Abstract
Increased production of tumor necrosis factor α (TNF) in the bone marrow (BM) in response to both oxidative stress and T cell activation contributes to the bone loss induced by estrogen deficiency, but it is presently unknown whether oxidative stress causes bone loss through T cells. Here we show that ovariectomy causes an accumulation in the BM of reactive oxygen species, which leads to increased production of TNF by activated T cells through up-regulation of the costimulatory molecule CD80 on dendritic cells. Accordingly, bone loss is prevented by treatment of ovariectomized mice with either antioxidants or CTLA4-Ig, an inhibitor of the CD80/CD28 pathway. In summary, reactive oxygen species accumulation in the BM is an upstream consequence of ovariectomy that leads to bone loss by activating T cells through enhanced activity of BM dendritic cells, and these findings suggest that the CD80/CD28 pathway may represent a therapeutic target for postmenopausal bone loss.
Keywords: CTLA-4Ig, reactive oxygen species, T cells, osteoporosis, tumor necrosis factor
Postmenopausal bone loss is induced by ovarian involution and the resulting increase in follicle-stimulating hormone production through multiple effects on bone marrow (BM) and bone cells (1–4), but the most relevant consequence of natural menopause and ovariectomy (ovx) is a cytokine-driven increase in osteoclast (OC) formation (2, 4). OC formation occurs when bone marrow macrophages (BMMs) are costimulated by the essential osteoclastogenic factors receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) (2), but after menopause TNF is produced in greater amounts in the BM and up-regulates OC formation (4, 5). TNF stimulates the production of RANKL and M-CSF by bone and BM cells (6, 7), augments the responsiveness of OC precursors to RANKL (8, 9), and induces additional osteoclastogenic cytokines such as IL-1, IL-6, and IL-7 (4). Studies with genetic and pharmacological models have demonstrated the pivotal role of TNF in ovx-induced bone loss in rodents and humans (10–13) and have provided insights on the estrogen (E)-regulated sources of TNF. Ovx up-regulates the production of TNF by BM T lymphocytes (8, 12) by increasing thymic T cell output (14) and by stimulating the antigen (Ag)-dependent activation and expansion of T cells in the BM (15). Attesting to the relevance of T cell-produced TNF, T cell-deficient nude mice are protected against the loss of cortical and trabecular bone induced by ovx (8, 12, 16). Furthermore, the capacity of ovx to induce bone loss is restored by adoptive transfer into nude mice of T cells from wild-type (WT) but not of those from TNF −/− mice (12).
T cell activation is caused by the presentation to T cells of Ag fragments bound to MHC molecules expressed on Ag-presenting cells (APCs) (17). Ovx up-regulates the capacity of BMMs to present Ag to CD4+ T cells (4, 15), but no information is available on how ovx activates CD8+ T cells and on whether ovx has additional stimulatory effects on BM DCs, the most potent professional APCs residing in the BM (18).
Ovx alters the generation of ROS and the antioxidant defense capacity of the cell (19, 20), leading to an accumulation of ROS, which stimulate the production of TNF in the BM. This effect is compounded by the capacity of ROS to stimulate OC formation and activity directly (20–22). However, little information is available regarding the cell lineage that produces TNF in response to ROS (19).
Because ROS activate Ag presentation by DCs (23) and activated T cells further stimulate Ag presentation by BMMs through production of IFNγ, a plausible hypothesis, tested herein is that ovx induces the activation of both CD4+ and CD8+ cells through ROS-mediated stimulation of Ag presentation by DCs. We show that the generation of ROS represents an “upstream” event that leads to bone loss by increasing the capacity of DCs to present Ag, thus promoting the production of TNF by activated T cells.
Results
Ovx Selectively Activates BM DCs Through Increased Costimulation by CD80.
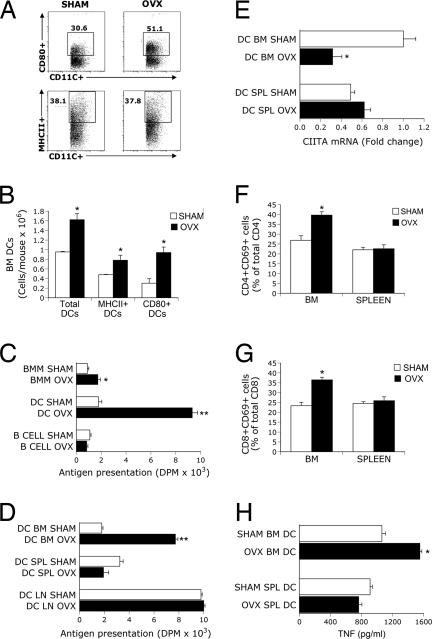
To assess the effects of ovx on the number and function of APCs, BM was harvested 2 weeks after sham operation or ovx, a time when the rate of bone loss is most rapid (8, 12, 16). Ovx did not alter the percentage of DCs (data not shown) nor that of DCs expressing MHCII (Fig. 1A), whereas it increased the percentage of DCs expressing CD80, a costimulatory molecule that binds to the T cell receptor CD28 (17). Confirming earlier reports (15), ovx caused a 2-fold increase in the total number of BMMs and BM B cells (data not shown). The total number of BM DCs was also ≈50% higher in ovx mice than in sham controls (Fig. 1B). Ovx caused only a small increase in the number of MHCII+ DCs, whereas the number of DCs expressing CD80 was ≈3.5-fold higher in ovx than in sham mice. Attesting to specificity, ovx did not affect the expression of the DC costimulatory molecules CD86, CD40, PDL-1, PDL-2, and OX40 (data not shown).
Fig. 1.
Effects (mean ± SD) of ovx on DC expression of MHCII and CD80, Ag presentation, and T cell activation. (A) Ovx up-regulates the percentage of CD80+ but not that of MHCII+ BM DCs. FACS analysis was carried out by gating CD11c+ cells and analyzing for MHCII and CD80 expression. Dot plot shows one of three independent experiments. (B) Ovx increases the number of BM DCs, MHCII+ DCs, and CD80+ DCs. (C) Ovx increases Ag presentation by BMMs and BM DCs. (D) Ovx increases Ag presentation by BM but not spleen (SPL) and lymph node (LN) DCs. (E) Ovx decreases CIITA mRNA expression in BM DCs. (F and G) Ovx increases the expression of the activation marker CD69 in CD4+ and CD8+ BM T cells. (H) TNF levels in conditioned medium from coculture of BM DCs, OT-II T cells, and ovalbumin; n = 4 mice per group in each one of triplicate experiments. *, P < 0.05; **, P < 0.001 compared with sham mice.
Characterization studies of BM DCs revealed that ovx increased the percentage of both CD80+ plasmacytoid (B220+) DCs, and CD80+CD8− conventional (B220−) DCs, but not that of CD80+CD8+B220− DCs [supporting information (SI) Fig. 5]. Furthermore, ovx did not alter the percentage of any CD80− DC lineage. Reflecting the generalized increase in BM cellularity induced by ovx, the total number of most BM DC subsets was higher in ovx than in sham mice (SI Table 2). However, the increase induced by ovx was larger for CD80+ conventional DCs than for the other DC lineage.
Ovx increased Ag presentation by BMMs and BM DCs by ≈2- and ≈5-fold, respectively (Fig. 1C). The effect of ovx on DCs was organ-specific because ovx increased the activity of BM DCs, but it failed to affect that of spleen and lymph node DCs (Fig. 1D).
The expression of class II transactivator (CIITA), a gene that encodes for a nuclear activator of MHCII transcription, is characteristically down-regulated as DCs acquire the capacity to present Ag (24). Attesting to an effect of ovx on DC activation, we found ovx to decrease the expression of CIITA mRNA by BM DC by ≈60% (Fig. 1E). Analysis of the T cell activation marker CD69 at 2 weeks revealed that ovx increases CD69 expression on BM CD4+ (Fig. 1F) and CD8+ T cells (Fig. 1G) but not in spleen T cells. Thus, the activation of DCs coincides, both spatially and temporally, with the activation of T cells.
To assess whether T cell TNF production coincides with DC activation, we cocultured γ-irradiated BM and spleen DCs from sham and ovx mice with the avian Ag ovalbumin and T cells from OT-II mice, a strain with T cells that harbor a transgenic T cell receptor responsive exclusively to ovalbumin. In this model ovalbumin-derived peptides presented by APCs to T cells induce T cell activation and T cell cytokine secretion. TNF was measured in the culture medium at the end of a 3-day culture period. Ovx increased the production of TNF by ≈40% in cocultures containing BM DC, whereas it had no effects in those containing spleen DCs (Fig. 1H). Together, the data demonstrate that increased Ag presentation by BM DCs leads to T cell activation and T cell TNF production.
Ovx Induces a Tissue-Selective Increase in Oxidative Stress in the BM.
Oxidative stress has been implicated in ovx-induced bone loss (19, 20, 22), although how ovx increases oxidative stress is unknown. To address this issue we measured the levels of APE-1/Ref-1, peroxiredoxin-1 (Prx-1), and glutathione (GSH) in BM, spleen, and liver. These two latter tissues were chosen as controls because they are known active sites of ROS production both under physiological and pathological conditions (25–27). APE-1/Ref-1 is an inhibitor of the NADPH oxidase that regulates the intracellular generation of ROS (28) and is also a redox coactivator of NF-κB (29). Prx-1 provides cytoprotection against cellular oxidative stress through its thioredoxin-dependent peroxidase activity (30), whereas GSH is essential to eliminate intracellular peroxide (31).
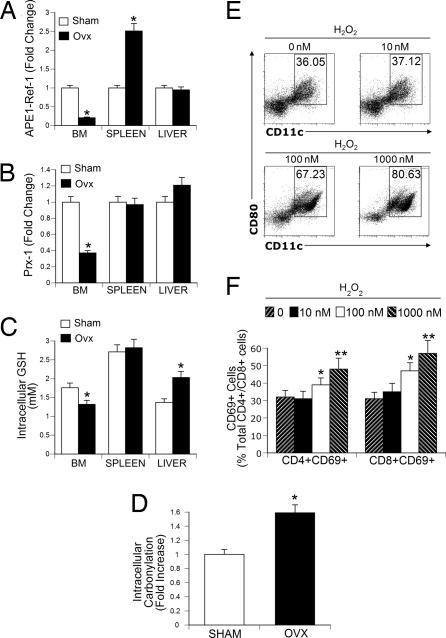
In the BM, ovx induced a 4-fold decrease in intracellular APE-1/Ref-1 (Fig. 2A), a nearly 3-fold decrease in Prx-1 levels (Fig. 2B), and a 30% decrease in intracellular GSH (Fig. 2C). Unexpectedly, ovx also caused an increase in APE-1/Ref-1 in the spleen and in GSH in the liver. Representative Western blots showing that ovx down-regulates the levels of APE-1/Ref-1 and Prx-1 in BM but not in spleen or liver are shown in SI Fig. 6.
Fig. 2.
Ovx induces oxidative stress and T cell activation in the BM. (A and B) Densitometric quantification of Western blots. (A) Effect of ovx on intracellular levels of APE-1/Ref-1. (B) Effect of ovx on BM Prx-1 levels. (C) Effect of ovx on GSH levels in the BM. (D) Ovx increases protein carbonylation in BM lysates. (A–D) All data are the mean ± SD of three independent experiments (n = 3 mice per group; *, P < 0.05, compared with the corresponding sham group). (E) H2O2 causes a dose-dependent increase in the relative number of mature BM DC (CD80+ MHCIIhi CD11c+ cells). Whole BM was cultured in α-MEM with 10% FBS for 24 h and treated with the indicated concentrations of H2O2. Cell viability was confirmed by trypan blue exclusion. FACS analysis was carried out by gating MHCIIhi cells and analyzing for CD11c and CD80 expression. Dot plot shows one of three independent experiments. (F) H2O2 increases CD69 expression in CD4+ and CD8+ BM T cells. (E and F) Data are the mean ± SD of three independent experiments (n = 4 mice per group; *, P < 0.05; **, P < 0.01 compared with unstimulated BM).
Protein carbonyl formation is a consequence of oxidative damage to cellular proteins (32). Thus, protein carbonylation was quantified in the BM to determine the extent of oxidative damage and was found to be significantly increased by ovx (Fig. 2D). Therefore, ovx induces a tissue-selective decrease in both ROS intracellular scavengers and inhibitors of ROS generation, leading to a local accumulation of ROS-induced protein damage in the BM.
Antioxidants Prevent the Activation of BM DCs and T Cells Induced by Ovx.
One relevant consequence of increased ROS production is induction of APC maturation and T cell activation (23, 33). We thus investigated whether ovx increases Ag presentation by DCs, T cell activation, and T cell TNF production, through oxidative stress. First, we assessed whether CD80 expression is directly modulated by ROS. Thus, BM from intact mice was treated in vitro with hydrogen peroxide (H2O2) for 24 h to increase the levels of ROS. Measurements of cell viability by trypan blue exclusion showed the viability of BM cells to be >90% and >75% after treatment with H2O2 at 100 nM and 1,000 nM, respectively. FACS analysis of gated MHCIIhi cells, a population of mature APCs, revealed that H2O2 caused a dose-dependent expansion of the pool of CD80+CD11c+ cells (Fig. 2E) and an increase in CD4 and CD8 T cell activation (Fig. 2F). These findings suggest that CD80-dependent T cell costimulation is up-regulated by ROS.
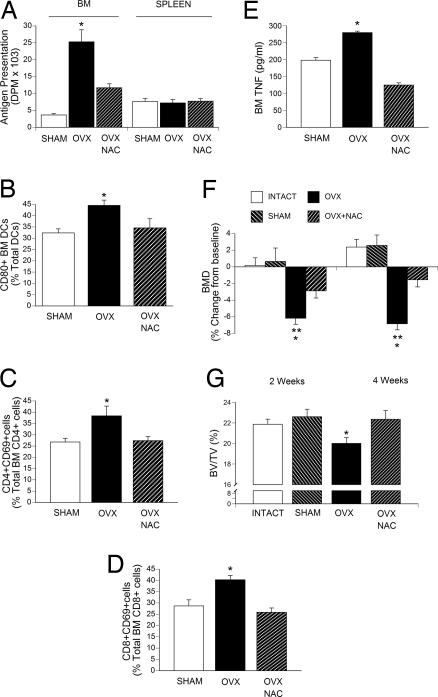
To determine whether ovx activates T cells through oxidative stress in vivo, ovx mice were treated for 4 weeks with N-acetylcysteine (NAC), an antioxidant that restores the intracellular pool of GSH, preventing increases in ROS. Measurements of GSH levels in the BM confirmed that NAC completely prevented the decrease in GSH levels induced by ovx (data not shown). Furthermore, NAC prevented the increase in Ag presentation by BM DCs (Fig. 3A), the increase in the relative number of CD80+ BM DCs (Fig. 3B), increases in the total number of BM CD69+CD4+ (Fig. 3C) and CD69+CD8+ T cells (Fig. 3D), and the increase in TNF production by ex vivo cultures of BM (Fig. 3E).
Fig. 3.
Effects (mean ± SD) of in vivo treatment of ovx mice with 100 mg/kg per day i.p. NAC for 4 weeks. (A) Ag presentation by BM and spleen DCs. (B) Percentage CD80+ BM DCs. (C) Percentage CD4+CD69+ BM T cells. (D) Percentage CD8+CD69+ BM T cells. (E) Levels of TNF in 24-h BM cultures stimulated with phorbol 12-myristate 13-acetate and ionomycin. (F) Femur BMD as measured in vivo by DXA at 2 and 4 weeks. (G) μCT measurements of percentage total volume occupied by trabecular bone volume (BV/TV) in vertebras harvested 4 weeks after surgery (n = 7 mice per group; *, P < 0.05 compared with sham mice; **, P < 0.05 compared with baseline).
Furthermore, in vivo measurement of bone mineral density by dual-energy x-ray absorptiometry (DXA), a technique that provides a combined assessment of cortical and trabecular bone, revealed that NAC treatment decreased the femoral bone loss induced by ovx (Fig. 3F). Ex vivo micro-computed tomography (μCT) measurements of vertebral trabecular bone, the skeletal site most affected by ovx, demonstrated that NAC prevents the loss of trabecular bone induced by ovx (Fig. 3G). Thus, the ovx-induced accumulation of ROS in BM stimulates immune cell activation and TNF production, whereas antioxidants prevent ovx-induced bone loss.
CTLA4-Ig Blocks Costimulation by CD80 Receptor and Protects Mice from Ovx-induced Bone Loss.
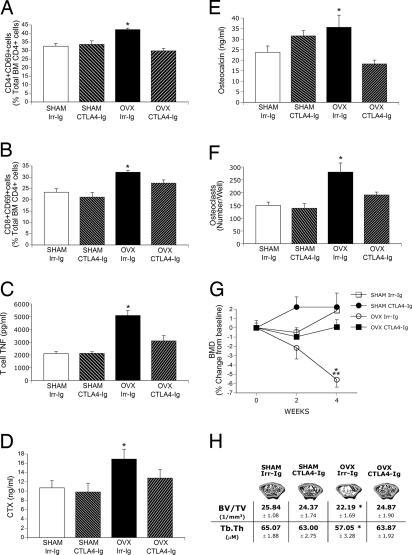
Up-regulation of CD80 expression on APCs and its binding to CD28 on T cells is one of the earliest events in Ag-dependent T cell activation (17). To test the relevance of CD80-mediated T cell activation on ovx-induced bone loss, mice were treated with 500 μg/day CTLA4-Ig, an immunosuppressant that inhibits the binding of CD80 (and CD86) to CD28, or 500 μg/day irrelevant (Irr) isotype-matched Ig. In a first experiment, mice were treated with CTLA4-Ig or Irr-Ig three times a week for 4 weeks. CTLA4-Ig prevented the increase in the number of CD4+CD69+ and CD8+CD69+ BM T cells (Fig. 4 A and B) as well as the increase in T cell TNF production induced by ovx (Fig. 4C), confirming that ovx activates T cells through Ag presentation. Measurements of serum C-terminal telopeptides (CTX), a marker of bone resorption (Fig. 4D), and serum osteocalcin, a marker of formation (Fig. 4E), revealed that the increase in both indices induced by ovx was prevented by CTLA4-Ig.
Fig. 4.
CTLA4-Ig prevents T cell activation, T cell TNF production, OC formation, and the bone loss induced by ovx. All data are shown as means ± SD. Ovx- and sham-operated mice were treated with CTLA4-Ig (500 μg/mouse) or isotype-matched Irr-Ig (500 μg/mouse) three times per week for 4 weeks. (A) Percentage CD4+CD69+ BM T cells. (B) Percentage CD8+CD69+ BM T cells. (C) TNF levels in 24-h BM T cell culture medium. (D) Serum CTX levels. (E) Serum osteocalcin levels. (F) No. of OCs in 8-day BM cultures stimulated with subsaturating doses of RANKL and M-CSF. (G) In vivo measurements of femoral BMD by DXA. (H) Epiphyseal trabecular volume over total volume and trabecular thickness (Tb.Th) as measured by μCT in femurs at 4 weeks from surgery (n = 7–8 mice per group; *, P < 0.05 compared with Irr-Ig-treated sham controls; **, P < 0.05 compared with baseline.
To investigate the effect of CTLA4-Ig on in vitro OC formation, whole BM was cultured in the presence of a suboptimal concentration of RANKL. BM from ovx mice generated a 2-fold higher number of OCs than BM from sham controls (Fig. 4F), and the ovx-induced increase in OC formation was prevented by in vivo CTLA4-Ig treatment. In vitro treatment of osteoclastogenic cultures of BMMs with CTLA4-Ig did not alter the production of mature OCs (data not shown), demonstrating that CTLA4-Ig inhibits OC formation in vivo without directly inhibiting OC differentiation.
In vivo prospective measurements of femoral bone mineral density by DXA showed (Fig. 4G) that CTLA4-Ig completely prevented the femoral bone loss induced by ovx. μCT measurements of femoral trabecular bone harvested at 4 weeks showed (Fig. 4H) that treatment with CTLA4-Ig afforded nearly complete protection against the loss of trabecular bone volume and trabecular thickness induced by ovx. Analysis of trabecular bone by histomorphometry (Table 1) confirmed that treatment with CTLA4-Ig prevents the loss of trabecular bone volume induced by ovx as well as the increase in bone resorption, as assessed by measurements of the number of OCs per bone surface and the percent eroded surfaces. Treatment with CTLA4-Ig also prevented the ovx-induced increase in the percent of bone surface covered by osteoid and the percent of bone surface covered by osteoblasts, two indices of bone formation.
Table 1.
Effects of CTLA4-Ig treatment on histomorphometric indices of trabecular bone resorption, formation, and volume in WT mice (n = 7–8 mice per group)
| Index | Sham Irr-Ig | Sham CTLA4-Ig | Ovx + Irr-Ig | Ovx CTLA4-Ig |
|---|---|---|---|---|
| BV/TV, % | 28.25 ± 0.78 | 28.03 ± 2.44 | 18.59 ± 1.74* | 31.82 ± 1.97 |
| N.Oc/BS, OC/mm | 0.29 ± 0.02 | 0.40 ± 0.09 | 0.62 ± 0.13* | 0.43 ± 0.10 |
| ES/BS, % | 0.72 ± 0.11 | 1.30 ± 0.42 | 2.85 ± 0.30* | 1.25 ± 0.32 |
| OS/BS, % | 1.27 ± 0.39 | 2.32 ± 0.52 | 5.95 ± 1.46* | 1.46 ± 0.52 |
| ObS/BS, % | 3.43 ± 0.35 | 6.11 ± 1.16 | 8.03 ± 1.14* | 4.52 ± 0.63 |
WT mice were treated with CTLA4-Ig or isotype-matched Irr-Ig (500 μg per day of each) three times a week for 4 weeks, and femurs were analyzed by histomorphometry. BV/TV (the percentage of total volume occupied by trabecular bone volume) is an index of bone structure. N.OC/BS (the no. of osteoclasts per mm of bone surface), and ES/BS (the percentage of eroded surfaces) are indices of bone resorption. OS/BS (the percentage of bone surface covered by osteoid) and ObS/BS (the percentage of bone surface covered by osteoblasts) are indices of bone formation. Data are expressed as mean ± SEM.
*, P < 0.05 compared with sham Irr-Ig-treated mice.
In a second set of experiments, CTLA4-Ig was injected during the 1st week after surgery and then discontinued, a protocol that ensures high circulating levels of the drug until the end of the 2nd week of follow-up (34). Subsets of mice were then killed at 2 and 4 weeks from surgery. In vivo bone density measurements by DXA as well as ex vivo μCT analysis of femurs showed a significant protection against ovx-induced bone loss by CTLA4-Ig treatment at 2 weeks. However, at 4 weeks, CTLA4-Ig-treated mice showed values of bone density similar to Irr-Ig-treated ovx mice, indicating that bone loss had resumed (SI Fig. 7).
Discussion
Among the mechanisms responsible for ovx-induced bone loss is an expansion of the pool of activated T cells that produce TNF (8, 12). Ovx increases CD4+ T cell activation by enhancing Ag presentation by BMMs through increased expression of MHCII, a phenomenon caused by a complex mechanism that involves increased production of IFNγ and IL-7 and blunted generation of TGFβ in the BM (15, 16, 35, 36). Here we show that additional effects of ovx are to expand the pool of BM plasmacytoid and conventional mature DCs and to enhance Ag presentation by BM DCs through up-regulation of the costimulatory molecule CD80. The latter leads to the activation in the BM of both CD4+ and CD8+ T cells. DCs are the most potent professional APCs (18), and ovx increases their antigen-presenting activity by ≈5-fold, whereas it enhances that of BMMs by ≈2-fold (15). Therefore, DCs play a pivotal role in the T cell activation induced by ovx.
A striking finding of this work is that ovx activates BM DCs without affecting DCs localized in other secondary lymphoid organs, a phenomenon that may explain why E deprivation triggers an effector T cell response in the BM but does not induce a systemic inflammatory and/or immune response. Accordingly, an effect of ovx on DCs was not demonstrated in an earlier study (15) because only DCs derived from lymph nodes were analyzed.
Our findings demonstrate that a mechanism by which ovx elicits a tissue-specific DC response is a change in redox state, a key microenvironment determinant (21). Multiple enzymatic pathways regulate the intracellular redox state through modulation of ROS levels (37). Ovx blunts the BM levels of GSH, a critical ROS scavenger, and those of APE-1/Ref-1 and Prx-1, proteins that contribute to limit the production of intracellular ROS (28). Furthermore, the finding in the BM of ovx mice of increased protein carbonylation, a marker of protein oxidation (32), attests to the functional relevance of the ovx-induced oxidative stress. Like the activation of DCs, the effects of ovx on the redox state are spatially limited to the BM a phenomenon noted previously by Lean et al. (19).
ROS comprise the superoxide anion (O2−̇), which has a short half-life and is an intermediate during the reduction of molecular oxygen to H2O2. The latter is the most abundant and long-lasting ROS. H2O2 plays a critical role in inflammation through its direct effects or by its transformation into OH• (38). This OH• is a more potent ROS than H2O2 but exerts its effects only locally because of its short half-life. Because ovx decreases Prx-1 and GSH levels, the data suggest that ovx increases H2O2 levels (30). The finding of an increase in protein carbonylation further suggests that a free radical process was involved. However, because we did not measure specific oxidant species, a contribution of other reactive oxygen species such as O2−̇ and of Prx-1 cannot be excluded.
Reports have suggested that stimulation of TNF production by OCs or BM cells is the mechanism by which ROS cause bone loss (19, 20, 39). However these studies did not provide conclusive information regarding the cellular source of TNF nor about the mechanisms by which ROS stimulates TNF production. The current work demonstrates that key effects of ovx, the up-regulation of Ag-dependent activation of T cells and the resulting T cell production of TNF, are mediated by ROS and abolished by treatment with antioxidants. We also found that oxidative stress, like ovx, causes an expansion of mature CD80+DCs and that antioxidants prevent the increase in the number of CD80+DCs and the bone loss induced by ovx. Together, these data suggest that the accumulation of ROS in the BM is the upstream event that leads to a tissue-specific activation of DCs and T cell-dependent overproduction of TNF.
The interaction of activated T cells with immature DCs in areas of inflammation is now known to promote the transdifferentiation of DCs into OCs (40, 41). Because ovx causes an increase in the number of several populations of BM DCs, the up-regulation of osteoclastogenesis induced by ovx is likely to be due, in part, to the transdifferentiation of immature DCs. However, the finding that ovx increases the percentage of CD80+ DCs but not that of immature (CD80−) DCs suggests that in ovx mice DCs stimulate OC formation primarily by increasing Ag presentation and T cell activation.
In the context of Ag recognition, the interaction of the costimulatory molecule CD80 on APCs with the T cell-expressed receptor CD28 is required for T cell activation. CTLA4-Ig is a fusion protein that promotes T cell anergy and apoptosis by binding to CD80 (and CD86) and blocking their interaction with CD28 (42, 43). We found that in vivo treatment with CTLA4-Ig prevents the increase in T cell activation and proliferation and TNF production resulting from E withdrawal, and it affords considerable protection against ovx-induced bone loss. Furthermore, because CTLA4-Ig does not directly target OCs and does not block the direct effects of ROS on OC maturation, the data argue against the hypothesis proposed by others that ROS induce bone loss primarily by directly promoting OC differentiation and/or by stimulating mature OCs to secrete TNF (19).
The bone-sparing activity of CTLA4-Ig demonstrates that activated T cells play a pivotal causal role in the mechanism by which ovx causes bone loss in WT mice. Thus, the current work provides confirmation in WT mice of previous reports demonstrating that ovx fails to induce trabecular and cortical bone loss in T cell-deficient nude mice (8, 12, 16). In contrast, another study (44) showed that nude mice lose trabecular bone after ovx, although they are protected against the loss of cortical bone. In the same investigation, ovx was found not to induce cortical bone loss in either the spine or the femur in TCRα −/− and RAG2 −/− mice, although it induced trabecular bone loss in both strains. The partially negative outcome of that study is likely explained by age and experimental design differences, the presence of residual T cells in some strains, and by ovx-independent alterations of bone resorption induced by either the concomitant lack of B cells or genetic compensations.
In summary, our data reveal that induction of oxidative stress is a localized upstream consequence of acute E deprivation, which leads to Ag-dependent activation of T cells through up-regulation of the costimulatory molecule CD80 on DCs. The downstream consequence of these events is increased T cell TNF production and bone loss. The capacity of CTLA4-Ig to prevent ovx-induced bone loss confirms the role of activated T cells in the bone-wasting effect of E deficiency and suggests that the CD80/CD28 pathway may represent a therapeutic target for postmenopausal bone loss.
Materials and Methods
Animal Protocol.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. Female C57BL/6 WT mice were sham operated or ovariectomized at 14 weeks of age, and killed 2–4 weeks later. At sacrifice, one femur and lumbar vertebra were excised for histomorphometric and μCT analysis, and BM was harvested from the remaining bones for ex vivo analysis. Uterine weight was determined at death to verify successful ovx.
CTLA4-Ig and NAC Treatment.
Mice (n = 7–8 per group) were injected i.p. with CTLA4-Ig or isotype-matched Irr human IgG, (500 μg of each per mouse, three times a week) for either 2 or 4 weeks, starting the day before surgery. In another set of experiments mice (n = 7 per group) were injected i.p. with NAC at 100 μg/kg per day, or vehicle, twice a day for 4 weeks.
Bone Mineral Density (BMD) Measurements.
Femoral BMD was measured in anesthetized mice at 0, 2, and 4 weeks by using a PIXImus2 bone densitometer as described previously (14–16). This technique provides a combined measurement of cortical and trabecular bone. The short-term reproducibility of this technique is 1.7% (45).
μCT Analysis.
The distal femoral epiphysis and vertebrae were analyzed at sacrifice by using a μCT 40 scanner (Scanco Medical, Bassersdorf, Switzerland) at a resolution of 12 μm, as described previously (36, 46). Femoral histomorphometric indices were calculated by using 40 slices proximal to the growth plate (480 μm total length) with the Image Processing Language package provided by the manufacturer. Vertebral indices were calculated by analyzing 30 slices of the L4 vertebra and by measuring three-dimensional distances directly in the trabecular network.
Quantitative Bone Histomorphometry.
Bone histomorphometry was performed as described previously (8, 10) at the University of Alabama at Birmingham Center for Metabolic Bone Disease–Histomorphometry and Molecular Analysis Core Laboratory. The measurements, terminology, and units used for histomorphometric analysis were those recommended by the Nomenclature Committee of the American Society of Bone and Mineral Research (47). Analysis of the epiphyseal region of the distal femur was performed by using trichrome-stained, plastic-embedded sections.
Measurements of APE-1/Ref-1, Prx1, and GSH.
APE-1/Ref-1 and Prx-1 were measured by Western blotting on samples (n = 3 mice per group) collected at 4 weeks from surgery as described (48) with anti-APE-1/Ref-1 mAb or with polyclonal anti-Prx1 Ab followed by secondary Ab conjugated to horseradish peroxidase. Blots were developed by using the ECL chemiluminescence procedure. GSH was measured by HPLC as described (31). Briefly, GSH was converted to N-dansyl,S-carboxymethyl-GSH and quantified relative to an internal standard (γ-glutamylglutamate) by HPLC with fluorescence detection.
Protein Carbonylation.
Measurements of carbonyl formation were carried out by using the OxyBlot protein oxidation detection kit (Intergen, Purchase, NY) following the manufacturer's protocol as described, n = 3 mice per group.
Serum CTX and Osteocalcin Measurements.
Serum CTX, a marker of bone resorption, and osteocalcin, a marker of bone formation, were determined in serum by ELISA with the mouse-specific kits Ratlaps and Rat-MID, respectively (NordicBioscience Diagnostics A/S, Herlev, Denmark).
Flow Cytometry.
Flow cytometry was performed as described (14) by labeling cells with anti-CD69-conjugated Peridinin-chlorophyll-protein complex (PerCP), anti-CD4-conjugated APC, and anti-CD8-conjugated phycoerythrin (PE). For BM DCs subset analysis, BM cells were also stained with anti-CD11c-conjugated APC, anti MHCII (Ia-b)-conjugated PE, anti-CD80-conjugated FITC, anti-CD8-conjugated PerCP, and anti-B220-conjugated FITC. Nonspecific staining was assessed by using FITC, APC, PerCP or PE-conjugated isotype-matched normal IgG mAbs. For each set of mice, BM cells from four mice were individually processed and stained cells were analyzed by a FACScan flow cytometer (BD Bioscience, San Jose, CA).
Purification of APCs.
BMMs and B cells were isolated from spleen or BM by immunomagnetic-positive selection as described previously (15). DCs were isolated by immunomagnetic-positive selection of CD11c+ of nonadherent mononuclear cells after an overnight adherence step on plastic to eliminate adherent cells. Cell phenotype was assessed by flow cytometry, and populations were found to be >98% pure.
Antigen Presentation Assay.
Ag presentation was measured as described previously (15, 16). Briefly, T cells from OT-II mice in C57BL/6 background were purified and cultured for 8 days in medium containing 40 units/ml IL-2 and 0.5 mg/ml ovalbumin. DC, BMMs or B cells (1 × 105 per well) were purified (n = 4 mice per group), γ-irradiated, and cocultured in a 96-well plate for 72 h with T cells at a ratio of 5:1 in medium containing 0.5 mg/ml ovalbumin. T cell proliferation induced by Ag presentation was quantitated by [3H]thymidine incorporation added during the last 18 h of culture.
T Cell TNF Production.
T cell TNF was assessed by ELISA (Quantikine; R & D Systems, Minneapolis, MN) in the supernatants of whole BM or purified BM T cells isolated from OT-II mice by immunomagnetic-positive selection of CD90+ cells.
RT-PCR.
CIITA was quantitated in DC by real-time PCR as described previously (15, 16). The primers used for CIITA were 5′-GAG AAG TTC ACC ATT GAG CCA TT A-3′ (forward) and 5′-GTC CCG GAT GGC AGG AA CG-3′ (reverse), mRNA was normalized against 18S mRNA by using the primers 5′-CAC GGC CGG TAC AGT GAA AC (forward) and 5′-AGA GGA GCG AGC GAC CAA AG-3′ (reverse). Dissociation curves revealed a single product in all cases. Fold change in cDNA concentration was calculated by using the 2-ΔΔCT method (15, 16, 49) with normalization of input using 18S.
In Vitro Osteoclast Formation.
1.2 × 106 BM cells were harvested from four mice per group and cultured in 48-well plates with 15 ng/ml RANKL (kindly provided by X. Feng, University of Alabama at Birmingham, AL) and 10 ng/ml M-CSF. Cells were stained for tartrate-resistant acid phosphatase (TRAP) at day 8, and TRAP+ cells with three or more nuclei were scored as OCs.
Statistical Analysis.
All cross-sectional data were analyzed by one-way ANOVA and Fisher's protected least-squares difference tests or by the Kruskal–Wallis test for data not normally distributed. Prospective data were analyzed by ANOVA for repeated measures. Simple comparisons were made by using a two-tailed unpaired Student's t test.
Supplementary Material
Acknowledgments
We are grateful to the University of Alabama at Birmingham Center for Metabolic Bone Disease–Histomorphometry and Molecular Analysis Core Laboratory, National Institutes of Health Grant P30-AR46031, for the histomorphometric analysis presented herein. This work was supported by National Institutes of Health Grant AG 28278 and by a grant from the Emory University Research Committee (to R.P.). F.G. was supported by Marie-Curie Fellowship 09097 from the European Commission.
Abbreviations
- APC
antigen-presenting cell
- Ag
antigen
- BM
bone marrow
- BMD
bone mineral density
- BMM
bone marrow macrophage
- CIITA
class II transactivator
- CTX
C-terminal telopeptides
- DC
dendritic cell
- DXA
dual x-ray absorptiometry
- E
estrogen
- GSH
glutathione
- H2O2
hydrogen peroxide
- M-CSF
macrophage colony-stimulating factor
- μCT
microcomputerized tomography
- NAC
N-acetylcysteine
- OC
osteoclast
- ovx
ovariectomy
- Prx-1
peroxiredoxin-1
- RANKL
receptor activator of nuclear factor-κB ligand
- TRAP
tartrate-resistant acid phosphatase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703610104/DC1.
References
- 1.Manolagas SC, Kousteni S, Jilka RL. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 2.Clowes JA, Riggs BL, Khosla S. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et al. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Weitzmann MN, Pacifici R. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 6.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 7.Sherman ML, Weber BL, Datta R, Kufe DW. J Clin Invest. 1990;85:442–447. doi: 10.1172/JCI114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimble R, Bain S, Pacifici R. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 11.Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, Garcia I. J Clin Invest. 1997;99:1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. J Bone Miner Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. Proc Natl Acad Sci USA. 2005;102:16735–16740. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Proc Natl Acad Sci USA. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Qian WP, Dark K, Toraldo G, Lin AS, Guldberg RE, Flavell RA, Weitzmann MN, Pacifici R. Proc Natl Acad Sci USA. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir ME, Sharpe AH. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Endocrinology. 2005;146:728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 22.Lean J, Kirstein B, Urry Z, Chambers T, Fuller K. Biochem Biophys Res Commun. 2004;321:845–850. doi: 10.1016/j.bbrc.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Kantengwa S, Jornot L, Devenoges C, Nicod LP. Am J Respir Crit Care Med. 2003;167:431–437. doi: 10.1164/rccm.200205-425OC. [DOI] [PubMed] [Google Scholar]
- 24.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, Hauser C, Fontana A, Reith W. J Exp Med. 2001;194:379–391. doi: 10.1084/jem.194.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon EY, Lee JH, Oh SY, Ryu SK, Kim HM, Kwak HS, Yoon WK. Free Radic Biol Med. 2006;40:2103–2111. doi: 10.1016/j.freeradbiomed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Loguercio C, Federico A. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 27.Farinati F, Cardin R, Bortolami M, Grottola A, Manno M, Colantoni A, Villa E. Mol Cell Endocrinol. 2002;193:85–88. doi: 10.1016/s0303-7207(02)00100-4. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki M, Suzuki S, Irani K. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 29.Tell G, Damante G, Caldwell D, Kelley MR. Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 30.Wood ZA, Poole LB, Karplus PA. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 31.Jones DP. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 32.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 33.Moon EY, Noh YW, Han YH, Kim SU, Kim JM, Yu DY, Lim JS. Immunol Lett. 2006;102:184–190. doi: 10.1016/j.imlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Srinivas NR, Shyu WC, Weiner RS, Tay LK, Greene DS, Barbhaiya RH. J Pharm Sci. 1995;84:1488–1489. doi: 10.1002/jps.2600841217. [DOI] [PubMed] [Google Scholar]
- 35.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. J Clin Invest. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkel T. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 38.Gloire G, Legrand-Poels S, Piette J. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Jagger CJ, Lean JM, Davies JT, Chambers TJ. Endocrinology. 2005;146:113–118. doi: 10.1210/en.2004-1058. [DOI] [PubMed] [Google Scholar]
- 40.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, Jurdic P, Servet-Delprat C. Blood. 2004;104:4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 41.Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. J Bone Miner Res. 2007;22:775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 42.Moreland L, Bate G, Kirkpatrick P. Nat Rev Drug Discov. 2006;5:185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 43.Ruderman EM, Pope RM. Arthritis Res Ther. 2005;7(Suppl 2):S21–S25. doi: 10.1186/ar1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SK, Kadono Y, Okada F, Jacquin C, Koczon-Jaremko B, Gronowicz G, Adams DJ, Aguila HL, Choi Y, Lorenzo JA. J Bone Miner Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 45.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. J Clin Invest. 2000;105:1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grassi F, Fan X, Rahnert J, Weitzmann MN, Pacifici R, Nanes MS, Rubin J. Endocrinology. 2006;147:4392–4399. doi: 10.1210/en.2006-0334. [DOI] [PubMed] [Google Scholar]
- 47.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 48.Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.