Abstract
Oxidized sterols consumed in the diet or formed on low-density lipoprotein (LDL) are toxic to endothelial cells and macrophages and are thought to have a central role in promoting atherogenesis. The ATP-binding cassette transporter ABCG1 was recently shown to promote efflux of cholesterol from macrophages to high-denisty lipoprotein (HDL). We show that HDL protects macrophages from apoptosis induced by loading with free cholesterol or oxidized LDL. The protective effect of HDL was reduced in Abcg1−/− macrophages, especially after loading with oxidized LDL. Similarly, HDL exerted a protective effect against apoptosis induced by 7-ketocholesterol, the major oxysterol present in oxidized LDL and atherosclerotic lesions, in Abcg1+/+, but not in Abcg1−/− macrophages. In transfected 293 cells, efflux of 7-ketocholesterol and related oxysterols was completely dependent on expression of ABCG1 and the presence of HDL in media. In contrast, ABCA1 and apoA-1 did not stimulate the efflux of 7-ketocholesterol into media. HDL stimulated the efflux of 7-ketocholesterol from Abcg1+/+, but not from Abcg1−/− macrophages. In Abcg1−/− mice fed a high-cholesterol diet, plasma levels of 7-ketocholesterol were reduced, whereas their macrophages accumulated 7-ketocholesterol. These findings indicate a specific role for ABCG1 in promoting efflux of 7-ketocholesterol and related oxysterols from macrophages onto HDL and in protecting these cells from oxysterol-induced cytotoxicity.
Keywords: atherosclerosis, ATP-binding cassette transporter, cholesterol efflux, oxysterol
The formation of atherosclerotic lesions is a complex process in part mediated by inflammatory and oxidative mechanisms (1, 2). Oxidative changes occurring in low-density lipoprotein (LDL) are widely regarded as having an important role in plaque development. LDL undergoes retention and aggregation in the subendothelial spaces of the arterial wall and may be oxidatively modified by various mechanisms (3, 4). Several lipid-derived bioactive molecules are thus generated in LDL, including oxysterols (5, 6), oxidized fatty acids (7), lysophospholipids (8), and aldehydes (9). Among the oxysterols that have been identified, those oxidized at the C7-position, such as 7-ketocholesterol, are most abundant in human atherosclerotic plaques (10). In addition, oxysterols may be present in the diet and incorporated into plasma lipoproteins (11). Oxysterols induce dysfunction or apoptosis in endothelial cells, smooth muscle cells, and macrophages (12–15). Apoptosis of macrophage foam cells is important in plaque development and breakdown and can be induced by uptake of oxidized LDL (oxLDL) or by loading with free cholesterol (FC) (16).
Plasma high-density lipoprotein (HDL) levels are inversely related to the risk of atherosclerotic cardiovascular disease (17, 18). HDL has antiinflammatory, antioxidant, antithrombotic, and vasodilatory properties that may be relevant to this relationship (19, 20). One of the most important atheroprotective roles of HDL is reverse cholesterol transport, in which excess cholesterol in macrophage foam cells undergoes efflux and then is transported to the liver for excretion in the bile (21). ATP-binding cassette transporter ABCA1 mediates cholesterol efflux to lipid-poor apolipoprotein A-1 (apoA-1) but only modestly increases cholesterol efflux to HDL (22–24). In contrast, ABCG1 promotes macrophage cholesterol efflux to HDL, including large HDL particles, from subjects with cholesteryl ester transfer protein deficiency (25), but not to lipid-poor apoA-1 (24, 26, 27).
In the present study, we investigated the role of HDL in protecting macrophages from apoptosis induced by loading with FC or oxLDL. HDL was found to reduce apoptosis induced by either method. Unexpectedly, the protection against oxLDL-induced apoptosis was specifically dependent on ABCG1, and ABCG1/HDL but not ABCA1/apoA-1 was able to promote efflux of 7-ketocholesterol from cells.
Results
ABCG1 Deficiency Increases Susceptibility to oxLDL-Induced Apoptosis.
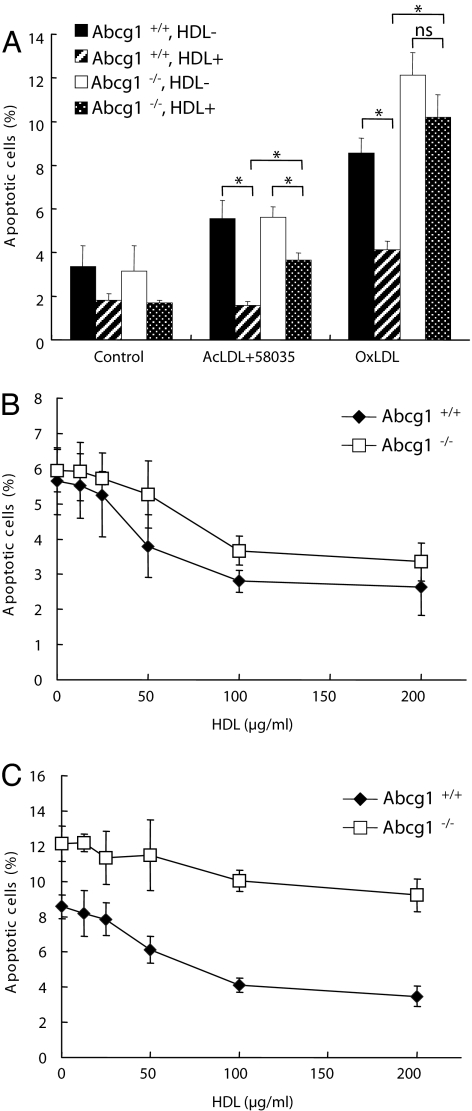
Two mechanistically distinct pathways relevant to macrophage apoptosis in atherosclerosis have been described, one involving the uptake of oxLDL and another initiated by loading with lipoprotein FC (16). To induce FC loading, macrophages were incubated with acetyl-LDL (acLDL) in the presence of an acyl-CoA:cholesterol acyltransferase inhibitor, 58035. In Abcg1+/+ macrophages, HDL reduced FC-induced apoptosis by 71% and oxLDL-induced apoptosis by 52% (Fig. 1A). To assess a possible role of ABCG1 in HDL-mediated protection, we carried out similar studies in Abcg1−/− macrophages. In the absence of HDL, Abcg1−/− macrophages showed similar levels of apoptosis to Abcg1+/+ macrophages in response to FC loading and modestly higher levels of apoptosis in response to oxLDL loading (Fig. 1A); the latter finding has been reported in ref. 28. In Abcg1−/− cells, the protective effect of HDL (100 μg/ml) was reduced by 50% in response to FC loading, but there was residual HDL protection (Fig. 1A). In contrast, HDL protection was markedly reduced in Abcg1−/− cells loaded with oxidized LDL (Fig. 1A; difference not significant). These findings were confirmed in an HDL dose-response experiment in which HDL (>50 μg/ml) provided significant protection from both FC- and oxLDL-induced apoptosis (Fig. 1 B and C). Whereas the protective effect of HDL on FC-induced apoptosis was only slightly reduced in Abcg1−/− cells (Fig. 1B), it was markedly impaired at all HDL concentrations for oxLDL-induced apoptosis (Fig. 1C). These finding suggested the possibility that HDL might promote efflux of specific bioreactive molecule(s) acquired from oxLDL in an ABCG1-dependent fashion.
Fig. 1.
ABCG1 deficiency increases susceptibility to oxLDL-induced apoptosis. (A) Peritoneal macrophages from Abcg1+/+ or Abcg1−/− mice were cultured in DMEM plus 5% LPDS containing AcLDL (100 μg/ml) plus 58035 (10 μg/ml) or oxLDL (100 μg/ml) with or without HDL (100 μg/ml) for 24 h. Apoptosis of macrophage was determined by annexin V staining and is expressed as a percentage of the total number of cells in at least three separate fields (containing ≈1,000 cells) from duplicate wells. (B and C) Concentration response of HDL (0–200 μg/ml) on apoptosis in response to FC loading (B) or oxLDL loading (C). Data are represented as the mean ± SE of three separate experiments. *, P < 0.05.
ABCG1 Deficiency Increases Susceptibility to 7-Ketocholesterol-Induced Apoptosis.
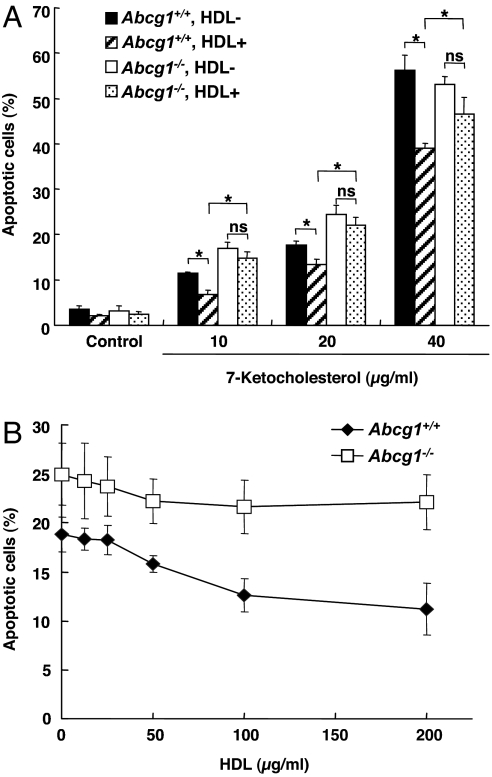
OxLDL contains a significant amount of oxysterols, of which 7-ketocholesterol is the most abundant and is known to induce apoptosis (12–15). In addition, 7-ketocholesterol is the most abundant oxysterol detected in human atherosclerotic plaques (10). Analysis of oxLDL showed a high content of 7-ketocholesterol (≈100 μg/mg protein), whereas acLDL used in FC loading experiments had a very low content of 7-ketocholesterol (<1 μg/mg protein). Therefore, we next investigated the effect of HDL and ABCG1 deficiency on 7-ketocholesterol-induced apoptosis. HDL significantly reduced apoptosis induced by 7-ketocholesterol at all concentrations of the oxysterol (10–40 μg/ml) (Fig. 2A), encompassing concentrations of 7-ketocholesterol comparable to those present in the oxidized LDL experiment. The protective effect of HDL on 7-ketocholesterol (20 μg/ml)-induced apoptosis showed maximum suppression at 100–200 μg/ml (Fig. 2B). However, in Abcg1−/− cells, the HDL protection was virtually abolished (Fig. 2 A and B).
Fig. 2.
ABCG1 deficiency increases susceptibility to 7-ketocholesterol-induced apoptosis. (A) Peritoneal macrophages from Abcg1+/+ or Abcg1−/− mice were cultured in DMEM plus 5% LPDS containing 7-ketocholesterol (10–40 μg/ml) with or without HDL (100 μg/ml) for 24 h. Apoptosis of macrophage was determined by annexin V staining and is expressed as a percentage of the total number of cells in at least three separate fields (containing ≈1,000 cells) from duplicate wells. (B) Concentration response of HDL (0–200 μg/ml) on apoptosis in response to 7-ketocholesterol. Data are represented as mean ± SE of three separate experiments. *, P < 0.05.
7-Ketocholesterol Is Selectively Exported to HDL in ABCG1-Transfected 293 Cells.
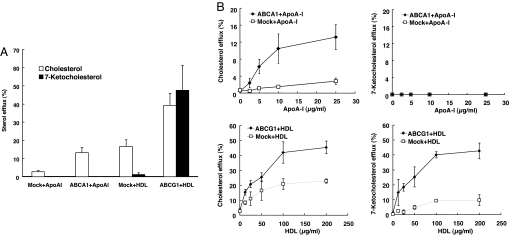
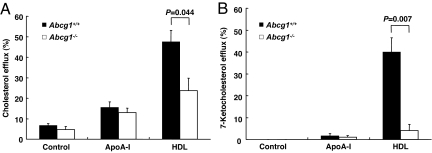
On the basis of these findings, we hypothesized that 7-ketocholesterol might be exported from cells to HDL by ABCG1. To test this hypothesis, ABCG1 or ABCA1 was overexpressed in HEK293 cells, and cholesterol and 7-ketocholesterol mass efflux was measured after loading cells with a mixture of cholesterol and 7-ketocholesterol. As described in refs. 22 and 24, ABCA1/apoA-1 and ABCG1/HDL promoted cholesterol efflux, and there was a significant background cholesterol efflux to HDL even in the absence of ABCG1 (Fig. 3A). In contrast, 7-ketocholesterol efflux was mediated by ABCG1 and HDL but not by ABCA1/apoA-1 and not by HDL in the absence of ABCG1, indicating a specific requirement for both ABCG1 and HDL in the efflux of 7-ketocholesterol (Fig. 3A). In cell media, the 7-ketocholesterol was found almost entirely in the HDL fraction (data not shown). Similar ABCG1-dependent efflux of 7-ketocholesterol was observed for HDL-2 and HDL-3 subfractions (data not shown).
Fig. 3.
7-Ketocholesterol is selectively exported to HDL in ABCG1-transfected 293 cells. (A) HEK293 cells were transiently transfected with plasmid constructs expressing ABCA1/ABCG1 or empty vector and incubated with a cholesterol and 7-ketocholesterol mixture (each 10 μg/ml) for 17 h. Transfected HEK293 cells were washed with PBS and incubated with DMEM plus 5% LPDS containing apoA-1 (10 μg/ml) or HDL (100 μg/ml) for 8 h. (B) Concentration response of apoA-1 (0–25 μg/ml) or HDL (0–200 μg/ml) for 8 h. Data are represented as the mean ± SE of an experiment performed in triplicate. Similar data were obtained in two separate experiments.
Next, we performed concentration dependence experiments with apoA-1 for ABCA1 and HDL for ABCG1. Cholesterol efflux mediated by ABCA1 to apoA-1 increased in a concentration-dependent manner and reached a maximum at 10–25 μg/ml (Fig. 3B). However, ABCA1 did not mediate 7-ketocholesterol efflux to apoA-1 at any concentration (Fig. 3B). ABCG1-dependent and -independent cholesterol efflux to HDL increased in a concentration-dependent manner between HDL concentrations of 10 and 100 μg/ml (Fig. 3B). In contrast, whereas efflux of 7-ketocholesterol mediated by ABCG1 increased in a concentration-dependent manner to HDL between 10 and 100 μg/ml, in cells transfected with mock vector, there was very low 7-ketocholesterol efflux to HDL (Fig. 3B). We also investigated the effects of SR-B1 on 7-ketocholesterol efflux in transfected cells. SR-B1 did not contribute to either 7-ketocholesterol or cholesterol mass efflux (data not shown). These experiments demonstrated a specific requirement for both ABCG1 and HDL in the efflux of 7-ketocholesterol from transfected 293 cells.
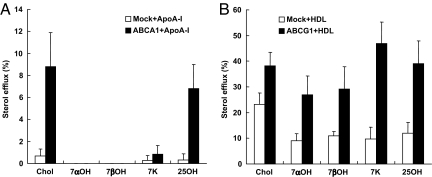
To further investigate the specificity of ABCG1 for efflux of different oxysterols, we measured efflux of 7β-hydroxycholesterol, 7α-hydroxycholesterol, and 25-hydroxycholesterol cholesterol. These experiments showed a specific role of ABCG1 for efflux of the different sterols modified at the C7-position, whereas 25-hydroxycholesterol was effluxed by both ABCA1 and ABCG1 (Fig. 4 A and B). 7β-Hydroxycholesterol is also found in oxLDL and human atherosclerotic lesions and can induce apoptosis in comparable fashion to 7-ketocholesterol (10).
Fig. 4.
7α- and 7β-hydroxycholesterol is selectively exported to HDL in ABCG1-transfected 293 cells. HEK293 cells were transiently transfected with plasmid constructs expressing ABCA1/ABCG1 or empty vector and incubated with 10 μg/ml of cholesterol (Chol), 7α-hydroxycholesterol (7αOH), 7β-hydroxycholesterol (7βOH), 7-ketocholesterol (7K), or 25-hydroxycholesterol (25OH) for 17 h. Transfected HEK293 cells were washed with PBS and incubated with DMEM plus 5% LPDS containing apoA-1 (10 μg/ml) (A) or HDL (100 μg/ml) (B) for 8 h. Data are represented as the mean ± SE of an experiment performed in triplicate. Similar data were obtained in two separate experiments.
7-Ketocholesterol Is Selectively Exported to HDL in oxLDL-Loaded Macrophages.
To further investigate the role of ABCG1 in efflux of 7-ketocholesterol, macrophages from Abcg1+/+ and Abcg1−/− were loaded with oxLDL, and then efflux to apoA-1 or HDL was examined. Abcg1−/− macrophages showed decreased efflux of cholesterol and 7-ketocholesterol efflux to HDL compared with Abcg1+/+ (Fig. 5 A and B). Although the efflux of cholesterol to HDL was reduced by 50%, the efflux of 7-ketocholesterol was reduced by 90% and was not significant (Fig. 5 A and B). As in transfected 293 cells, there was no efflux of 7-ketocholesterol to apoA-1 in either Abcg1+/+ or Abcg1−/− cells (Fig. 5B). We also examined cellular cholesterol and 7-ketocholesterol mass after efflux to apoA-1 or HDL. In Abcg1+/+ macrophages, after efflux to HDL, both cholesterol and 7-ketocholesterol mass were significantly reduced by 27% [supporting information (SI) Fig. 7A] and 41% (SI Fig. 7B), respectively. In Abcg1−/− macrophages, the corresponding numbers were 21% and 3% (SI Fig. 7 A and B). Together, these data indicate that ABCG1 deficiency partially reduced the mass efflux of cholesterol to HDL, as reported in ref. 29, but more dramatically abolished the efflux of 7-ketocholesterol.
Fig. 5.
Mass sterol efflux to apoA-1 or HDL in Abcg1+/+ or Abcg1−/− macrophages. Peritoneal macrophages from Abcg1+/+ or Abcg1−/− mice were incubated with 50 μg/ml of oxLDL for 17 h. Macrophages were washed with PBS and incubated in DMEM plus 5% LPDS with apoA-1 (10 μg/ml) or HDL (50 μg/ml) for 8 h. Cholesterol (A) and 7-ketocholesterol (B) efflux were determined. Data are represented as the mean ± SE of an experiment performed in triplicate. *, P < 0.05 as compared with control.
7-Ketocholesterol Concentrations in Abcg1−/− Macrophages and Lipoproteins in Vivo.
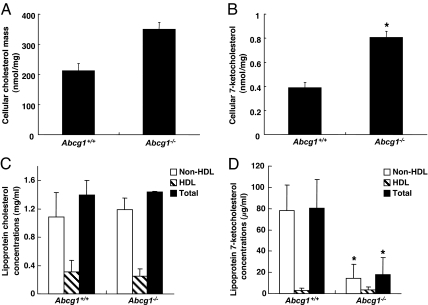
To determine whether 7-ketocholesterol would accumulate in macrophages in vivo, we carried out a bone marrow transplantation from Abcg1−/− mice into LDLR−/− mice by using a similar protocol to that used in the recent atherosclerosis studies (30). Peritoneal macrophages were harvested from transplanted mice after 12 weeks on a high-fat/cholesterol diet. Abcg1−/− macrophages showed a significantly increased content of 7-ketocholesterol (2.1-fold; P < 0.05) and a lesser increase in cholesterol (1.6-fold; not significant) (Fig. 6 A and B).
Fig. 6.
7-Ketocholesterol concentrations in Abcg1−/− macrophages and lipoproteins in vivo. (A and B) Cellular sterol mass in Abcg1+/+ or Abcg1−/− mice from a bone marrow transplantation (Abcg1+/+ or Abcg1−/− → LDLR−/−). Abcg1+/+ or Abcg1−/− macrophages were harvested from LDLR−/− mice transplanted with Abcg1+/+ or Abcg1−/− bone marrow and were fed a Western diet for 12 weeks. Pooled Abcg1+/+ or Abcg1−/− macrophages were incubated in DMEM plus 10% FBS for 2 h. Then, cells were washed with PBS, and cellular lipids were extracted. (A and B) Cellular cholesterol (A) and 7-ketocholesterol (B) mass were determined. Data are represented as the mean ± SE of an experiment performed in triplicate. (C and D) Abcg1+/+ or Abcg1−/− mice fed a high-fat/cholesterol diet for 4 weeks. Lipoprotein cholesterol (C) and 7-ketocholesterol (D) concentrations were determined. Data are represented as the mean ± SE (n = 4). *, P < 0.05 as compared with Abcg1+/+.
We also investigated the distribution of 7-ketocholesterol in serum and lipoproteins in ABCG1 total knockout mice. Abcg1+/+ and Abcg1−/− mice were fed a high-fat/cholesterol diet for 4 weeks, and we determined lipoprotein cholesterol and 7-ketocholesterol concentrations. Plasma cholesterol concentrations and the lipoprotein distribution of cholesterol in Abcg1−/− mice were essentially the same as in Abcg1+/+ mice (Fig. 6C). Plasma 7-ketocholesterol concentrations from Abcg1−/− mice were significantly lower than those from Abcg1+/+ mice (Fig. 6D). The decreased plasma 7-ketocholesterol concentrations in Abcg1−/− mice were due to the reduction of non-HDL [i.e., very LDL (VLDL) plus LDL] 7-ketocholesterol concentrations (≈80%), not to the reduction of HDL concentrations (Fig. 6 C and D). Differential accumulation of 7-ketocholesterol in VLDL plus LDL rather than in HDL could reflect transfer to VLDL plus LDL after efflux to HDL or the ability of ABCG1 to directly promote sterol efflux to LDL (29).
Discussion
The apoptosis of macrophages is thought to have an important role in the evolution of atherosclerotic plaques, and apoptotic macrophages are concentrated in areas of plaque rupture (16). Macrophage apoptosis can be induced either by loading with free cholesterol or by uptake of oxLDL (16, 31, 32). We show that HDL at physiologically relevant concentrations has a potent protective role against macrophage apoptosis induced by either method. The ability to protect against oxLDL-induced apoptosis specifically requires expression of macrophage ABCG1 and reflects the ability of ABCG1 and HDL to promote efflux of 7-ketocholesterol and related oxysterols from macrophages.
Oxysterols such as 7-ketocholesterol are abundant in human atherosclerotic plaques and are concentrated in plaques relative to circulating monocytes (10, 33). Cellular accumulation of 7-ketocholesterol leads to increased production of reactive oxygen species and induces apoptosis in various cell types, including macrophages, endothelial cells, and smooth muscle cells (12–15). In addition, oxysterols induce inflammatory gene expression in monocyte–macrophages (34–37). Therefore, 7-ketocholesterol and related oxysterols probably have an important role in the pathogenesis of atherosclerosis.
In addition to promoting cholesterol efflux from foam cells, HDL has been proposed to have antioxidant and antiinflammatory properties. Most of the research in this area has focused on the ability of HDL to break down proinflammatory oxidized phospholipids derived from LDL or from cells by virtue of the presence of enzymes such as lipoprotein-associated phospholipase A-2 (also known as PAF acethyhdrolase) and paraoxonase in HDL (38, 39). Our study reveals an aspect of HDL function mediated via cellular ABCG1, i.e., the efflux of toxic oxysterols from cells, thereby protecting macrophages from oxysterol-induced apoptosis. The subsequent detoxification steps could involve esterification of oxysterols by lecithin:cholesterol acyltransferase (40) or by rapid clearance of oxysterols from plasma by the liver, followed by conversion into bile acids (41).
In the present study, we demonstrate that ABCG1 has a necessary and sufficient role in promoting efflux of 7-ketocholesterol efflux from cells to HDL by using both ABCG1 overexpressing cells (Fig. 3) and Abcg1−/− macrophages (Fig. 5). Cholesterol efflux can be mediated by both ABCA1- and ABCG1-dependent pathways, whereas 7-ketocholesterol and related oxysterols modified at the C7 position are selectively exported to HDL by ABCG1. Combined knock-down of ABCA1 and ABCG1 in macrophages reduced cholesterol efflux to HDL only by ≈70%, suggesting the existence of additional pathways such as that mediated by apolipoprotein E or passive cholesterol efflux (30). Surprisingly, even though 7-ketocholesterol is more polar and water soluble than cholesterol, 7-ketocholesterol was unable to undergo efflux from macrophages by passive diffusional mechanisms. Perhaps the free-energy barrier to sterol desorption from the membrane into water is too high for passive efflux to make a significant contribution (42). The restricted efflux pathway of 7-ketocholesterol that appears to be almost completely dependent on ABCG1 and the presence of lipoproteins in media could explain the relative enrichment of this oxysterol in atherosclerotic plaques (10).
Baldan et al. (28) reported that Abcg1−/− macrophages are more susceptible to apoptosis in response to oxLDL and also that atherosclerotic lesions from mice transplanted with Abcg1−/− bone marrow have more apoptotic cells. Our studies provide a mechanism to understand these important in vivo observations, involving efflux of specific toxic oxysterols to HDL. Macrophages isolated from LDLR−/− mice transplanted with Abcg1−/− bone marrow showed an increased content of 7-ketocholesterol (Fig. 6B), whereas plasma lipoproteins were deficient in 7-ketocholesterol (Fig. 6D) in mice fed a high-cholesterol diet, suggesting that decreased efflux and oxysterol accumulation are relevant to the mechanism of increased macrophage apoptosis in vivo. Transplantation of Abcg1−/− bone marrow into atherosclerosis-susceptible mice results in no change, a small increase, or a small decrease in early atherosclerotic lesions (28, 30, 43). The smaller lesions reported from two different laboratories (28, 30) may be secondary to compensatory up-regulation of ABCA1 and apolipoprotein E secretion and to increased macrophage apoptosis in response to ABCG1 deficiency. Our studies also provide insight into the mechanism of up-regulation of ABCA1 in ABCG1−/− macrophages because several of the oxysterols that are effluxed by ABCG1 (and probably additional oxysterols that were not evaluated in our study) may be also liver X receptor activators. Thus, accumulation of oxysterols in ABCG1-deficient macrophages may explain both the increased apoptosis and up-regulation of liver X receptor target genes, and both mechanisms may be relevant to decreased atherosclerosis in early lesions (30).
Increased macrophage apoptosis in early lesions can result in smaller lesions as a result of efficient phagocytosis of apoptotic cells by healthy macrophages; however, in advanced lesions, apoptosis of macrophages and other cells may cause increased inflammation and destabilization of atherosclerotic plaques (16, 44). Thus, it is likely that efflux of 7-ketocholesterol via macrophage ABCG1 has a protective role in advanced atherosclerotic plaques. Moreover, oxysterol accumulation in endothelial cells leads to reactive oxygen species production and inactivation of NO-dependent vascular relaxation (45, 46). Because ABCG1 appears to be significantly expressed in arterial endothelium (47), the HDL/ABCG1 pathway could also have a protective effect on endothelial function. Therapies that increase HDL levels, such as niacin and cholesteryl ester transfer protein inhibitors, are probably activating the ABCG1–oxysterol efflux pathway in macrophages and possibly endothelial cells, likely with beneficial effects on atherosclerosis.
Materials and Methods
Materials.
Cholesterol, 7-ketocholesterol, 7β-hydroxycholesterol, 25-hydroxycholesterol, the acyl-CoA:cholesterol acyltransferase inhibitor 58035, and lipoprotein-deficient serum (LPDS) were purchased from Sigma (St. Louis, MO). 7β-Hydroxycholesterol was from Steraloids (Newport, RI). HDL (density 1.063–1.21 g/ml) were isolated by preparative ultracentrifugation from normolipidemic human plasma and stored in PBS. Human acLDL was from Biomedical Technologies (Stoughton, MA). Human oxLDL was prepared as described in ref. 48. Human apoA-1 was obtained from Biodesign International (Saco, ME).
Plasmid Constructs and Transfection.
Plasmid constructs expressing mouse ABCA1 and ABCG1 were described in refs. 22 and 24. HEK293 cells were plated into 12-well, collagen-coated plates and were transfected with various plasmid constructs with Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Isolation of Mouse Peritoneal Macrophages.
Abcg1−/− mice were purchased from DeltaGen (San Mateo, CA) and maintained as described in ref. 30. C57BL/6J mice (Abcg1+/+) were obtained from The Jackson Laboratory (Bar Harbor, ME). Peritoneal macrophages from Abcg1−/− mice and wild-type control mice were harvested with PBS after 3 days of i.p. injection of thioglycolate broth medium. The pooled macrophages from each strain were plated into 12-well plates (2 × 106 macrophages per well) for cellular sterol efflux assay or 48-well plates (5 × 105 macrophages per well) for apoptosis assay. Macrophages were maintained in culture medium (DMEM supplemented with 10% FBS) for 24 h and then carried out following assays.
Macrophages in Bone Marrow Transplantation.
Bone marrow transplantation was performed as reported in ref. 30. Abcg1+/+ or Abcg1−/− macrophages were harvested from LDLR−/− mice transplanted with Abcg1+/+ or Abcg1−/− bone marrow and fed a Western diet for 12 weeks. Peritoneal macrophages from Abcg1+/+ or Abcg1−/− were harvested with PBS after 3 days of i.p. injection of thioglycolate broth medium. Abcg1+/+ or Abcg1−/− macrophages were plated into 12-well plates (2 × 106 macrophages per well). Macrophages were incubated in DMEM plus 10% FBS for 2 h. Then, macrophages were washed with PBS, and cellular lipids were extracted with hexane:isopropanol (3:2, vol/vol). Cellular sterol mass was determined as described below.
Cellular Mass Sterol Efflux Assay.
HEK293 cells were incubated in DMEM plus 10% FBS supplemented with cholesterol and a 7-ketocholesterol mixture (each 10 μg/ml) for 17 h. For the sterol specificity experiments, 10 μg/ml cholesterol, 7-ketocholesterol, 7α-hydroxycholesterol, 7β-hydroxycholesterol, or 25-hydroxycholesterol was used instead of the mixture. Macrophages were incubated in DMEM plus 10% FBS supplemented with 50 μg/ml acLDL or oxLDL for 17 h. The next day, cells were washed with PBS and then incubated in DMEM plus 5% LPDS supplemented with or without human apoA-1 or HDL for 8 h. After the efflux period, media and cells were collected separately, and lipids were extracted with hexane:isopropanol (3:2, vol/vol) with stigmastanol as the internal standard. Sterol mass of media and cells were determined using gas chromatograpy as described in ref. 25. Percentages of sterol mass efflux were calculated by the ratio of sterol mass in the medium to total (medium plus cellular) sterol mass.
Mouse Lipoprotein Sterol Mass Analysis.
Abcg1+/+ or Abcg1−/− mice fed a high-fat/cholesterol diet (1.25% cholesterol, 7.5% cocoa butter, and 0.5% sodium cholate, wt/wt) (TD88051, Harlan Teklad, Madison, WI) for 4 weeks. HDL fraction was obtained by precipitation of non-HDL by using a commercial kit (HDL cholesterol E; Wako Chemical, Richmond, VA). Plasma and HDL lipids were extracted with hexane:isopropanol (3:2, vol/vol). Cholesterol and 7-ketocholesterol mass were measured as described above. Non-HDL sterol concentrations were determined by subtraction of plasma and HDL sterol concentrations.
Apoptosis Assay.
Macrophages were incubated in DMEM plus 5% LPDS supplemented with acLDL (100 μg/ml) plus acyl-CoA:cholesterol acyltransferase inhibitor 58035, oxLDL (100 μg/ml), or 7-ketocholesterol (10–40 μg/ml) and with or without HDL (100 μg/ml) for 24 h. Apoptosis assays were performed by staining macrophages with Alexa 488-labeled annexin V and propidium iodide by using Vybrant Apoptosis Assay kit (Invitrogen) as described in ref. 31. Cells were viewed immediately by using an Olympus (Melville, NY) IX-70 inverted fluorescent microscope equipped with a mercury 100-W lamp (CHIU Technical, Kings Park, NY), fluorescent filters (Chroma, Brattleboro, VT), an Olympus LCPlanF1 ×20 objective, Imaging software (Roper Scientific, Tucson, AZ), and a Cool Snap CCD camera (RS Photometrics, Tucson, AZ). Representative fields were photographed for each condition. The number of Annexin V-positive cells were counted and expressed as a percentage of the total number of cells in at least three separate fields (containing ≈1,000 cells) from duplicate wells.
Statistical Analysis.
Statistical analysis was performed using Student's t test. Results are represented as a mean ± SE.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant HL 54591.
Abbreviations
- LDL
low-density lipoprotein
- oxLDL
oxidized LDL
- FC
free cholestorol
- HDL
high-density lipoprotein
- apoA-1
apolipoprotein A-1
- acLDL
acetyl-LDL
- LPDS
lipoprotein-deficient serum.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704602104/DC1.
References
- 1.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 3.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevanian A, Hodis HN, Hwang J, McLeod LL, Peterson H. J Lipid Res. 1995;36:1971–1986. [PubMed] [Google Scholar]
- 6.Chang YH, Abdalla DS, Sevanian A. Free Radical Biol Med. 1997;23:202–214. doi: 10.1016/s0891-5849(96)00626-0. [DOI] [PubMed] [Google Scholar]
- 7.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 8.Stiko A, Regnström J, Shah PK, Cercek B, Nilsson J. Arterioscler Thromb Vasc Biol. 1996;16:194–200. doi: 10.1161/01.atv.16.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Pakala R, Katagiri T, Benedict CR. Atherosclerosis. 2001;155:37–44. doi: 10.1016/s0021-9150(00)00526-8. [DOI] [PubMed] [Google Scholar]
- 10.Brown AJ, Jessup W. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 11.Vine DF, Mamo JCL, Beilin LJ, More TA, Croft KD. J Lipid Res. 1998;39:1995–2004. [PubMed] [Google Scholar]
- 12.Dimmeler S, Haendeler J, Galle J, Zeiher AM. Circulation. 1997;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- 13.Müller K, Dulku S, Hardwick SJ, Skepper JN, Mitchinson MJ. Atherosclerosis. 2001;156:133–144. doi: 10.1016/s0021-9150(00)00641-9. [DOI] [PubMed] [Google Scholar]
- 14.Heinloth A, Brüne B, Fischer B, Galle J. Atherosclerosis. 2002;162:93–101. doi: 10.1016/s0021-9150(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 15.Okura Y, Brink M, Itabe H, Scheidegger KJ, Kalangos A, Delafontaine P. Circulation. 2000;102:2680–2686. doi: 10.1161/01.cir.102.22.2680. [DOI] [PubMed] [Google Scholar]
- 16.Tabas I. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 17.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. J Am Med Assoc. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 18.Rhoads GG, Gulbrandsen CL, Kagan A. N Engl J Med. 1976;294:293–298. doi: 10.1056/NEJM197602052940601. [DOI] [PubMed] [Google Scholar]
- 19.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 20.Assmann G, Gotto AM., Jr Circulation. 2004;109(Suppl 1):III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 21.Rader DJ. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Silver DL, Costet P, Tall AR. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 23.Oram JF, Lawn RM, Garvin MR, Wade DP. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 24.Wang N, Lan D, Chen W, Matsuura F, Tall AR. Proc Natl Acad Sci USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan AM, Oram JF. J Biol Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 28.Baldan A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Arterioscler Thromb Vasc Biol. 2006;26:2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 29.Wang N, Ranalletta M, Matsuura F, Peng F, Tall AR. Arterioscler Thromb Vasc Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 30.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Arterioscler Thromb Vasc Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 31.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Tabas I. Cell Death Differ. 2004;11:S12–S16. doi: 10.1038/sj.cdd.4401444. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Wasowicz E, Handler B, Fleischer L, Kummerow FA. Atherosclerosis. 2000;149:191–197. doi: 10.1016/s0021-9150(99)00343-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Hulten LM, Wiklund O. Arterioscler Thromb Vasc Biol. 1997;17:317–323. doi: 10.1161/01.atv.17.2.317. [DOI] [PubMed] [Google Scholar]
- 35.Erridge C, Webb DJ, Spickett CM. Free Radical Biol Med. 2007;41:260–266. doi: 10.1080/10715760601070091. [DOI] [PubMed] [Google Scholar]
- 36.Leonarduzzi G, Gamba P, Sottero B, Kadl A, Robbesyn F, Calogero RA, Biasi F, Chiarpotto E, Leitinger N, Sevanian A, et al. Free Radical Biol Med. 2005;39:1152–1161. doi: 10.1016/j.freeradbiomed.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Rosklint T, Ohlsson BG, Wiklund O, Noren K, Hulten LM. Eur J Clin Invest. 2002;32:35–42. doi: 10.1046/j.1365-2362.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 38.Lenten BJV, Navab M, Shih D, Fogelman AM, Lusis AJ. Trends Cardiovasc Med. 2001;11:155–161. doi: 10.1016/s1050-1738(01)00095-0. [DOI] [PubMed] [Google Scholar]
- 39.Navab M, Anantharamaiah GM, Reddy ST, Lenten BJV, Ansell BJ, Fogelman AM. Nat Clin Pract Endocrinol Metab. 2006;2:504–511. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 40.Szedlacsek SE, Wasowicz E, Hulea SA, Nishida HI, Kummerow FA, Nishida T. J Biol Chem. 1995;270:11812–11819. doi: 10.1074/jbc.270.20.11812. [DOI] [PubMed] [Google Scholar]
- 41.Lyons MA, Samman S, Gatto L, Brown AJ. J Lipid Res. 1999;40:1846–1857. [PubMed] [Google Scholar]
- 42.Small DM. Proc Natl Acad Sci USA. 2003;100:4–6. doi: 10.1073/pnas.0237205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJC, Van Eck M. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 44.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf D, Tontonoz P, et al. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Cai H, Harrison DG. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 46.Chikani G, Zhu W, Smart EJ. Am J Physiol Endocrinol Metab. 2004;287:E386–E389. doi: 10.1152/ajpendo.00106.2004. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell BJ, Denis M, Genest J. Circulation. 2004;110:2881–2888. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 48.Brown AJ, Dean RT, Jessup W. J Lipid Res. 1996;37:320–335. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.