Abstract
A half-century's worth of research has established the existence of numerous event-related potential components measuring different cognitive operations in humans including the selection of stimuli by covert attention mechanisms. Surprisingly, it is unknown whether nonhuman primates exhibit homologous electrophysiological signatures of selective visual processing while viewing complex scenes. We used an electrophysiological technique with macaque monkeys analogous to procedures for recording scalp event-related potentials from humans and found that monkeys exhibit short-latency visual components sensitive to sensory processing demands and lateralizations related to shifting of covert attention similar to the human N2pc component. These findings begin to bridge the gap between the disparate literatures by using electrophysiological measurements to study the deployment of visual attention in the brains of humans and nonhuman primates.
Keywords: macaque, monkey, visual search, electroencephalogram, local field potentials
Ever since Caton's experiments with animals (1) inspired Berger's (2) discovery of the electroencephalogram (EEG) in humans, the literatures on animal and human electrophysiology have developed largely independently. This chasm is principally because of methodological differences. Invasive single-unit recording in animals have yielded a rich literature describing the functional characteristics of individual neurons (3–6). In contrast, decades of noninvasive EEG and event-related potential (ERP) research with humans have revealed many aspects of large ensemble activity during different cognitive processes (7–14). This has resulted in gaps in our understanding of how the brains of humans and nonhuman primates process information during cognitively demanding tasks. For example, it is unknown whether macaque monkeys exhibit electrophysiological indices of covert visual attention homologous to those described in human subjects. The need for studies directly comparing electrophysiological measures of dynamic information processing between species has been identified as a major weakness in our understanding of brain function (15). In this study, we sought to bridge this gap by recording ERPs from monkeys performing a difficult visual search task to obtain data directly comparable with studies of human subjects.
This work builds on pioneering studies that have sought to establish nonhuman primate homologues of a number of human ERP components indexing other cognitive processes. For example, research has established homologues of the human P3 component (16–19), early sensory potentials (also known as visual evoked potentials or VEPs, e.g., refs. 20 and 21–23), and the mismatch and selection negativities in cross modal and auditory oddball tasks (24–28). Additionally, a number of other studies have explored the neural substrates of the electrical activity in the brain that gives rise to the observed EEG and averaged ERPs, typically by using cross-modal tasks (24, 25, 29–31).
This study addresses a critical weakness in our knowledge regarding mechanisms of covert visual attention by recording ERPs from macaque monkeys performing attention-demanding visual search (Fig. 1A). This is a task that has been studied heavily by using human ERP recordings. Studies of attention in nonhuman primates commonly record spiking activity with the ultimate goal of understanding how human brains function. However, it is not known whether macaque monkeys exhibit electrophysiological indices of attentional selection like those discovered in humans. As described in Materials and Methods, electrodes with impedance comparable with those of typical low-impedance human EEG recordings were implanted s.c. in the skull. This technique ensures that the layers of tissue and bone through which the signals propagate are as similar as possible across species while bypassing substantial differences in the cranial musculature of macaque monkeys and humans. To examine the topographic distribution of ERP effects, we placed electrodes across the monkey skulls in locations that approximate those typically used in human studies. This method affords the most direct comparison possible of the electrophysiological signals recorded from the two species and thereby provides a strong test of the validity of the macaque monkey model for understanding attentional selection in the human brain.
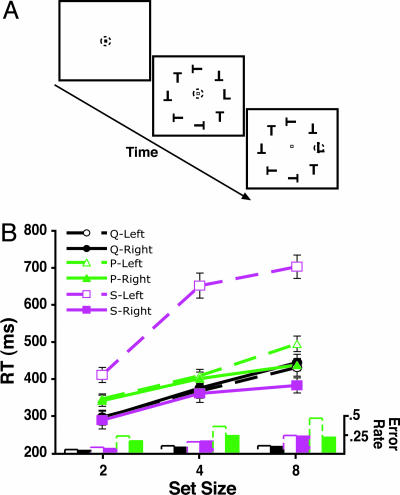
Fig. 1.
Example of the visual search task and the behavioral results from this task. (A) Example of a stimulus sequence with correct eye-movement behavior during a single trial in the visual search task. The dashed circle represents the monkey's eye position. The monkeys fixated a central fixation point for 750-1500 ms before a visual search array was presented. Each array was composed of two, four, or eight elements, and the target location was randomly selected on each trial. After making a saccade directly to the target, the monkey had to fixate the target for 500 ms before the array was extinguished and reward delivered. (B) RT of the eye-movement responses shown separately for each monkey and left-versus-right visual field targets. Monkey S exhibited RTs similar to those of monkeys Q and P for right visual field targets but, was significantly slower for targets appearing in the left visual field. The error bars represent the standard error of the mean across the 22 search sessions for each monkey. There was no evidence for a speed–accuracy tradeoff because accuracy also exhibited a significant effect of set size for each monkey (P < 0.05). These data are from target-present trials because no RT was measurable on nontarget trials.
If macaque monkeys are like humans, then the ERPs recorded during a visual search task will vary with set size in specific ways. First, we were primarily interested in whether monkeys exhibit a posterior, lateralized component related to a shift of covert attention to the target. This component, known as the N2pc in humans, is defined as a more negative potential recorded from posterior electrodes contralateral to an attended item relative to ipsilateral electrodes and is typically observed 175–200 ms after search array onset. Human ERP studies have shown that this component is maximal when a target is flanked by distractor objects and must be discriminated in advance of an eye-movement response to the target location (32). From the human ERP literature, we know that when visual search is efficient, with fast reaction times, this component has a shorter duration and larger amplitude than in conditions in which visual search is less efficient (33), such as when set size increases during search for multifeature targets. This reduction in amplitude with increasing mean reaction time is presumably because of greater variability when attention is focused on the target object. A more variable onset time of an underlying cognitive operation causes an ERP component to have a smaller amplitude but broader distribution (34). Therefore, if this component exists in monkeys, it should have a more variable onset with a larger array of possible target items. A secondary goal was to examine the early visual ERPs recorded from monkeys. The early visually evoked human ERP components are known to be sensitive to the strength of the incoming sensory signal (34). As a result, monkey homologues of these components should exhibit larger amplitude deflections when more sensory stimulation is delivered such as when more stimuli are present in a visual search array.
Results
Behavioral Data.
The behavioral results from the eye-movement responses to targets indicated that the set size manipulation significantly influenced reaction time (P < 0.0001) and accuracy (P < 0.0001) for all three monkeys (Fig. 1B). There was no evidence of a speed–accuracy tradeoff because accuracy declined with set size as reaction times became longer. This pattern of performance is consistent with findings from humans (35).
Monkeys Q and P were more proficient than monkey S and exhibited no effect of target location or an interaction of location with set size (F < 1.0). As shown in Fig. 1, however, Monkey S responded to targets presented in the right visual field 100–400 ms before those in the left visual field, resulting in a significant effect of target location [F (1, 21) = 60.79, P < 0.0001] and an interaction of set size and target location [F (2, 42) = 68.65, P < 0.0001]. In humans, such a bias for a target in one visual field is known not only to delay RTs but also the onset of the N2pc component of the search array-evoked ERP waveform (12). Thus, this unexpected asymmetry of performance provides an opportunity to test the hypothesis that bias for an object in one visual field yields ERP effects similar to those known from human studies.
Electrophysiological Data.
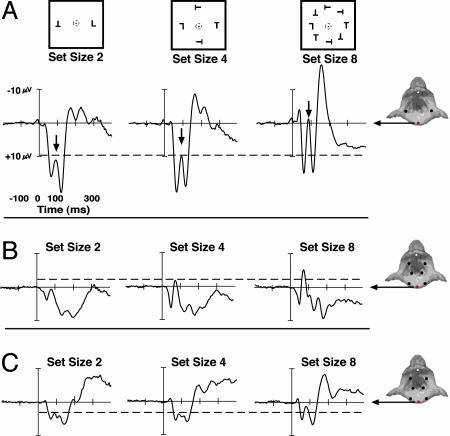
In humans, the amplitudes of the first negative and positive components elicited after the presentation of a visual stimulus (the P1 and N1) increase when a higher contrast or larger stimulus is shown (34, 36). Consistent with previous studies using different stimuli and tasks (16, 18, 37), monkeys exhibit early visually evoked components. The present findings extend previous reports in that we find only two of these components were sensitive to the number of objects in the search array (Fig. 2). Both the first and second negative-going components were significantly modulated by the strength of sensory stimulation and increased monotonically across set size in all three monkeys (P < 0.001). Interestingly, these sensory-modulated negative-going components were flanked by positive-going deflections that did not show this parametric response to the set size manipulation. This presents an unexpected dissociation between the subset of the early components that behave as those observed in humans and those that are dissimilar. It is possible that these positive components have a lower saturation threshold than the negative waveforms or that these components index an overlapping cognitive process that is unknown in human electrophysiology. Another interesting parallel between these findings from macaques and the human literature is that, although always present, the early sensory components vary significantly in amplitude across individuals (34). We found that the same pattern of early components was observed across monkeys, although the amplitudes of those components varied just as they do across human observers (Fig. 2).
Fig. 2.
ERP waveforms recorded from posterior midline electrode (red) on monkey Q (A), monkey P (B), and monkey S (C) across set size 2, 4, and 8. The relevant active electrode on each monkey is shown in red, and the common frontal reference electrode is shown in white on a diagram of a monkey head. ERP waveforms recorded from both monkeys show a complex of early negative-going components sensitive to the amount of sensory stimulation as a function of set size (marked by the arrows on the waveforms from monkey Q). For reference, the dashed horizontal lines mark the amplitude of the set size 4 peak of the first negativity.
The ERP component of primary interest to us was a homologue of the human N2pc component. This component maps onto a covert shift of perceptual attention to an object in the left or right visual field during visual search (12, 32, 38–40). The N2pc is defined as a difference between the contralateral and ipsilateral hemispheres relative to the visual field that contains the object to which attention is deployed. In the visual search task, the monkeys were required to focus gaze on the central fixation point until an eye movement could be made directly to the target location. This ensured that attention was covertly shifted to the target before shifting gaze to that object (41).
Consistent with our predictions, we found that waveforms recorded from electrodes contralateral to the target became more positive than ipsilateral electrodes ≈110–170 ms after the presentation of the search array in monkeys Q and P (Fig. 3 A and B); data from monkey S will be presented below. In addition, the amplitude of this difference was modulated by the number of objects in the search array. As set size increased, the amplitude of the difference between the most posterior contralateral and ipsilateral electrodes decreased, resulting in an interaction of electrode position, target hemifield, and set size for monkey Q [F (2, 42) = 5.94, P < 0.05] and monkey P [F (2, 42) = 8.39, P < 0.001]. This is identical to the reduction in amplitude of the human N2pc component when visual search is made more difficult (33). This amplitude reduction is attributed to greater variability in the onset of the shift of attention to the target when conditions make visual search more demanding and reaction times longer at larger set sizes (34). The measurable onset of the putative monkey homologue of the N2pc was also slowed as set size increased (see dashed lines marking measured onset of the contralateral component in Figs. 3 and 4).
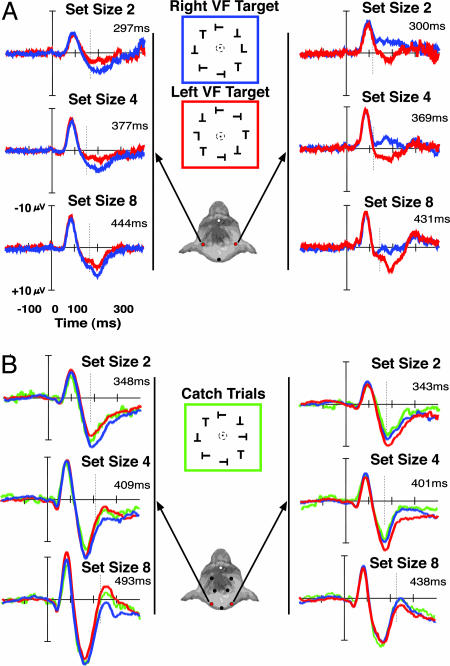
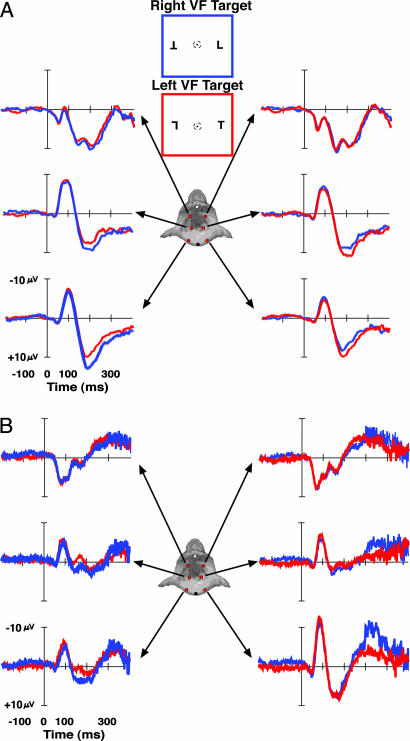
Fig. 3.
ERP waveforms recorded from the left posterior (left columns) and right posterior electrodes (right columns) from monkey Q (A) and monkey P (B) for right (blue) and left (red) visual field targets across set sizes 2, 4, and 8. For monkey P (B), waveforms elicited by catch trials containing no target are shown in green. The nontarget catch trials were 33% of all trials across set sizes, and accuracy averaged 53.8%, 70.3%, and 69.9% correct for set size 2, 4, and 8, respectively, across all sessions. After a visually evoked negativity, a contralateral positivity was observed beginning ≈125 ms poststimulus for lateralized targets but not when targets appeared on the horizontal midline (data not shown), as in human observers. The amplitude of the positivity was parametrically modulated by the set size of the visual search array presented. Dashed vertical lines mark the onset of the contralateral positivity and are absent when statistical significance was not achieved. The number above the waveform indicates the mean saccadic response latency for contralateral targets.
Fig. 4.
ERP waveforms recorded from monkey S for right (blue) and left (red) visual field targets across set size. After a visually evoked negativity, a contralateral positivity was observed beginning ≈125 ms poststimulus for right visual field targets but after ≈250 ms poststimulus for left visual field targets in monkey S. The amplitude of the positivity was parametrically modulated by the set size of the visual search array presented. The delay of the contralateral positivity in monkey S was consistent with the longer RTs to targets in the left visual field, suggesting that attention was shifted more slowly to left visual field targets. Dashed vertical lines mark the onset of the contralateral positivity and are absent when statistical significance was not achieved.
For monkey P, more catch trials were included to provide a comparison between the waveforms elicited by nontarget arrays and waveforms contralateral and ipsilateral to the hemifield containing the search target. In previous work with human subjects, it was shown that nontarget arrays elicited waveforms similar to those ipsilateral to the target (42). The waveforms recorded from nontarget and target-present arrays during visual search support our conclusions that targets elicit a contralateral positivity (Fig. 3B). Specifically, the waveforms from nontarget catch trials were significantly different from those elicited by arrays with a contralateral target (P < 0.05) but not ipsilateral target arrays (P > 0.5). These findings provide an additional link between the present study and previous work recording ERPs from humans (42).
We now turn to the data from monkey S. Recall that this monkey had RTs for right visual field targets similar to monkeys P and Q, whereas RTs for left visual field targets were significantly slower (Fig. 1B). This idiosyncratic behavioral effect suggests that the monkey avoided shifting attention to left visual field targets, probably because of some peculiarity during training. Nevertheless, consistent with this behavioral bias, the onset of the contralateral positivity for targets in the left visual field was delayed by approximately the same amount as the RTs (Fig. 4). We suspect that this idiosyncratic bias can be eliminated with additional task learning such as receiving additional reward for localizing left visual field targets.
The higher-density array of electrodes in monkeys P and S provided information about the topography of this ERP component; the positivity contralateral to the target hemifield was maximal at the electrodes over the occipital–temporal cortex and decreased in amplitude at more anterior electrodes (Fig. 5), mirroring the posterior-to-anterior distribution of the N2pc in humans (42). Both monkeys P and S exhibited the largest voltage differences contralateral to the target at the most posterior pair of lateralized electrodes. The contralateral positivity decreased monotonically from posterior to anterior electrode pairs. In monkey S, this held for both the earlier contralateral positivity elicited by right visual field targets as well as the longer latency component elicited by left visual field targets. This resulted in a significant interaction of time window (125–200 vs. 250–350), electrode position, target hemifield, and set size [F (10, 210) = 7.32, P < 0.0001] in monkey S. Monkey P had no visual field bias but showed the same posterior focus of the contralateral positivity, resulting in an interaction of electrode site, target hemifield, and set size [F (10, 210) = 10.92, P < 0.0001].
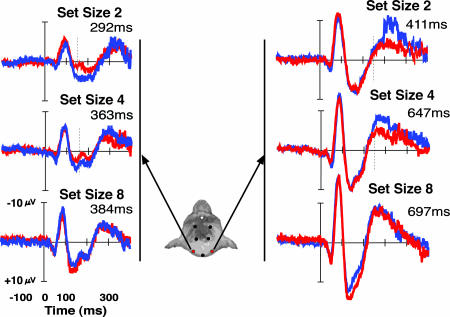
Fig. 5.
ERP waveforms recorded from monkey P (A) and monkey S (B) for right (blue) and left (red) visual field targets across all pairs of lateralized electrodes for search arrays with two items. The amplitude of the contralateral positivity was maximal and significant at the most posterior pair of electrodes (P < 0.01) and decreased progressively at more anterior electrodes. For monkey S, both the early positivity contralateral to right visual field targets and the later positivity elicited by left visual field targets show this pattern. This mirrors the scalp distribution of the human N2pc. Set size 2 arrays are shown because they elicited the largest amplitude effects.
Planned comparisons of each pair of electrodes showed that the interaction of left versus right electrode, target hemifield, and set size was significant for the most posterior pair of lateral electrodes [F (2, 42) = 8.39, P < 0.001; F (2, 42) = 5.56, P < 0.01] but not the central [F (2, 42) = 1.75, P > 0.15; F (2, 42) = 2.60, P > 0.08] or frontal pairs [F (2, 42) = 0.55, P > 0.5; F (2, 42) = 1.65, P > 0.2] for monkeys P and S, respectively. Thus, this lateralized positivity exhibits a posterior-to-anterior distribution that parallels that of the human N2pc component (39, 42).
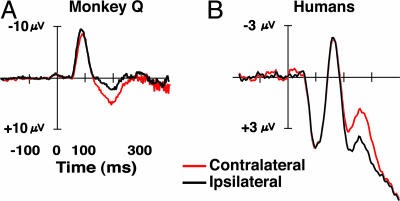
For illustration purposes, Fig. 6 shows the contralateral attention effects from analogous electrode sites in monkey Q and from a group of 10 human subjects who were also shown visual search arrays containing a form-defined target (12). In summary, the macaque contralateral positivity exhibits a spatial distribution and sensitivity-to-task manipulations corresponding to what define the human electrophysiological index of the deployment of covert visual attention during visual search. Hence, the difference in the latency of this functionally equivalent component across species should provide an interesting avenue for future study.
Fig. 6.
Comparison of average ERP waveforms recorded from posterior pair of electrodes from monkey Q (A) with average ERP waveforms from electrodes T5/6 in 12 humans (B) performing a similar visual search task. Waveforms are collapsed across left and right electrodes relative to the hemifield containing the target item. Waveforms from the monkey were recorded while viewing visual search arrays with a set size of 2. Waveforms from humans were recorded while locating and identifying a target appearing in search arrays of Landolt-C-like objects. Adapted from ref. 40 with permission.
Discussion
Recording surface ERPs from macaque monkeys performing visual search, we found attention-related lateralized components homologous to those recorded from humans performing the same type of task. This discovery substantiates the relationship between single-unit neurophysiological measures in macaques and scalp potentials in humans during the deployment of covert visual–spatial attention and thereby bridges an empirical gap known for over half a century (e.g., 15, 43). Previous work has started the bridge (e.g., refs. 16, 17–19, 24, 25, and 29–32), but the present findings are the most direct homology to date including similarities in scalp distribution and systematic sensitivity to cognitive demands.
We found that the monkey homologue of the N2pc is elicited ≈50–75 ms earlier than the human N2pc (e.g., ref. 40). This finding could be due to two factors. First, through extensive practice, the overtrained monkeys may be able to shift attention to targets faster than human subjects. Second, signals may propagate faster in the smaller macaque brain. Previous studies in which visually evoked potentials were recorded from monkeys not performing a task have reported similar latency differences between monkey and human potentials (20–23). This supports the idea that signals simply propagate more quickly through the smaller monkey brain as does the observation of similar lateralized-component onsets across animals with different performance proficiencies.
Of particular note, we found that the polarities of the lateralized ERP components observed in monkeys were opposite those found in humans. However, this can be understood easily, given the anatomical differences between the brains of macaques and humans. ERPs are generated by tissue that affords open electrical fields between the dendritic arbors and the soma of the pyramidal cells, so the cortex is the principle generator of such electrical activity (7). Humans and macaque monkeys have radically different patterns of cortical folding. Source estimation procedures suggest that the human N2pc may be generated predominantly in extrastriate visual areas such as V4 (32, 39, 44) in addition to possible contributions from posterior parietal cortex (45, 46). Whereas monkey V4 is located on a superficial gyrus, comparative neuroimaging research has proposed that the human homolog of macaque area V4 is in an area that has both sulci and gyri (47). Thus, it is plausible that the monkey homologue of the human N2pc component is generated by cortical tissue that is simply inverted relative the orientation of the functionally homologous tissue in humans, resulting in a polarity inversion of the contralateral ERP component. In fact, the human C1 component is of opposite polarity when it is evoked by an upper versus a lower visual field stimulus activating neurons on opposite banks of the calcarine sulcus (48). Similarly, the lateralized readiness potential inverts in polarity for hand-versus-foot movement responses and this is attributed to generators in tissue that exhibits a somatosensory map of the peripheral nervous system in which the hand and the foot are on lateral and medial cortical surfaces, respectively (49, 50). Understanding the neuroanatomical causes of the polarity and timing differences between functionally equivalent ERP components in monkeys and humans will be a fundamental step toward knowing how ERP components are generated.
To summarize, we have developed a technique for reliably and repeatedly recording ERPs from macaque monkeys performing demanding visual search. This will provide a direct empirical link between attention mechanisms studied in the monkey and human electrophysiological literatures. Future simultaneous recordings of extracranial ERPs, intracranial local-field potentials, and neural action potentials are necessary to understand the neural mechanisms of cognition expressed on multiple spatial scales. This combination of electrophysiological techniques can potentially solve the inverse problem of dipole generator localization that that has plagued human electrophysiology (e.g., refs. 51 and 52).
Materials and Methods
Data were collected from three male macaque monkeys (Macaca radiata, 4.5–9 kg) and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Vanderbilt Institutional Animal Care and Use Committee. The general surgical procedures have been described (53).
The electrode implants were constructed from Teflon-coated braided stainless steel wire and solid-gold terminals. Implanted wires were cut to 8.5 cm, the wire ends exposed, and gold amphenol pins were crimped to both ends. One end of the wires were inserted into a plastic connector, whereas the gold pin on other end was ground down until ≈1 mm of the pin remained. During aseptic surgery, 1-mm holes were drilled into the surface of the skull (≈3–5 mm thick), allowing the terminal end of the electrode to be tightly inserted. The inserted gold pin was then covered with a small amount of acrylic cement. After all of the EEG electrodes were implanted, the plastic connector was attached to exposed acrylic to allow access to the channels. The electrode leads that were not embedded in the acrylic were covered by skin that was sutured back over the skull. This allowed for the EEG electrodes to be minimally invasive once implanted. Unlike recordings from skull screws that extend to the dura mater through the skull, recordings from these electrodes approximate those used in human electrophysiological studies because the signals must propagate through the layers of brain, dura, and skull.
The impedance of the EEG electrodes once implanted was 2–5 kΩ measured at 30 Hz, just as those of low-impedance EEG electrodes typically used in human studies. We implanted eight electrodes in monkeys P and S, spanning the frontal, parietal, and occipital bones (Figs. 2 Lower and 3 Lower), approximating sites F3, F4, C1, C2, OL (midway between O1 and T5), OR (midway between O2 and T6), and Oz of the modified human 10/20 system (40, 54). The frontal-most electrode site was placed immediately behind the brow ridge (stereotaxic coordinates: 56 mm anterior, 0 mm lateral/medial) and served as the common reference site for all three monkeys. Because of existing implanted chambers and headpost on monkey Q, the skull was less accessible, but we implanted the common reference and occipital electrode (approximating Oz) in addition to a pair of lateralized electrodes approximating human 10/20 locations T5 and T6 (0 mm anterior/posterior, 25 mm lateral, see Figs. 2 Upper and 3 Upper).
The changes in electrical potential were recorded by using a multichannel data acquisition system (Plexon, Dallas, TX) with a preamplifier band pass of 0.7–170 Hz and digitized at 1,000 Hz. The voltage data and eye position signals were streamed continuously to disk for offline analysis. Eye position was monitored by using a scleral search coil system (55), which provides greater sensitivity than the electrooculogram (EOG) typically used to monitor eye position during human ERP studies. Stimulus presentation and reward delivery were controlled by a cluster of computers running the Reflective Computing TEMPO client–server software package and the Psychophysics Toolbox for Matlab on a Macintosh computer (Apple, Cupertino, CA) (56).
The stimuli used in the visual search task were white (23.8 cd/m2) uppercase san serif Ts and Ls (1.3° × 1.3°) randomly oriented either upright, rotated 90° to the left, 90° to the right, or inverted; each was composed of one vertically and horizontally oriented rectangle. The arrays of search items were presented on a black background (>0.01 cd/m2). Across trials, the set size of the search array varied randomly among 2, 4, and 8 items. The items were centered 10° from the center of the fixation point in the middle of the monitor. When the array contained two objects, they were always presented at opposite locations on the symmetric circle of eight possible locations relative to the fixation point, and when the array contained four items, each was separated by an unfilled location.
Each trial began with the presentation of a filled-square fixation point (0.4° × 0.4°, 23.8 cd/m2). Once the monkey achieved and maintained fixation within ≈2.0° of the center of the screen for 500–1,000 ms (randomly jittered), the search array was presented, and the square fixation point changed simultaneously to an unfilled square of the same size. The monkey's task was to continue to fixate in the center of the screen until he could make an eye movement directly to the target object. Monkeys were permitted one saccade to fixate the target; otherwise the trial was aborted, the array was removed and no reward was given. When the monkey made a saccade to the target and maintained fixation within a 3.0° × 3.0° window centered on the target, a reward, in the form of a drop of juice, was delivered. If no saccade was made within 2,000 ms of the onset of the array, the trial timed out, and no reward was given. Across days, the identity of the target changed such that each of the possible search elements served as both targets and distractors. Randomly interleaved among the target-present trials were a percentage of catch trials (10–33%) to discourage guessing. On catch trials, the monkeys were rewarded for maintaining fixation in the center of the screen for a randomly jittered duration (between 750 and 1,250 ms, with a rectangular distribution) after the presentation of the search array. Except where noted, these analyses will focus on trials in which a target was present. In each session, the monkey correctly localized the lateralized targets on at least 900 trials, and ERP averages from each monkey were collected during 22–24 sessions, resulting in within-monkey grand averages of at least 20,000 trials.
Behavioral and ERP analyses were performed offline by using custom Matlab scripts. Trials were rejected from the ERP averages if an incorrect response was made, any channel saturated the amplifier, or the monkey failed to maintain fixation. Thus, trials were included in the analysis only if one saccade was correctly made to the target location. To examine the ERPs preceding correct eye-movement responses of the monkeys, code was used to detect the onset of a saccade based on velocity and change-of-position criteria (53). All data after saccade initiation and preceding it by 20 ms were discarded to ensure that the data contributing to the averages were not contaminated by small changes in eye position before saccade detection. To identify potentially lateralized effects relative to target location, trials in which the target appeared on the vertical meridian were not included in analyses of behavior or ERPs. Activity from trials of each type was averaged millisecond-by-millisecond time-locked relative to the onset of the stimulus array.
Behavioral and ERP analyses within each animal used the means of the individual sessions as the random factor. Amplitude measurement windows for the first positive and negative component were measured from the occipital electrode (Oz) from 75–125 ms and 125–175 ms poststimulus, respectively, and the second positive deflection from 175–225 ms poststimulus. The lateralized N2pc-like activity was measured as the difference between the most posterior ipsilateral and contralateral electrode sites during two intervals: from 125 to 250 ms and 250 to 350 ms poststimulus. The first window included the voltage deflections contralateral to targets across all sessions from all animals. The second allowed us to analyze the latency difference observed in monkey S for left and right visual field targets. Onset latency of the contralateral, positive component was measured by calculating the time point at which the amplitude of the waveforms elicited by ispilateral and contralateral targets in a 17-ms sliding window were significantly different (P < 0.05) for 20 consecutive data points (34). All significance values were corrected for nonsphericity by using the Greenhouse–Geisser ε-correction (57). The ERP averages shown are baseline corrected but unfiltered beyond the amplifier bandpass settings because of the power inherent in the large number of trials obtained (>20,000 per monkey).
Acknowledgments
We thank Steve Luck for valuable comments on this work and anonymous readers for important suggestions during the review process. This work was supported by National Research Service Award F32 EY015043 (to G.F.W.), by Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience, a Vanderbilt University Discovery Grant, and National Institutes of Health Grants R01-EY08890, P30-EY08126, P30-HD015052.
Abbreviations
- EEG
electroencephalogram
- ERP
event-related potential
- RT
reaction time.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Caton R. Br Med J. 1875;2:278. [Google Scholar]
- 2.Berger H. Arch Psychiatrie Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- 3.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 4.Evarts EV, Magoun HW. Science. 1957;125:1147–1148. doi: 10.1126/science.125.3258.1147. [DOI] [PubMed] [Google Scholar]
- 5.Hubel DH, Henson CO, Rupert A, Galambos R. Science. 1959;129:1279–1280. doi: 10.1126/science.129.3358.1279. [DOI] [PubMed] [Google Scholar]
- 6.Wurtz RH, Goldberg ME. Science. 1971;171:82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- 7.Hillyard SA, Picton TW. In: Handbook of Physiology: Section 1. The Nervous System: Volume 5. Higher Functions of the Brain, Part 2. Plum F, editor. Bethesda: Waverly Press; 1987. pp. 519–584. [Google Scholar]
- 8.Kutas M, Hillyard SA. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- 9.Hillyard SA, Squires KC, Bauer JW, Lindsay PH. Science. 1971;172:1357–1360. doi: 10.1126/science.172.3990.1357. [DOI] [PubMed] [Google Scholar]
- 10.Vogel EK, Machizawa MG. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 11.Knight RT. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 12.Woodman GF, Luck SJ. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- 13.Gehring WJ, Willoughby AR. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 14.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 15.Bullock TH. Neural Comp. 2003;15:2013–2027. doi: 10.1162/089976603322297269. [DOI] [PubMed] [Google Scholar]
- 16.Paller KA, McCarthy G, Roessler E, Allison T, Wood CC. Electroencephalogr Clin Neurophysiol. 1992;84:269–279. doi: 10.1016/0168-5597(92)90008-y. [DOI] [PubMed] [Google Scholar]
- 17.Arthur DL, Starr A. Science. 1984;223:186–188. doi: 10.1126/science.6691145. [DOI] [PubMed] [Google Scholar]
- 18.Glover A, Ghilardi MF, Bodis-Wollner IMO, Mylin LH. Electroencephalogr Clin Neurophysiol. 1991;1991:65–72. doi: 10.1016/0168-5597(91)90045-y. [DOI] [PubMed] [Google Scholar]
- 19.Javitt DC, Schroeder CE, Steinshneider M, Arezzo JC, Vaughan HG. Electroencephalogr Clin Neurophysiol. 1992;83:87–90. doi: 10.1016/0013-4694(92)90137-7. [DOI] [PubMed] [Google Scholar]
- 20.Lamme VA, Van Dijk BW, Spekreijse H. Vision Res. 1992;32:797–807. doi: 10.1016/0042-6989(92)90022-b. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder CE, Tenke CE, Givre SJ. Electroencephalogr Clin Neurophysiol. 1992;84:219–231. doi: 10.1016/0168-5597(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HGJ. Vision Res. 1991;31:1143–1157. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- 23.Marel HVD, Dagnelie G, Spekreijse H. Electroencephalogr Clin Neurophysiol. 1984;57:354–368. doi: 10.1016/0013-4694(84)90159-7. [DOI] [PubMed] [Google Scholar]
- 24.Mehta AD, Schroeder CE. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- 25.Mehta AD, Schroeder CE. Cereb Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- 26.Pincze Z, Lakatos P, Rajkai C, Ulbert I, Karmos G. Cogn Brain Res. 2002;13:249–253. doi: 10.1016/s0926-6410(01)00105-7. [DOI] [PubMed] [Google Scholar]
- 27.Pincze Z, Lakatos P, Rajkai C, Ulbert I, Karmos G. Clin Neurophysiol. 2001;112:778–784. doi: 10.1016/s1388-2457(01)00509-0. [DOI] [PubMed] [Google Scholar]
- 28.Javitt DC, Steinshneider M, Schroeder CE, Vaughan HGJ, Arezzo J. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder CE, Foxe JJ. Cognit Brain Res. 2002;14:187–198. doi: 10.1016/s0926-6410(02)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.Fu KM, Foxe JJ, Higgins BA, Murray MM, Javitt DC, Schroeder CE. J Cognit Neurosci. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- 31.Shah AS, Bressler SL, Knuth KH, Ding M, Mehta AD, Ulbert I, Schroeder CE. Cereb Cortex. 2004;14:476–483. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- 32.Luck SJ, Girelli M, McDermott MT, Ford MA. Cognit Psychol. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- 33.Luck SJ, Hillyard SA. Percep Psychophys. 1990;48:603–617. doi: 10.3758/bf03211606. [DOI] [PubMed] [Google Scholar]
- 34.Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 35.Wolfe JM, Cave KR, Franzel SL. J Exp Psychol: Hum Percep Perform. 1989;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- 36.Wijers AA, Lange JJ, Mulder G, Mulder LJM. Psychophysiology. 1997;34:553–565. doi: 10.1111/j.1469-8986.1997.tb01742.x. [DOI] [PubMed] [Google Scholar]
- 37.Pineda JA, Nava C. Behav Brain Res. 1993;53:177–187. doi: 10.1016/s0166-4328(05)80277-3. [DOI] [PubMed] [Google Scholar]
- 38.Woodman GF, Luck SJ. Psychol Sci. 2003;14:605–611. doi: 10.1046/j.0956-7976.2003.psci_1472.x. [DOI] [PubMed] [Google Scholar]
- 39.Luck SJ, Hillyard SA. J Exp Psychol: Hum Percep Perform. 1994;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- 40.Woodman GF, Luck SJ. J Exp Psychol: Hum Percep Perform. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman JE, Subramaniam B. Percep Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- 42.Luck S. J, Hillyard S. A. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 43.Li C-L, McLennan H, Jasper H. Science. 1952;116:656–657. doi: 10.1126/science.116.3024.656. [DOI] [PubMed] [Google Scholar]
- 44.Luck SJ. Curr Opin Neurobiol. 1994;4:183–188. doi: 10.1016/0959-4388(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 45.Fuggetta G, Pavone EF, Walsh V, Kiss M, Eimer M. J Neurophysiol. 2006;95:3277–3280. doi: 10.1152/jn.01273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hopf J-M, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, Heinze HJ. Cereb Cortex. 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- 47.Orban GA, Van Essen DC, Vanduffel W. Trends Cognit Sci. 2004;8:315–320. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Clark VP, Fan S, Hillyard SA. Hum Brain Mapp. 1995;2:170–187. [Google Scholar]
- 49.Miller J, Riehle A, Requin J. J Exp Psychol: Hum Percep Perform. 1992;18:1121–1138. doi: 10.1037//0096-1523.18.4.1121. [DOI] [PubMed] [Google Scholar]
- 50.Coles MGH. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 51.Adrian ED, Yamagiwa K. Brain. 1935;58:323–351. [Google Scholar]
- 52.Helmholtz HV. Ann Physik Chemie. 1853;89:211–233. 354–377. [Google Scholar]
- 53.Schall J, Hanes DP, Thompson KG, King DJ. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jasper HH. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [Google Scholar]
- 55.Robinson DA. IEEE Transact Biomed Engineer. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 56.Brainard DH. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 57.Jennings JR, Wood CC. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]