischemia, and the alterations in NOS-dependent vasodilation were measured and correlated with assays of eNOS activity and cardiac BH4 concentrations. Ischemia time-dependently decreased cardiac BH4 content with 85, 95, or 97% irreversible degradation after 30, 45, or 60 min of ischemia, respectively. Paralleling the decreases in BH4, reductions of eNOS activity were seen of 58, 86, or 92%, and NOS-derived superoxide production was greatly increased. Addition of 10 μM BH4 enhanced eNOS activity in nonischemic hearts and partially restored activity after ischemia. It also suppressed NOS-derived superoxide production. Impaired coronary flow during postischemic reperfusion was improved by BH4 infusion. Thus, BH4 depletion contributes to postischemic eNOS dysfunction, and BH4 treatment is effective in partial restoration of endothelium-dependent coronary flow. Supplementation of BH4 may therefore be an important therapeutic approach to reverse endothelial dysfunction in postischemic tissues.
Keywords: ischemia reperfusion injury, nitric oxide, nitric oxide synthase uncoupling, superoxide, vascular function
Nitric oxide synthase (NOS) converts l-arginine and O2 to nitric oxide (NO) and l-citrulline. This enzymatic process consumes NADPH and requires Ca2+/calmodulin, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4) as NOS cofactors. Endothelial NO synthase (eNOS) contributes to the regulation of vasomotor tone and blood pressure by producing NO that activates soluble guanylate cyclase in vascular smooth muscle, resulting in vasorelaxation (1–3).
Endothelial dysfunction is a prognostic marker of cardiovascular disease (4). It has been suggested that limited availability of BH4 contributes to eNOS dysfunction in hypercholesterolemia, diabetes, atherosclerosis, hypertension, and heart failure (5–9). It was also observed previously that eNOS function is impaired in ischemic hearts (10). In vivo coronary artery occlusion triggers endothelial dysfunction and decreased eNOS-dependent vasoreactivity, although reactivity is preserved to exogenous NO (11, 12). Endothelial-dependent coronary vasoreactivity is impaired in hearts subjected to periods of global ischemia and reperfusion (10). Endothelium-dependent vasodilators induce a relatively high increase in coronary flow in control hearts or in those made ischemic for short times, but longer periods of ischemia result in an abrupt decline in vasodilatory response.
In addition to impairing eNOS-mediated NO formation, BH4 depletion may have additional detrimental effects in postischemic hearts. We (13) and others (14) have directly demonstrated that BH4 depletion not only prevents NO formation from eNOS but also results in markedly enhanced superoxide (•O2−) formation. Thus, BH4 has an important role in regulating eNOS function because BH4 depletion can switch the enzyme from synthesis of NO to •O2−.
BH4 is highly redox-sensitive and readily oxidized. With oxidative stress, intracellular BH4 content might transiently fall below a critical threshold (15), leading to NOS uncoupling (13) and preferential production of •O2− rather than NO.
In the ischemic and reperfused heart, there is a marked increase in oxygen radical generation (16, 17). Radical generation occurs within both the endothelium and myocytes (18–20). EPR studies have provided direct detection of free radicals in the ischemic and reperfused heart and demonstrated that •O2− and hydroxyl radicals are formed (16, 21, 22). These radicals and their secondary oxidant products could readily oxidize BH4.
Currently, it is not known how BH4 levels are altered by the process of ischemia and whether these alterations trigger postischemic vascular dysfunction and loss of NO production from eNOS. Therefore, the goal of this work is to determine the alterations in BH4 that occur after periods of myocardial ischemia and their role in ischemia-induced NOS dysfunction and loss of endothelial-mediated vasodilation. We observe that with ischemic periods longer than 30 min, near total depletion of BH4 occurs. This BH4 depletion causes a loss of NOS activity and induces •O2− production from the enzyme with a resultant loss of endothelial-dependent vasodilation.
Results
Effect of Ischemia/Reperfusion on Coronary Flow (CF).
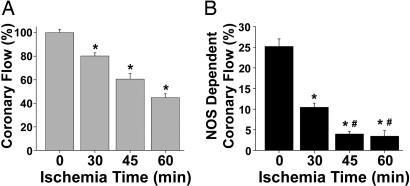
After hearts were subjected to periods of global ischemia (30, 45, or 60 min) followed by 30 min of reflow, basal CF values were measured and compared with the preischemic values. CF was decreased after ischemia, and this reduction in flow was proportional to the duration of ischemia (Fig. 1A).
Fig. 1.
Effect of ischemia/reperfusion on total and NOS-dependent CF. (A) CF, calculated as a percentage of basal, preischemic flow, was measured in hearts before ischemia and after periods of ischemia followed by 30 min of reperfusion. Longer periods of ischemia were associated with more severe reductions of CF. *, P < 0.0001 vs. preischemic control. (B) NOS-dependent CF was measured in control hearts (0 min of ischemia) with infusion of 1 mM l-NAME for 10 min after the 15-min equilibration period. NOS-dependent CF was similarly measured in hearts subjected to 30, 45, or 60 min of ischemia followed by 30 min of reperfusion with infusion of 1 mM l-NAME for 10 min at the end of the 30-min reperfusion period. NOS-dependent flow was calculated as the percentage of basal, preischemic, CF inhibited by l-NAME. NOS-dependent CF was decreased in the postischemic heart, which was a function of the ischemic duration. *, P < 0.001 vs. preischemic NOS-dependent flow; #, P < 0.01 vs. 30 min of ischemia, n = 5–7 hearts per group.
NOS Dependence of Coronary Flow in the Normal and Postischemic Heart.
To evaluate whether the loss of CF in the postischemic heart correlates with the onset of NOS dysfunction, a series of studies were performed measuring NOS-dependent CF. For these studies, the NOS inhibitor nitro-l-arginine methyl ester (l-NAME) (1 mM) was infused for 10 min after the equilibration period in hearts not subjected to ischemia or after 30 min of reperfusion in hearts subjected to 30, 45, or 60 min ischemia.
Although l-NAME infusion decreased CF before the onset of ischemia by 25 ± 2% (n = 7), a less prominent decrease was induced by this NOS inhibitor after ischemia and reperfusion. After 30 min of ischemia followed by 30 min of reperfusion, l-NAME decreased CF by 10.5 ± 1% (n = 5) and had a modest effect on basal postischemic CF when hearts were subjected to longer ischemic times (45 min, 4 ± 0.6% decrease, n = 5; 60 min, 3.4 ± 1.4% decrease, n = 5). These values were calculated as a percentage of basal, preischemic flow (Fig. 1B). Thus, loss of eNOS-dependent CF was largely responsible for the reduction in flow observed after 30 min of ischemia and also contributes to the loss of flow after longer ischemic durations. These functional observations suggest that eNOS-dependent NO production is greatly impaired in postischemic hearts (10).
NOS Activity After Myocardial Ischemia.
To characterize the alterations in cardiac NOS function, NOS activity was monitored in vitro by the l-[14C]arginine to l-[14C]citrulline conversion assay (23). NOS activity was measured from control hearts and hearts subjected to 15–90 min of global ischemia before and after addition of 10 μM BH4. NOS activity declined after all ischemic periods, being markedly impaired in hearts subjected to >30 min of ischemia. At 30 min of ischemia, activity was 42% of the preischemic control levels, and after 45, 60, and 90 min of ischemia it was <24% of control, suggesting that the duration of ischemia directly correlates with loss of NOS activity. BH4 restored eNOS activity above control values in hearts subjected to 15 or 30 min of ischemia and partially restored the enzyme activity in hearts subjected to longer ischemic periods (Fig. 2), suggesting basal BH4 insufficiency. Moreover, when we infused 50 μM liposomal BH4 in control nonischemic hearts, we were able to increase basal CF by ≈11%, reflecting a 44% increase in NOS-dependent flow, suggesting that normal cellular BH4 levels are NOS-limiting.
Fig. 2.
Effect of the duration of ischemia on cardiac eNOS activity. Hearts were homogenized at the end of normal perfusion or after different durations of ischemia, and eNOS activity was measured by l-[14C]arginine to l-[14C]citrulline conversion. Loss of NOS activity paralleled the duration of ischemia and was fully restored by BH4 with shorter ischemic times but only partially with longer durations. *, P < 0.001 + BH4 vs. basal eNOS activity, n = 5 hearts per point.
Effect of Ischemia on Cardiac BH4 Content.
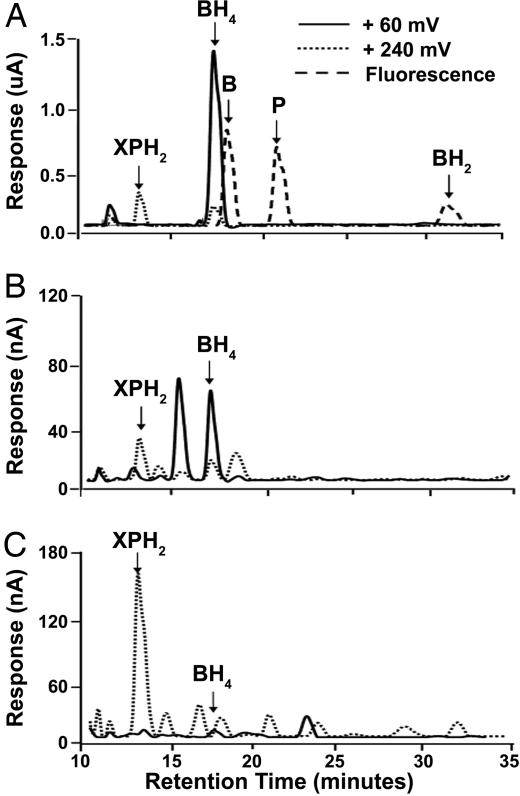
The results above suggest that eNOS activity was impaired in ischemic hearts and BH4 completely or partially restored this activity when added back to the heart homogenates. Subsequently, we measured cardiac BH4 content and its degradation after ischemia. BH4 and its metabolites 7,8-dihydrobiopterin (BH2), biopterin (B), pterin (P), and dihydroxanthopterin (XPH2) were assayed by HPLC with electrochemical detection (Fig. 3). These studies demonstrate that BH4 is depleted during ischemia (Figs. 4 and 5). BH4 levels were slightly lower in hearts subjected to 15 min of ischemia compared with controls. However, ischemic durations longer than 15 min caused a sharp decline in BH4 levels, which became almost undetectable after 60 min of 37°C global ischemia (≈95% depletion at ischemia times ≥30 min). The steep decline of BH4 cardiac content with prolonged ischemia is paralleled by an abrupt increase in XPH2. These data suggest that the degradation of the essential cofactor BH4 might cause a loss of NOS function. The fact that BH4 depletion parallels the decline of eNOS activity and that its addition to ischemic heart homogenates partially restores the activity of the enzyme to control nonischemic values, indicates that BH4 depletion limits NOS function in the postischemic heart. Interestingly, BH4 was largely oxidized to form XPH2, an irreversibly oxidized product with side-chain cleavage, and there was no detectable levels of either the 2- or 4-electron oxidized derivatives BH2, or B that could be potentially reduced back to BH4 (Fig. 3). Thus, BH4 is irreversibly degraded in the postischemic heart, which causes a loss of NOS activity.
Fig. 3.
Pathways of BH4 catabolism. BH4 is labile and readily oxidized and degraded to different biopterin and pterin derivatives through enzymatic or nonenzymatic processes.
Fig. 4.
Chromatograms of BH4 and its degradation products. (A) Chromatogram of a mixture of P and B derivative standards (10 μM XPH2, 10 μM BH4, 50 μM B, 50 μM P, and 20 μM BH2). (B) Chromatogram of a nonischemic rat heart homogenate. (C) Chromatogram from a heart after 60 min of ischemia. With ischemia, a marked loss of BH4 was seen with an increase in XPH2.
Fig. 5.
BH4 levels are depleted in ischemic hearts. At ischemic time intervals longer than 15 min, BH4 levels are markedly depleted. The decline of BH4 is paralleled by an increase in XPH2. (n = 5 hearts per point.)
•O2− Generation After Myocardial Ischemia.
Because BH4 depletion can trigger •O2− generation from NOS, experiments were performed to determine whether NOS-derived •O2− generation occurs. •O2− generation was assayed in nonischemic control hearts and hearts subjected to 30 min ischemia. Only trace •O2− generation was seen in control hearts and was not altered by the NOS inhibitor l-NAME. However, in postischemic hearts, •O2− production increased by 10-fold, and 1 mM l-NAME inhibited this increase by ≈50% (Fig. 6). With the addition of 10 μM BH4, this •O2− generation was also decreased significantly. Superoxide dismutase (SOD) quenched the observed fluorescence signal in ischemic hearts by 99%, confirming that it was derived from •O2−.
Fig. 6.
Superoxide generation in control and ischemic heart homogenates. In control (Ctr) nonischemic hearts there was only trace superoxide production, not inhibitable by l-NAME. Superoxide generation in hearts subjected to 30 min ischemia (I30) was increased 10-fold compared with Ctr, and 10 μM BH4 or 1 mM l-NAME inhibited this process. SOD almost totally quenched the observed signal in ischemic hearts, confirming it was derived from superoxide. *, P < 0.05 vs. I30; #, P < 0.001 vs. I30, n = 4.
BH4 Partially Restores Endothelial-Dependent Coronary Flow.
To determine whether postischemic BH4 supplementation can restore eNOS-dependent CF, experiments were performed in which 50 μM BH4 (in liposomal formulation) was infused for 10 min in control hearts and hearts subjected to 30, 45, and 60 min of ischemia and 30 min of reperfusion. With BH4 infusion in nonischemic hearts, an increase in CF of 11% was seen, with a 44% increase in NOS-dependent flow, confirming that even under control nonischemic conditions cellular BH4 levels limit NOS-dependent flow (Fig. 7). In hearts treated with BH4, CF augmentation was calculated as a percentage of eNOS-dependent flow, determined by 1 mM l-NAME infusion in normal nonischemic hearts. In hearts reperfused after 30, 45, or 60 min of ischemia, BH4 significantly enhanced NOS-dependent flow (Fig. 7). With prior administration of l-NAME, this BH4-mediated vasodilation was not seen (data not shown). Thus, BH4 treatment can partially restore the loss of NOS-dependent CF in postischemic hearts. In contrast to the restoration of CF seen with BH4 administration, comparable BH2 administration was totally ineffective in increasing CF.
Fig. 7.
Restitution of NOS-dependent CF. NOS-dependent flow was measured as described in Fig. 1, in nonischemic (0 ischemia time) or postischemic hearts subjected to 30, 45, or 60 min of ischemia and 30 min of reperfusion. Filled bars, values of NOS-dependent flow seen before BH4 infusion; white bars, increase in flow after BH4 treatment, 50 μM BH4 (in liposomal formulation) infused for 10 min (mean ± SE, n = 5–7 hearts per group).
Discussion
In the setting of acute myocardial infarction, it is critical to achieve rapid revascularization with reperfusion of the area at risk. Although reperfusion terminates ischemia, it induces a new form of injury termed “reperfusion injury” that is associated with alterations in cardiac myocytes and vasculature. High levels of reactive oxygen radicals and related oxidants are formed and are central mediators of postischemic injury. Previous studies have demonstrated in the heart and the isolated cells of which the heart is comprised that radical generation is greatly enhanced in both cardiac myocytes and endothelium (16, 18,21–22). In the postischemic heart, alterations in endothelial vasodilatory function occur because of a loss of NO production from eNOS, which in turn limits CF and triggers a range of problems including platelet and leukocyte adhesion and aggregation, leading to capillary plugging and vascular reocclusion (24, 25). Thus, impaired endothelial function is of critical importance in patients with acute coronary syndromes, and restoration of normal endothelial vasodilator function would tend to prevent problems of microvascular occlusion after acute recanalization.
Endothelial vasodilator function is largely derived from the production of NO that is synthesized by the enzyme eNOS, which converts the substrate l-arginine to l-citrulline and NO. This enzyme is one of a family of three well characterized mammalian NO synthases, eNOS, inducible NOS (iNOS), and neuronal NOS (nNOS), that require the substrates NADPH and O2 as well as the cofactor BH4. eNOS depends on the binding of Ca2+ and calmodulin for its activation, and its activity in NO production can also be modulated by the available levels of substrates and cofactors, phosphorylation status, and its binding to other proteins including caveolin and HSP90 (26–28).
Over the last decade, it has been appreciated that each of the NOS isoforms not only produces NO but can also become uncoupled to produce •O2− and H2O2. Initially, it was reported for nNOS and iNOS that depletion of l-arginine triggered •O2− generation from the isolated enzymes and in cells (29–31). In 1998, we and others observed that the redox sensitive cofactor BH4 is not only critical for NO generation from eNOS but that its depletion triggered prominent •O2− production from the enzyme (13, 14). Subsequently, it has been suggested that BH4 is a critical regulator of a broad range of cardiovascular disease including hypercholesterolemia, diabetes, atherosclerosis, and hypertension, which are all characterized by impaired endothelial-dependent vasodilation.
Although it was known that endothelial function is impaired in the postischemic heart with eNOS dysfunction (10), the mechanisms behind this dysfunction were only partially elucidated, and it was unclear whether there are therapeutic approaches that can restore this critical function. In view of the importance of BH4 in eNOS function and its known sensitivity to oxidant stress (32, 33), we hypothesized that: (i) BH4 depletion may contribute to the loss of eNOS and endothelial function observed in the postischemic heart and, (ii) that BH4 administration would be effective in restoring this function. To examine precisely the role of BH4 in postischemic endothelial dysfunction, studies were performed in an isolated heart model where NOS-dependent CF could be readily measured and NOS activity and BH4 levels assayed as a function of ischemic duration. The recovery of CF upon reperfusion progressively decreased in hearts subjected to increasing periods of ischemia. We observed that NOS-dependent flow accounts for 25 ± 2% of preischemic CF (Fig. 1B), which is consistent with a previous study of global ischemia and reperfusion in isolated rat hearts that reported endothelial-dependent NOS activity accounts for ≈30% of control, preischemic CF (10). The 20% decline in postischemic CF after 30 min of ischemia (Fig. 1A) was largely because of the loss of NOS-dependent flow, whereas after 45 min of ischemia about half of the decline was NOS-dependent with ≈85% loss of NOS-dependent CF seen (Fig. 1B).
Measurements of NOS activity showed that even in control hearts, NOS function is limited by BH4 availability. From our studies, we estimate that basal BH4 concentrations are ≈1 μM in the nonischemic heart, consistent with values reported for blood and kidney of ≈0.4 or 2 μM (34). Because BH4 levels of up to 10 μM would be required for saturation of NOS, the observed levels would be expected to limit NOS function (35). Furthermore, in the absence of added BH4 a ≈60% loss of NOS activity was seen after 30 min of ischemia and >90% loss after 60 min (Fig. 2). Addition of BH4 totally reversed the loss of NOS activity seen after 30 min but only partially reversed loss of activity after 45 or 60 min of ischemia. This finding is consistent with the observation of the present work that a marked loss of BH4 occurs after 30 min of ischemia, with >90% depletion after 45 min. The diminished restoration of NOS activity after ischemic durations of 45 min or longer is also consistent with prior immunoblotting studies showing no loss of eNOS protein at 30 min, but 38% and 60% losses after 60 min or 90 min, respectively (10). The loss of BH4 was accompanied by a rise in the irreversible metabolite XPH2 (Figs. 4 and 5). Thus, BH4 was found to be irreversibly oxidized with side-chain cleavage (Fig. 3). Interestingly, no formation of the potentially reversible oxidation product BH2 or B was seen.
Recently, we have observed that exposure of BH4 to oxidants such as •O2− and peroxynitrite results in rapid conversion to XPH2. The oxidation of BH4 to the quinoid BH2 occurs naturally during hydroxylation of the aromatic amino acids (36) and can occur by autooxidation (37) and oxidative stress (33). Dihydropterin reductase (DHPR) is the enzyme that converts the quinoid BH2 back to BH4 (Fig. 3) by using NADH preferentially, and this enzyme is present in the heart. It is known that DHPR activity is greatly decreased at acidic pH (38). Because in the globally ischemic heart pH drops to 5.5 (39), this acidosis could lead to DHPR inactivation. The quinoid BH2 is highly unstable, and in the absence of functional DHPR, it breaks down to either BH2 or PH2 (Fig. 3). The BH2 can be converted back to BH4 by dihydrofolate reductase (DHFR) in an NADPH dependent manner and the PH2 can subsequently breakdown to XPH2 (40). Although the activity of DHFR has not been measured in the ischemic heart, we observed that postischemic BH2 administration was totally ineffective in increasing CF and did not replete BH4. This finding suggests that the function of DHFR is impaired in postischemic myocardium, which could be the result of loss of enzyme activity or depletion of its requisite substrate NADPH.
The loss of BH4 is likely the result of oxidative degradation secondary to the formation of oxidants and oxygen free radicals that are enhanced during myocardial ischemia and subsequent reperfusion. Although the dissociation rate constants for BH4 release from NOS at neutral pH are slow, reported as 0.01–0.3 min−1 (41–43), BH4 release would likely be greatly accelerated under the acidotic conditions of the ischemic heart. Furthermore, oxidation of BH4 can occur to both free and NOS-bound biopterin with rapid loss of BH4 and XPH2 formation (J.L.Z., R.B., W. Chen, and A.J.C., unpublished results).
In the endothelium, the enzyme xanthine oxidase (XO) is an important central mechanism of •O2− and secondary oxidant formation in cells subjected to hypoxia and reoxygenation. XO-derived free radicals have been shown to trigger endothelial injury in reoxygenated bovine or human endothelial cells (18, 19). In rat hearts, XO has been shown to give rise to prominent radical generation that is largely stimulated by the high levels of its substrates hypoxanthine and xanthine that accumulate because of ATP degradation during ischemia (44, 45). More recently, a vascular NADPH oxidase has been identified and also implicated as a source of endothelial radical generation (46). In addition, as considered above, eNOS itself under conditions of oxidant stress with BH4 depletion generates •O2− that can also react with NO formed from the enzyme to produce the potent oxidant peroxynitrite that rapidly reacts with and consumes BH4. It has been demonstrated previously that NO generation and accumulation in the ischemic heart are increased markedly through NOS-dependent formation and NOS-independent nitrite reduction (39, 47). Upon reperfusion, this NO has been shown to react with •O2− to form peroxynitrite, which induces cellular oxidation and nitration (48). Thus, after an initial oxidant stress that partially depletes cellular BH4 levels, a vicious cycle of further •O2− and peroxynitrite production would be triggered that would further deplete BH4.
This process, once initiated, would tend to self-accelerate until all cellular BH4 is depleted. With complete BH4 depletion, eNOS would lose the ability to synthesize NO and effectively become solely an NADPH oxidase generating •O2−. After this transformation, activation of eNOS would no longer trigger vasodilation but vasoconstriction instead. Indeed, in the current work and a prior report, with activation of NOS after reperfusion following periods of ischemia of longer than 45 min, which correspond to near total BH4 depletion, stimulators such as histamine or bradykinin induced vasoconstriction rather than vasodilation (10).
With the large depletion of BH4 observed after ischemia, uncoupling of NOS occurred with prominent •O2− production from the enzyme. After 30 min of ischemia, a >10-fold increase in •O2− generation was seen, and this increase was inhibited >50% by the NOS inhibitor l-NAME. This NOS-derived •O2− generation was also inhibited by BH4. Thus, the depletion of BH4 in the postischemic heart triggers •O2− generation from NOS. Because BH4 is also a potent antioxidant and radical scavenger, its depletion would sensitize the heart and its vasculature to further oxidant stress.
In these studies, •O2− was detected from the washed membrane fraction to enrich eNOS and facilitate measurement of its •O2− generation. Although l-NAME inhibited the •O2− signal from this fraction by >80%, in whole rat hearts subjected to ischemia and reperfusion, only ≤30% inhibition of •O2−-derived radical generation is observed with l-NAME treatment (48). Clearly, other pathways of radical generation predominate in the whole heart. These pathways, such as XO and the mitochondrial electron transport chain, likely provide the oxidative stress responsible for the BH4 depletion that triggers •O2− generation from eNOS (16, 20, 44). Interestingly, we have observed previously that inhibition of XO enhances recovery of CF, suggesting that XO-derived radicals and oxidants contribute to postischemic endothelial dysfunction (44). From measurements of the NOS activity and •O2− generation in the membrane fraction, we estimate that at 30 min of ischemia >75% of the enzyme is converted from NO to •O2− generation, consistent with the marked BH4 depletion measured (Figs. 2 and 5).
When postischemic hearts were supplemented with BH4, prominent restitution of NOS-dependent CF was seen upon reperfusion after 30, 45, or 60 min of ischemia, demonstrating that cardiac BH4 treatment was effective in partially restoring NOS dysfunction. Although significant enhancement in NOS-dependent CF was seen, clearly with increasing periods of ischemia lesser restoration was achieved. Thus, although a loss of BH4 contributes to the loss of endothelial-dependent CF and NOS function, with longer periods of ischemia other processes also contribute to this dysfunction.
In summary, endothelial-dependent vasodilator function is impaired in the postischemic heart. This impairment occurs secondary to a loss of NO production from NOS with shift of the enzyme to the production of •O2−. This shift is triggered by the near total depletion of BH4 that was irreversibly oxidized to XPH2. Administration of exogenous BH4 restored NO production from NOS and enhanced postischemic recovery of NOS-dependent CF. Thus, cellular levels of BH4 are a key factor modulating eNOS activity, NO production, •O2− production, and vascular reactivity in the postischemic heart. Our results suggest that supplementation of BH4 or manipulation of its metabolism might be an important therapeutic approach to reverse the loss of endothelial vasodilatory and NOS function in the postischemic heart as well as in other oxidant-associated cardiovascular diseases.
Methods
Isolated Heart Perfusion.
Sprague–Dawley rats (300–350 g) were heparinized and anesthetized with 50 mg/kg pentobarbital, i.p. Hearts were excised, and their aortas were cannulated and perfused retrograde in Langendorff mode at constant pressure of 80 mmHg (1 mmHg = 133 Pa) with modified Krebs–Henseleit buffer (37°C), saturated with 95% O2/5% CO2. The perfusion buffer consisted of 120 mM NaCl/5.9 mM KCl/1.2 mM MgCl2/2.5 mM CaCl2/17 mM glucose/25 mM NaHCO3/1 mM EDTA, and it was filtered before use. A fluid-filled balloon connected to a pressure transducer was inserted into the left ventricle, and the end-diastolic pressure was adjusted to 8 mmHg. Data were acquired by using a PowerLab/400 system (ADInstruments, Colorado Springs, CO). CF was measured with a flow probe inserted directly above the cannula and connected to a T206 flow meter (Transonic Systems, Inc., Ithaca, NY). Left ventricular developed pressure, perfusion pressure, heart rate, and CF were monitored.
Materials.
Reagents were purchased from Sigma (St. Louis, MO), unless noted otherwise.
Experimental Protocols.
After 10–15 min of equilibration, hearts were subjected to periods of global 37°C ischemia of 15, 30, 45, 60, or 90 min. At the end of the given ischemic period, hearts were freeze-clamped and homogenized. BH4 levels, NOS activity, and •O2− production were then measured from heart homogenates as described below.
Three groups of hearts were subjected to 30, 45, and 60 min of ischemia followed by 30 min of reperfusion. At the end of reperfusion, 50 μM BH4 was infused for 10 min, and CF changes were measured. In preliminary experiments, we determined that BH4 infusion was most effective when we prepared BH4 along with 0.2 mg/ml ascorbate in a liposomal emulsion containing 50 mg/ml phosphatidylcholine in water.
Additional groups of hearts were studied to measure endothelial-dependent CF. These hearts were subjected to control perfusion or given ischemic durations followed by 30 min of reperfusion, and NOS-dependent CF was determined by infusing 1 mM l-NAME for 10 min, either at the end of the equilibration period before ischemia or after 30 min of reperfusion. Five to seven hearts were studied in each of these groups.
Detection of BH4 and Pterin Derivatives by Reverse-Phase HPLC.
Hearts were rapidly frozen in liquid nitrogen, finely ground, and suspended in water containing diethylenetriamene pentaacetate and 1 mg/ml ascorbate to prevent autooxidation. The suspension was homogenized, and the samples were loaded into Centricon filters (3,000 molecular weight cutoff; Millipore, Billerica, MA) and centrifuged for 60 min at 10,000 × g, 4°C. The filtrate was dried under vacuum and then resuspended in 50-μl mobile phase, and 20 μl was loaded into sample vials and injected on the HPLC column by using an ESA (Chelmsford, MA) refrigerated autosampler. For HPLC, a reverse-phase column was used, with the mobile phase consisting of 100 mM KH2PO4/25 mM octyl sodium sulfate/0.6 mM EDTA/2% methanol. The pH was adjusted to 2.5 with concentrated phosphoric acid, and just before use, DTT was added to give a final concentration of 0.16 mM. The chromatographic separation was performed using a 5-μm ODS-80TM reverse phase column (4.6 mm × 25 cm; Tosoh Bioscience, Stuttgart, Germany) with flow rate set at 1.3 ml/min. BH4 and XPH2 were electrochemically detected with an ESA coularray HPLC system, with the analytical electrodes set at 0.06 and 0.35 V and the guard cell set at 0.8 V. B, P, and BH2 were measured by HPLC fluorescence.
Measurements of NOS Activity.
Heart tissue was processed for NOS measurements in a manner similar to that detailed previously (10). Hearts were rapidly frozen in liquid nitrogen, finely ground, and suspended in 3 ml of ice-cold buffer consisting of 50 mM Tris, pH 7.4/0.1 mM EDTA/0.1 mM EGTA/12 mM mercaptoethanol/2 mM PMSF/4 μM leupeptin. The suspension was homogenized and centrifuged at 100,000 × g for 60 min, at 4°C. The particulate fraction was subsequently washed in 3 ml of ice-cold buffer containing 1 M KCl for 5 min and centrifuged at 100,000 × g for 30 min at 4°C. The supernatant was discarded, and the pellet was rinsed several times with buffer to remove the excess KCl.
NOS activity was measured from the conversion rate of l-[14C]arginine to l-[14C]citrulline (23). The reaction mixture contained 3 mM NADPH/200 μM CaCl2/30 μM EDTA/30 μM EGTA/100 nM calmodulin in Tris buffer. The reaction was initiated by the addition of purified l-[14C]arginine (317 mCi/mmol) to produce a 10 μM final concentration and was carried out for 8 min at 37°C. The reaction was quenched with 3 ml of ice-cold stop buffer (20 mM Hepes/2 mM EDTA, pH 5.5). Experiments were also performed in the presence of either 5 mM EGTA or 250 μM l-NAME. l-[14C]Citrulline content was determined by liquid scintillation counting after separation from the reaction mixture, by passage through a column of the cation exchange resin Dowex AG 50W X-8 (500 μl of the Na+ form). NOS activity was determined by subtracting total counts from l-NAME-blocked counts and normalized for protein content (measured by the Bradford method) and conversion time (10). All activity detected was calcium-dependent and could be blocked by EGTA, confirming that it was from eNOS.
Measurements of Superoxide Production.
Measurements were performed in control hearts and hearts subjected to 30 min of ischemia (±10 μM BH4), with homogenates prepared as noted above. Forty-five microliters of each sample was mixed with 45 μl of NOS assay buffer, and hydroethidine (HE) was added, for a final concentration of 10 μM. After addition of HE all samples were placed in a Spectra MAX GEMINI plate reader (510 nm and 590 nm excitation and emission wavelengths; Molecular Devices, Sunnyvale, CA) to measure the formation of the •O2−-derived product 2-HE with this production measured over 5 min. We subtracted background fluorescence from all of the counts and normalized measurements for protein content. Measurements were repeated by using 1 mM l-NAME and Cu-Zn SOD (500 units per reaction) to determine specificity of •O2− generation.
Statistical Analysis.
Results were expressed as mean ± SE. Statistical significance was estimated by one-way ANOVA. If the F ratio exceeded a critical value of P < 0.05, variance analysis was followed by Newman–Keuls post hoc analysis, to compare the group means. A value of P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL63744 (to J.L.Z.), HL65608 (to J.L.Z.), HL38324 (to J.L.Z.), HL77575 (to Y.X.), and HL86965 (to Y.X.).
Abbreviations
- B
biopterin
- BH2
7,8-dihydrobiopterin
- BH4
tetrahydrobiopterin
- CF
coronary flow
- DHFR
dihydrofolate reductase
- DHPR
dihydropterin reductase
- eNOS
endothelial nitric oxide synthase
- l-NAME
nitro-l-arginine methyl ester
- NOS
nitric oxide synthase
- •O2−
superoxide
- P
pterin
- SOD
superoxide dismutase
- XO
xanthine oxidase
- XPH2
dihydroxanthopterin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Palmer RM, Ferrige AG, Moncada S. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs EA. Br J Pharmacol. 2006;147:193–201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 5.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott-Burden T. Circulation. 1995;91:248–250. doi: 10.1161/01.cir.91.1.248. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Harrison DG. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 8.Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL. J Biol Chem. 1997;272:21420–21426. doi: 10.1074/jbc.272.34.21420. [DOI] [PubMed] [Google Scholar]
- 11.VanBenthuysen KM, McMurtry IF, Horwitz LD. J Clin Invest. 1987;79:265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta JL, Nichols WW, Donnelly WH, Lawson DL, Saldeen TG. Circ Res. 1989;64:43–54. doi: 10.1161/01.res.64.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Tsai AL, Berka V, Zweier JL. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T Harrison DG. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 16.Zweier JL, Flaherty JT, Weisfeldt ML. Proc Natl Acad Sci USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolli R, Jeroudi MO, Patel BS, Dubose CM, Lai EK, Roberts R, Mccay PB. Proc Natl Acad Sci USA. 1989;86:4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier JL, Kuppusamy P, Lutty GA. Proc Natl Acad Sci USA. 1988;85:4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zweier JL, Broderick R, Kuppusamy P, Thompsongorman S, Lutty GA. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]
- 20.Zweier JL, Talukder HM. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Zweier JL. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 22.Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. J Biol Chem. 1989;264:18890–18895. [PubMed] [Google Scholar]
- 23.Bredt DS, Snyder SH. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosio G, Weisman HF, Mannisi JA, Becker LC. Circulation. 1989;80:1846–1861. doi: 10.1161/01.cir.80.6.1846. [DOI] [PubMed] [Google Scholar]
- 25.Duilio C, Ambrosio G, Kuppusamy P, Dipaula A, Becker LC, Zweier JL. Am J Physiol. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 26.Gautier C, Negrerie M, Wang ZQ, Lambry JC, Stuehr DJ, Collin F, Martin JL, Slama-Schwok A. J Biol Chem. 2004;279:4358–4365. doi: 10.1074/jbc.M305048200. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Gachhui R, Crooks C, Wu CQ, Lisanti MP, Stuehr DJ. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- 28.Gratton JP, Bernatchez P, Sessa WC. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Zweier JL. Proc Natl Acad Sci USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Y, Roman LJ, Masters BSS, Zweier JL. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 31.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 32.Vasquez-Vivar J, Whitsett J, Martasek P, Hogg N, Kalyanaraman B. Free Radic Biol Med. 2001;31:975–985. doi: 10.1016/s0891-5849(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 33.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 34.Sawabe K, Wakasugi KO, Hasegawa H. J Pharmacol Sci. 2004;96:124–133. doi: 10.1254/jphs.fp0040280. [DOI] [PubMed] [Google Scholar]
- 35.Wei CC, Crane BR, Stuehr DJ. Chem Rev. 2003;103:2365–2383. doi: 10.1021/cr0204350. [DOI] [PubMed] [Google Scholar]
- 36.Fisher DB, Kaufman SJ. J Biol Chem. 1973;248:4300–4304. [PubMed] [Google Scholar]
- 37.Davis MD, Kaufman S, Milstien S. Eur J Biochem. 1988;173:345–351. doi: 10.1111/j.1432-1033.1988.tb14004.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiefer PM, Varughese KI, Su Y, Xuong NH, Chang CF, Gupta P, Bray T, Whiteley JM. J Biol Chem. 1996;271:3437–3444. doi: 10.1074/jbc.271.7.3437. [DOI] [PubMed] [Google Scholar]
- 39.Zweier JL, Wang PH, Samouilov A, Kuppusamy P. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 40.Armarego WL, Randles D, Taguchi H. Eur J Biochem. 1983;135:393–403. doi: 10.1111/j.1432-1033.1983.tb07666.x. [DOI] [PubMed] [Google Scholar]
- 41.Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, Mayer B. J Biol Chem. 1994;269:13861–13866. [PubMed] [Google Scholar]
- 42.List BM, Klosch B, Volker C, Gorren AC, Sessa WC, Werner ER, Kukovetz WR, Schmidt K, Mayer B. Biochem J. 1997;323:159–165. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh DK, Wu C, Pitters E, Moloney M, Werner ER, Mayer B, Stuehr DJ. Biochemistry. 1997;36:10609–10619. doi: 10.1021/bi9702290. [DOI] [PubMed] [Google Scholar]
- 44.Thompson-Gorman SL, Zweier JL. J Biol Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- 45.Xia Y, Zweier JL. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 46.Suh YA, Arnold RS, Lassegue B, Shi J, Xu XX, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 47.Zweier JL, Wang PH, Kuppusamy P. J Biol Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- 48.Wang PH, Zweier JL. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]