Abstract
Color constancy refers to the unchanging nature of the perceived color of an object despite considerable variation in the wavelength composition of the light illuminating it. The color contrasts between objects and their backgrounds play a crucial role in color constancy. We tested a patient whose right striate cortex had been removed and demonstrated that he made no use of color contrast in judging color appearance but instead made judgments based simply on wavelength comparison. This was shown by presenting pairs of colored stimuli against a background color that gradually changed across space. When presented with such displays, both normal observers and those with cerebral achromatopsia (cortical color blindness) judge the color appearance of such stimuli on the basis of the chromatic contrast the stimuli make against their background rather than on the physical wavelengths of the light emitted from them. However, our patient made no such use of color contrast but, instead, made color discriminations simply on the basis of wavelength composition. This is consistent with recent findings from monkey electrophysiology that identify cells in early cortical visual areas that signal local contrast and so contribute to the likely mechanism for achieving color constancy.
Keywords: constancy, visual cortex, neuropsychology
The stable appearance of colored surfaces, despite variations in the wavelength distribution of the light illuminating them, implies that color appearance is not simply determined by the composition of reflected light. Color appearance is more closely related to a constant property of a surface, its spectral reflectance. The relative reflectances of adjacent surfaces at any specific wavelength of light determine the relative intensities of light reflected from them at that wavelength, regardless of the spectral composition of the light illuminating them. Ratios of cone signals (the ratio of responses of cone photoreceptors that integrate light intensities over differing ranges of wavelengths) are approximately invariant under changes of illumination (1). If the reflectance properties of one surface are known, or can be reliably estimated by other means, then the properties of all other surfaces seen under the same illuminant can be inferred on the basis of these cone ratios or color contrasts [this is the essence of Land's “retinex” color constancy algorithm (2)]. One important step in this process is therefore extracting the relative activity in each class of retinal cones elicited by adjacent surfaces. Questions about the role striate cortex, the first visual area of the higher mammalian brain, plays in color perception have been reignited recently (3–7), revisiting earlier classic work (8). Single-cell electrophysiological data from monkeys suggest that striate cortex is indeed involved, specifically signaling color contrasts at surface boundaries. These cells in striate cortex are excited by light of one distribution of wavelengths and inhibited by light of another distribution falling in one small region of space. Adjacent or surrounding regions show the opposite pattern of responses. The cells therefore respond optimally to borders with a specific chromatic contrast that fall across the center and surround regions of their spatial receptive fields. These are important findings because they suggest color-specific processing starts earlier in the visual system than previously thought. The earliest mechanisms of color constancy, our ability to recognize objects' colors regardless of the color of light illuminating them, might occur in striate cortex. If so, patients without striate cortex should lose color contrast processing. They should behave as if objects change color as the light illuminating them changes, their apparent color depending on the wavelengths of light they reflect, rather than the properties of their surface material. Brightness discrimination, however, may still show some aspects of constancy because cells selective for luminance contrast across borders are found subcortically in the thalamus and even in the retina.
A person's response to carefully tailored visual illusions (Fig. 1) can reveal whether he or she discriminates between stimuli on the basis of the physical properties of light reaching his or her eyes from those stimuli (wavelength and intensity) or the contrasts the stimuli make with their backgrounds. If the background luminance of an image varies gradually across it, then two stimuli presented against it at different positions but having identical luminances will be perceived by a normal observer as being different, presumably as a consequence of their different immediate backgrounds (Fig. 1a). Two stimuli that differ in brightness but have the same contrast relative to their background will, however, be perceived as similar (Fig. 1b). This illusion is also effective for color using stimuli matched for chromatic contrast in terms of the changes they elicit in the excitation of different retinal cone types, as can be seen in Fig. 1 e and f. In our task, the participant was presented successively with two pairs of discs, either colored or achromatic, against a graduated background and asked to report which of the two pairs of discs were most similar to one another. In one pair, the discs had identical cone contrast with their, albeit different, backgrounds and were of a similar appearance to a normal observer (as in Fig. 1 b and f). In the other pair, the discs were physically identical to one another but were chosen such that the cone contrast of the discs, against their respective backgrounds, was of equal but opposite magnitude (as in Fig. 1 a and e). Although the discs were identical, they nevertheless appeared different to the normal observer. As a control condition, the same two pairs of discs were presented but against a uniform, rather than graduated, background (as in Fig. 1 c and d and analogous color conditions).
Fig. 1.
Stimuli. (a and b) Pairs of discs presented against a graduated background. Most observers judge the pair in b as being more similar to one another than those in a. (c and d) Exactly the same pairs of discs as those in a and b, respectively, but against uniform backgrounds. This shows that the pair in a are physically identical, although they appear different because they differ in contrast relative to their immediate backgrounds. (e and f) The same illusion in the color domain. The upper and lower discs are spectrally identical in e, although they appear quite different from one another when seen against the graduated background. In f, the color of the lower disc has been adjusted to make its contrast against its background, and hence its appearance, more similar to the upper disc. Note that print color reproduction may not represent the stimuli accurately.
To assess the role of striate cortex in color contrast processing, we presented our task to a patient, D.B., who had large portions of right striate cortex surgically removed in 1973 for treatment of venous contusions (9). D.B., aged 64 at the time of testing, still has a left visual field defect as a consequence but retains a residual ability to make visual discriminations between stimuli presented within his scotoma (10). D.B. does not report perceiving color when colored stimuli are presented to him (and did not do so during this experiment) but can discriminate between stimuli differing in color using forced-choice methodology. D.B. participated after giving informed consent. His participation in these studies was approved by the Oxford University Department of Experimental Psychology Committee on Ethical Aspects of Research.
D.B. performed the task with stimuli presented in his left visual field, where striate cortex could play no part in his response, and, acting as his own control, with stimuli presented in his normal right visual field. We predicted that his responses to luminance stimuli would be identical in both fields but that he would respond to colored stimuli solely on the basis of their wavelength, not their contrast, in his left field. His task on each trial was to indicate in which of two successively presented stimuli the pair of discs matched each other better. In one interval, the discs were constructed to be contrast matches when presented against the graduated background; in the other, they were physical matches.
Results
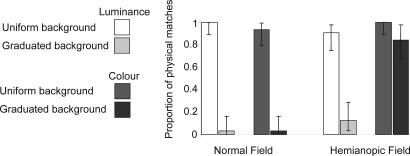
D.B.'s performance is illustrated in Fig. 2. In his left, undamaged, visual field, his judgments of both luminance and chrominance matches are changed when stimuli are presented against a graduated background. In his damaged visual field, a graduated background affects his luminance matching judgments but not his chrominance judgments. As a statistical test, we assessed the likelihood of obtaining D.B.'s pattern of responses in his damaged field by chance given the probabilities with which he made “match” judgments against graduated and uniform backgrounds in his undamaged field. We pooled color and luminance conditions in computing the undamaged-field baseline probabilities to avoid the statistical problems an expected probability of zero (for contrast-based matches of luminance against a uniform background) would produce. Applying these expected probabilities [p(contrast match | uniform background) = 0.031, p(physical match | uniform background) = 0.969, p(contrast match | graduated background) = 0.969, p(physical match | graduated background) = 0.031], we computed the likelihood of the obtained hemianopic field judgments using multinomial distribution functions (tests based on χ2 are ruled out by the low expected frequencies encountered). The likelihood that D.B.'s luminance judgments in his affected visual field are made on the same basis as his judgments in his good field does not differ from chance. The likelihood that his chrominance judgments in his affected visual field are made on the same basis as his judgments in his good field is vanishingly small (<1 in 1033).
Fig. 2.
Results. Percentage of trials on which the physically matching pair of stimuli was selected as more similar to one another than the contrast matching pair. In D.B.'s right hemianopic visual field, he judges a pair of discs such as those seen in Fig. 1e as being similar, although, in both his normal and damaged fields, he is subject to the luminance illusion corresponding to that which makes us see the discs presented in Fig. 1e as different. Error bars are 95% binomial confidence intervals.
Discussion
Normal subjects reliably judge contrast matches as being perceptually similar against a graduated background and physical matches as perceptually similar against a uniform background (11). The results of this simple experiment clearly show that damage to striate cortex leads to quite different responses to wavelength from those mediated by undamaged cortex, either in D.B.'s unaffected visual field or by normal observers. These results also contrast with the behavior of patients suffering from cerebral achromatopsia, which is cortical color blindness as a result of damage to cortex in the vicinity of the lingual and fusiform gyri. We previously showed that such a patient (who had spared striate cortex) discriminated between chromatic-contrast cone ratios but could not match colors presented against differing backgrounds (12), that is, he was subject to the same form of illusion as normal observers when comparing stimuli against graduated backgrounds. We assume that this latter patient retains access to the responses of chromatically double-opponent cells in striate cortex signaling local chromatic contrast and can make use of these signals in perception of form and motion (13) but that he has lost later stages of color processing necessary for achieving color constancy across an entire visual scene.
In contrast to his wavelength processing, D.B.'s luminance judgments were similar in his normal and damaged visual fields. Cells in the lateral geniculate nucleus (and retina) demonstrate spatial opponency for luminance but are spatially synergistic, not opponent, for wavelength. It is not until striate cortex that cells are found with chromatic spatial opponency that are selective for spatial chromatic contrasts. Extraction of luminance contrast signals should not, therefore, be compromised by damage to striate cortex, as is reflected by D.B.'s luminance judgments.
A variety of pathways may mediate wavelength discrimination in the absence of striate cortex, perhaps involving projections to extrastriate areas bypassing striate cortex (14). Our results show that any such extrastriate areas nevertheless depend on striate cortex for the computation of chromatic contrast in a manner analogous to recent electrophysiological discoveries of cellular responses in the monkey.
Materials and Methods
Stimuli were generated by using a Cambridge Research Systems (Kent, U.K.) VSG2/5 driving a gamma-corrected Sony (Tokyo, Japan) GDM-F520 21-inch color monitor. The display consisted of two 1° diameter discs presented 15° above and below the horizontal meridian either 10° into the left or the right visual field from a central black fixation cross. Displays were characterized in terms of cone contrast space based on Smith–Pokorny (15) cone fundamentals. S-cone activation was kept constant and as low as possible for all displays. Discs and background variation were therefore defined entirely in terms of changes in L and M cone activations, either so that all modulation was along an isoluminant “red–green” axis or so that all modulation was along a constant L:M ratio isochrominant axis. The display background was either uniform or varied linearly from top to bottom in cone contrast from a midpoint of 0.388, 0.474 in CIE 1934 x, y color space, with a luminance of 20.5 cd·m−2. For isoluminant displays, the graduated background varied from +14% to −14% cone contrast from the midpoint, for isochrominant display the variation was between +80% and −80%. The locations at which the discs were displayed corresponded to contrasts of ±7% and ±40% on the isoluminant and isochrominant graduated backgrounds, respectively. The uniform backgrounds had the same color as that in the graduated backgrounds at the first of these locations (+7% and +40% from the graduated midpoint). For the “physical match” condition, the contrasts of the discs relative to their immediate backgrounds were computed so that, on the graduated background, physically identical stimuli at the upper and lower locations would have contrasts of identical magnitude but opposite signs relative to their immediate backgrounds. For the “contrast match” condition, both discs had the same positive contrast relative to their immediate backgrounds on the graduated background.
Testing was carried out in blocks of 32 trials. There were two blocks of luminance trials and two blocks of color trials for left and right visual fields carried out in a counterbalanced order. Within each block, there was a randomized presentation of equal numbers of graduated background and uniform background trials and equal numbers of trials where the physical and contrast match discs were presented first. In each interval, the background and fixation cross alone were presented for 1,000 ms, the discs then appeared and remained present for 1,500 ms. A warning tone (one beep for the first interval, two for the second) sounded 500 ms before the presentation of the discs. At the end of the second interval, a different tone indicated that a response should be made. The subject indicated which of the two intervals appeared to show the better matching pair using a button box. Once the response had been registered, the next trial started immediately. The subject's fixation was monitored by direct visual inspection throughout the experimenter. No deviations from fixation were detected during the experiment. Any failure to fixate would, in any case, tend to bias results toward the null hypothesis, i.e., results from left and right fields would become more similar. It is also the case that, if any functional striate cortex survives in D.B.'s right hemisphere, that too would bias results toward the null hypothesis.
Acknowledgments
We thank D.B. for his willing cooperation and A. Sahraie and C. Trevethan for their help and advice. This work was supported by Medical Research Council Grant G0000679.
Footnotes
The authors declare no conflict of interest.
References
- 1.Foster DH, Nascimento SMC. Proc R Soc London Ser B. 1994;257:115–121. doi: 10.1098/rspb.1994.0103. [DOI] [PubMed] [Google Scholar]
- 2.Land EH, McCann JJ. J Opt Soc Am. 1971;61:1–11. doi: 10.1364/josa.61.000001. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EN, Hawken MJ, Shapley R. Nat Neurosci. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- 4.Conway BR. J Neurosci. 2001;21:2768–2783. doi: 10.1523/JNEUROSCI.21-08-02768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapley R, Hawken MJ. Curr Opin Neurobiol. 2002;12:426–432. doi: 10.1016/s0959-4388(02)00349-5. [DOI] [PubMed] [Google Scholar]
- 6.Conway BR, Hubel DH, Livingstone MS. Cereb Cortex. 2002;12:915–925. doi: 10.1093/cercor/12.9.915. [DOI] [PubMed] [Google Scholar]
- 7.Johnson EN, Hawken MJ, Shapley R. J Neurophysiol. 2004;91:2501–2514. doi: 10.1152/jn.01043.2003. [DOI] [PubMed] [Google Scholar]
- 8.Michael CR. J Neurophysiol. 1978;41:572–588. doi: 10.1152/jn.1978.41.3.572. [DOI] [PubMed] [Google Scholar]
- 9.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Brain. 1974;98:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 10.Weiskrantz L. Brain. 1987;110:77–92. doi: 10.1093/brain/110.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Kentridge RW, Cole GG, Heywood CA. Prog Brain Res. 2004;144:161–169. doi: 10.1016/S0079-6123(03)14411-1. [DOI] [PubMed] [Google Scholar]
- 12.Kentridge RW, Heywood CA, Cowey A. Neuropsychologia. 2004;42:821–830. doi: 10.1016/j.neuropsychologia.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Heywood CA, Kentridge RW, Cowey A. Exp Brain Res. 1998;123:145–153. doi: 10.1007/s002210050555. [DOI] [PubMed] [Google Scholar]
- 14.Cowey A, Stoerig P. Exp Brain Res. 1989;75:631–638. doi: 10.1007/BF00249914. [DOI] [PubMed] [Google Scholar]
- 15.Smith VC, Pokorny J. Vision Res. 1975;15:161–175. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]