Abstract
In monkeys infected with simian immunodeficiency virus (SIV), changes in body temperature and locomotor activity occur after the acute retroviral syndrome stage of the disease. However, alterations to the circadian rhythm of these factors in SIV-infected monkeys have not been reported. To determine whether the circadian rhythm of body temperature and locomotor activity are disrupted during SIV infection, we analyzed the temperature and activity patterns of SIV-infected monkeys through different stages of the disease, progressing to SIV encephalitis by using the cosinor model for circadian oscillation. We found that SIV infection resulted in significant impairments of the amplitude and mean of the circadian rhythm of body temperature and activity and in the acrophase of the circadian rhythm for temperature. These alterations were not related to changes observed in the acute febrile response induced after viral inoculation. In animals killed once marked circadian anomalies were evident, microglia infiltration and macrophage accumulation in the hypothalamus were observed. Together, these results clearly demonstrate that SIV infection compromises aspects of circadian regulation in monkeys, with important implications for physiological functions, including cognition, in HIV-infected individuals.
Keywords: AIDS, CNS, dementia, HIV
Cognitive and motor abnormalities due to CNS dysfunction are common in HIV-infected patients and can result in marked impairment of their quality of life. Although reduced in incidence, the prevalence of such disorders has increased in the current era of antiretroviral therapy (1). Similarly, in the nonhuman primate model for AIDS, simian immunodeficiency virus (SIV) infection of macaques also leads to CNS disorders. Because HIV/SIV does not infect neurons, the untoward effects of HIV on neurons are indirect and may occur through release of proinflammatory molecules that disrupt normal neuronal function or neurotoxic viral proteins (2–4). Nonetheless, the exact mechanisms that lead to cognitive and motor dysfunction during HIV/SIV infection are unknown.
Interestingly, in HIV-infected individuals, disruptions have been observed in both homeostatic regulation and circadian rhythmicity of body temperature, activity, sleep patterns, and hormone secretion (5–10). The master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, is the neural structure controlling the circadian rhythms of the behavioral and physiological states (11–13). It has been hypothesized that impairments in circadian control mechanisms could cause disorders in numerous functions regulated by the CNS, as well as alterations in cognitive processes. For instance, deterioration of the control of circadian rhythm in patients with Alzheimer's disease (AD) (14, 15) leads to disruptions in the secretion of melatonin and body temperature rhythm alterations (16, 17). These, in turn, affect the distribution of the sleep/wake patterns and are associated with cognitive impairments (18). Therefore, it is possible that disturbances in circadian rhythms are partially responsible for the CNS disorders reported in HIV patients.
Although the effect of SIV infection on the circadian rhythms have not been studied in detail, we have previously shown that monkeys infected with SIV develop an acute febrile response, accompanied by hypolocomotion and up-regulation of proinflammatory cytokines 2 wk after inoculation of the virus (19, 20). Milder disruptions in temperature and activity occur after the resolution of this acute phase and are not reversed by treatment with antiretroviral drugs that reduce plasma viral load. This result suggests that the virus/host interaction may induce long-lasting alterations in the mechanisms associated with the regulation of body temperature and activity (21).
To determine whether the circadian rhythms of body temperature and gross locomotor activity are compromised during SIV infection and the subsequent progression to CNS disease, we analyzed the temperature and activity patterns of monkeys through different stages of the disease. Three parameters characterize a circadian rhythm: amplitude (half the difference between the minimum and maximum); midline estimating statistic of rhythm (MESOR) (the mean of the circadian oscillation); and acrophase (time of maximum value). Alterations in these measures indicate impairments in the regulation of the circadian system (22). Here we report significant changes in these three variables of the circadian rhythms of body temperature and activity in SIV-infected monkeys that progress to SIV-induced CNS disease, which is manifested as encephalitis. These results clearly show that infection with SIV can lead to disruption of the circadian regulation of both body temperature and locomotor activity in monkeys.
Results
Effects of SIV Infection on the Circadian Rhythm of Body Temperature.
To assess whether SIV infection and the subsequent progression of disease affects circadian rhythms, we analyzed radiotelemetry data from five uninfected control rhesus monkeys and five infected rhesus monkeys that developed SIV encephalitis (SIVE) as the manifestation of simian AIDS. Six stages were examined in the infected animals: (i) before SIV inoculation (preinfection) when the monkeys were clinically normal; (ii) the period corresponding to the acute retroviral syndrome (acute), an acute febrile period associated with increasing viremia; (iii) after the resolution of the acute stage (steady state), a relatively asymptomatic period associated with a stable plasma viral load; and (iv) three time points as the disease progressed (progressing stages 1, 2, and 3).
The steady stage varied in length, but signs and symptoms of disease progression occurred ≈1 month before animals required euthanasia. Therefore, we used the time before termination to better synchronize the stages for the individual animals, and we defined this period as progressing stages 1, 2, and 3, occurring 4.5, 2.5, and 0.5 wk before necropsy. Thus, except for the febrile period in the acute stage, all stages were defined without regard to the telemetry data. The data from uninfected control animals were matched on the basis of elapsed time between the stages defined for the infected animals. During these latter stages, the SIV-infected monkeys exhibited increased plasma viral load, loss of body weight (Table 1), and noticeable behavioral changes (e.g., withdrawn behavior, decreased food and water intake).
Table 1.
Body weights and plasma viral load in five SIV-infection stages
| Stage | Body weight, kg | Plasma viral load, log10/ml |
|---|---|---|
| Preinfection | 6.82 ± 1.34 | NA |
| Acute | 6.77 ± 1.11 | 7.84 ± 0.49 |
| Steady state | 6.89 ± 1.34 | 6.88 ± 0.54 |
| Progressing 1 | ND | 7.70 ± 0.49 |
| Progressing 2 | ND | 8.19 ± 0.42 |
| Progressing 3 | 6.35 ± 0.78 | 8.32 ± 0.22 |
The data are means ± SD. The values are those closest to the time points used for circadian analysis, except for the acute stage, for which the peak viral load is given as that occurring between days 10 and 17 after inoculation. NA, not applicable; ND, not determined due to missing data points.
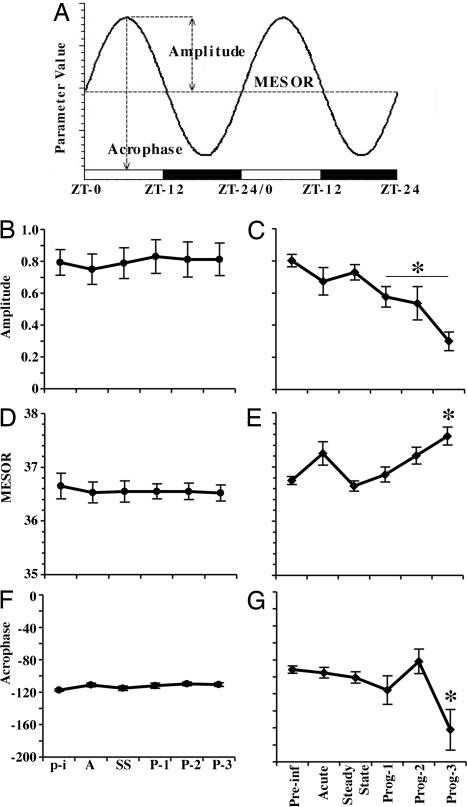
The data were analyzed by using cosinor analysis (fitting the circadian changes to a cosine curve) for evaluating alterations of the circadian rhythm of body temperature by measuring the amplitude, MESOR, and acrophase (Fig. 1A) of the rhythm (22). Analysis of uninfected monkeys, kept under the same environmental conditions in the same housing room, revealed no significant differences in the circadian rhythm of body temperature during the study period (Fig. 1 B, D, and F). However, in the SIV-infected group, the group mean cosinor analysis indicated a progressive decrease in the temperature amplitude in SIVE monkeys compared with baseline, reaching a significant reduction of 28%, 33%, and 62% [F(5,35) = 36.73, P < 0.0001] during the progressing stages 1, 2, and 3, respectively (Fig. 1C), whereas a significant difference in the MESOR occurred in the progressing stage 3 [F(5,35) = 9.73, P < 0.01] (Fig. 1E). A significant delay of 5 h (from −91.5 to −162.24°) [F(5,35) = 8.27, P < 0.01] occurred in the acrophase during the progressing stage 3, compared with preinfection (−91.5°) (Fig. 1G).
Fig. 1.
Effects of SIV infection on the circadian rhythm of body temperature. (A) Diagrammatic representation of cosinor analysis and the circadian parameters measured in this study. (B–G) Effects of SIV infection on the circadian rhythm of body temperature. In uninfected animals, the amplitude (B), MESOR (D), and acrophase (F) in the uninfected control group did not show significant changes during the study. In the SIV-infected group, the amplitude (C), MESOR (E), and acrophase (G) showed significant alterations through the different stages of the disease. For the uninfected controls, time periods equivalent to those measured in the SIV-infected group are labeled p-i (corresponding to preinfection), A (acute), SS (steady state), and P-1, P-2, and P-3 (progressing stages 1, 2, and 3, respectively). Each point in the graphs represents the mean values (± SEM) of a 24-h period. Asterisks indicate significant differences compared with the preinfection stage (P ≤ 0.01); n = 5 in each group.
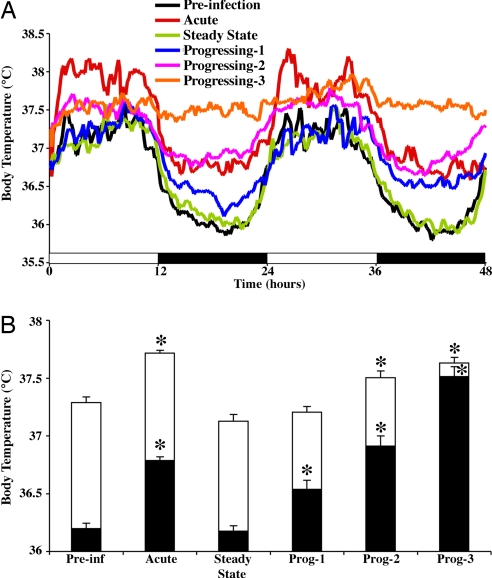
Because light provides a strong environmental time cue (zeitgeber) to the circadian rhythm, the patterns of body temperature during the day and night were also analyzed in the monkeys. Body temperature recordings revealed that before SIV infection, the animals had a well defined body temperature distribution across the light and dark phases (Fig. 2A), revealing average values of 37.28 ± 0.089°C and 36.19 ± 0.089°C, respectively. A detailed analysis of the changes in body temperature across states of the disease revealed that in the light phase, SIV-infected monkeys had a slight, but significant, increase of 0.42, 0.21, and 0.34°C during the acute stage and progressing stages 2 and 3, respectively [F(5,719) = 211.6, P < 0.0001], compared with the preinfection stage. A greater change in temperature was found in the dark phase, in which the infected animals showed an increase of 0.58, 0.34, 0.71, and 1.31°C during the acute stage and progressing stages 1, 2, and 3, respectively [F(5,719) = 737.3, P < 0.0001], compared with the preinfection stage (Fig. 2B).
Fig. 2.
Effects of SIV on body temperature through different stages of the disease. (A) Data from the 48-h monitoring periods revealing the acute febrile response, followed by recovery of body temperature control but then progressive disruption in disease progression. The data are expressed as the means of five monkeys. (B) The effects on temperature during the light (white bars) and dark (black bars) phases (12 h each) of the cycle. The data are expressed as the means ± SEM of five monkeys. Asterisks indicate significant differences compared with the preinfection stage (P ≤ 0.01). Prog, progressing stage.
Effects of SIV Infection on the Circadian Rhythm of Gross Locomotor Activity.
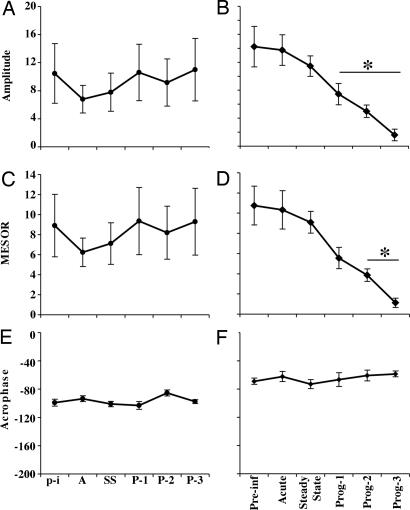
We similarly evaluated the circadian rhythm of gross locomotor activity of SIV-infected animals through analysis of the telemetry data for locomotor activity. As observed in the temperature data, no significant differences in the circadian rhythm of activity were identified in the control uninfected group (Fig. 3 A, C, and E). However in the SIV-infected group, the amplitude of the rhythm was significantly decreased 44, 63, and 88% [F(5,35) = 34.18, P < 0.001] during progressing stages 1, 2, and 3, respectively, compared with baseline (Fig. 3B). The MESOR was significantly decreased 50%, 65%, and 90% [F(5,35) = 9.83, P < 0.001] during progressing stages 1, 2, and 3, respectively, compared with baseline (Fig. 3D). However, the acrophase of the rhythm did not show significant changes compared with baseline (Fig. 3F).
Fig. 3.
Effects of SIV infection on the circadian rhythm of activity. (A, C, and E) In uninfected monkeys, the amplitude (A), MESOR (C), and acrophase (E) did not show significant changes along the study. (B, D, and F) For the SIV-infected monkeys, the amplitude (B) and MESOR (D) of locomotor activity circadian rhythm showed significant alterations during the progression of the disease to SIVE; the acrophase (F) did not. Stages were matched between the uninfected and SIV-infected groups, as described in the legend to Fig. 1. Each point represents the mean values (± SEM) of a 24-h period. Asterisks indicate significant differences compared with the preinfection stage (P ≤ 0.01); n = 5 in each group.
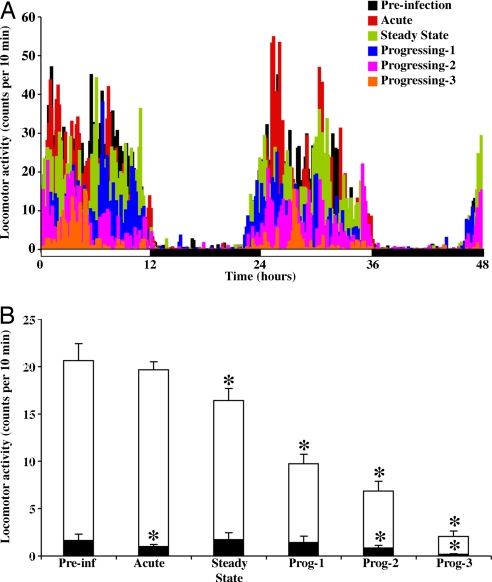
Gross locomotor activity patterns were analyzed through the different stages of the SIV infection. The results indicated that locomotor activity during the preinfection stage showed a well defined distribution through the light and dark phase (Fig. 4A), resulting in average values of 20.62 ± 1.78 and 1.63 ± 0.67, respectively. However, a detailed analysis revealed that, in the light phase, SIV-infected monkeys had a significant gradual decrease of 20, 53, 67, and 90% during the steady-state stage and progressing stages 1, 2, and 3, respectively [F(5,719) = 167.4, P < 0.0001], compared with the preinfection stage. During the dark phase, gross locomotor activity remained low during the different stages of the disease but did show significant reductions of 38%, 48%, and 88% during the acute stage and progressing stages 2 and 3, respectively [F(5,719) = 9.31, P < 0.0001], compared with the preinfection stage (Fig. 4B).
Fig. 4.
Effects of SIV on locomotor activity through different stages of the disease. (A) Data from the 48-h monitoring periods, expressed as the means of five monkeys. A progressive decrease in activity, mainly during the light phase, was observed in SIVE monkeys. (B) The effect on activity levels during the light (white bars) and dark (black bars) phase (12 h each) of the cycle. The data are expressed as the means ± SEM of five monkeys. Asterisks indicate significant differences compared with the preinfection stage (P ≤ 0.01).
CNS Histopathology in Animals with Circadian Abnormalities.
The animals analyzed all manifested SIVE, with the brain containing multifocal perivascular macrophage accumulation, activated microglia and astrocytes, infiltrating immune cells (predominantly macrophages as well as lesser numbers of lymphocytes), and the pathognomic multinucleated giant cells. Abundant SIV was present in the brain, demonstrated by immunohistochemistry for the Gag protein, in situ hybridization for the Nef gene, and the quantitative branched DNA assay, the latter averaging 6.26 ± 0.40 log10 genome equivalents per microgram of brain RNA. However, the hypothalamus was not directly examined microscopically in these animals.
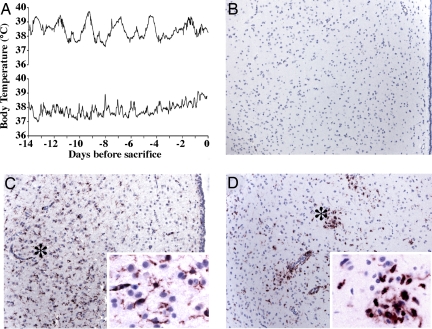
To assess the hypothalamus in the setting of this circadian anomaly, the telemetric body temperature recordings were followed in an additional group of SIV-infected animals, and two were killed when the characteristic circadian abnormalities developed (Fig. 5A). The histopathological and viral findings in the brain were similar to those described above. Importantly, the hypothalamus was also affected. Most prominently, immunohistochemical staining for CD163, a macrophage marker that also uniquely identifies activated microglia in the setting of SIV and HIV (23), revealed increased numbers of macrophages and activation of microglia in several regions of the hypothalamus, including the subparaventricular zone (SPZ) (Fig. 5).
Fig. 5.
Hypothalamic histopathology in the setting of altered circadian body temperature rhythm. (A) Body temperature data in rhesus monkey 521 (upper trace) and 523 (lower trace) were monitored until a disruption in circadian parameters was observed, at which time, animals were killed. (B–D) Histopathology of the hypothalamus, illustrated in the SPZ. The third ventricle is shown at right, with the ependymal lining visible in B and C. Photomicrographs [original magnification: ×10; ×60 (C Inset and D Inset)] of CD163 immunohistochemistry in control uninfected animal 556 (B) and SIV-infected animals 521 (C) and 523 (D). CD163-reactive ramified microglia (notable in C, region with * shown in C Inset) and macrophages (notable in D, region with * shown in D Inset) are not found in control animals (e.g., B).
Discussion
The principal finding of this study is that SIV-infected monkeys that developed CNS disease, manifested as encephalitis, displayed progressive pathological alterations in the circadian rhythms of body temperature and gross locomotor activity. These alterations were manifested as impairments in three variables: amplitude, MESOR, and acrophase, the disruption of which are indicators of defects in the regulation of the circadian rhythm system (22). These impairments developed before clinical symptoms were observed and became more severe as the disease progressed. Although an acute febrile response develops early after inoculation, the subsequent changes in temperature regulation are not representative of a febrile response but, instead, are due to an impairment in circadian control systems resulting from the viral infection. Other studies of humans and experimental animals report that the febrile response induced by parasitic and bacterial diseases do not alter the circadian rhythmicity of body temperature, supporting our hypothesis that the control of the circadian rhythm is affected by SIV infection (24–26).
During the acute phase of disease, the body temperature increased during both the day and night, which we interpret as the febrile response to infection. However, we observed that after recovery from this phase and the subsequent normalization of temperature, both the temperature and the circadian rhythm were altered over the progression of infection. Interestingly, the temperature changes were first manifest during the progressing stage 1 at nighttime (the dark phase), when body temperature is normally at its lowest, and to a much greater degree as disease progressed. These alterations were evidenced by significant changes in the measured circadian parameters, first, in the amplitude and, later, in the MESOR and acrophase.
Changes in locomotor activity were also present. Although only a slight disruption was found during the acute phase (a lowering of the already low level of nighttime movement), the daytime locomotion began dropping during the steady-state phase, and continued to decline as the disease progressed (and was accompanied by a decline in nighttime movement). These results confirm our previous observations about the effects of the SIV infection on locomotor activity (27, 28). The circadian analysis here revealed alterations in the amplitude and MESOR of the activity rhythm during disease progression. In contrast, the acrophase, which underwent notable alteration at the final time point measured for temperature, did not differ for activity. These results clearly demonstrate that the homeostatic regulation of activity and temperature are differentially altered in the SIV-infected monkeys of this study. Because the basal ganglia are a frequent target for HIV/SIV-induced damage, alterations in the basal ganglia of animals infected with SIV cannot be ruled out as a factor for hypolocomotion.
Thus there are two effects of SIV infection: the initial fever, which resolves, followed by alterations in both temperature and activity level, with distinctive circadian changes. Because the SIV does not infect neurons, alterations of the SCN-associated pathways controlling the circadian rhythms of infected monkeys are indirect and may be caused by the release of several factors during the infection. Cytokines, which are induced in the CNS in SIV and HIV encephalitis, may be particularly important because there is evidence that proinflammatory cytokines can induce disruptions in the synaptic transmission in the SCN, as well as reduce the mRNA levels of the clock gene Period in the SCN (29, 30). On the other hand, viral proteins can also have effects. Tat has been found to reset the circadian rhythm of spontaneous action potentials recorded in the SCN and to affect the circadian rhythm of the wheel-running activity in mice (31). The surface envelope protein gp120 causes a significant reduction in glucose utilization in the SCN (32).
Although viral proteins may have an effect, host molecules are prime candidates in circadian disruptions, as well as the cognitive and motor disorders in SIV/HIV infection. Indeed, the steady state may actually be an active state in the brain. In a chronic setting, we observed behavioral (cognitive and motor) abnormalities and increased levels of several IFN-induced genes in the brains of SIV-infected monkeys (33). In addition, host lymphoid cells (predominantly virus specific) infiltrate and accumulate in the infected brain (34, 35). Interestingly, peripherally administered IFN does result in IFN-induced gene expression in the hypothalamus and alters circadian parameters (30). In the chronic/steady-state phase, because of the active host/pathogen interaction, both CNS and peripherally produced immune mediators may affect the hypothalamus. Such relatively low-level but consistent production of these mediators may lead to the decreased activity, as well as the behavioral abnormalities found in our and other studies of otherwise asymptomatic infected animals (33, 36, 37). However, in the setting of advanced disease, there is a proinflammatory transcriptional profile in the brain (38), and a direct effect of such molecules produced locally is likely, resulting in the progressive anomalies in temperature regulation. Intriguingly, physiological activation of the supraoptic nucleus of the hypothalamus leads to distinct changes in local microglia (39). We hypothesize that the converse, the activation of microglia and subsequent production of immune mediators, alters the physiology of hypothalamic neurons and leads to the circadian changes we report in advanced stages of disease.
Like the SIV-infected monkeys of this study, HIV-infected individuals experience disruptions in the circadian rhythmicity of several functions, including body temperature, activity, sleep patterns, and hormone secretion (5, 7–9). In addition, it has been reported that HIV patients who develop AIDS have a reduced number of vasopressin- and oxytocin-expressing neurons in the SCN and paraventricular nucleus, respectively (40, 41), indicating that hypothalamic neurons are compromised during the progression of the HIV disease. Circadian impairment can lead to neurobehavioral symptoms such as those found in HIV individuals, including motor and cognitive disorders and fatigue, and their recognition and possible treatment may be helpful in this now-chronic disease.
Such changes are in no way unique to HIV/SIV-induced disease. Alterations in the circadian rhythm of body temperature, activity, and sleep have been reported in AD patients (17, 18), suggesting impairments in the SCN in this neurodegenerative disorder (14, 15). The possible influence of circadian rhythm on cognitive functions has been also observed in AD patients whose behavioral symptoms of dementia are exacerbated in the afternoon and evening, a phenomenon known as “sundowning” (42). Intriguingly, such AD patients have increased MESOR values, decreased amplitude, and delayed acrophase for the circadian rhythm of body temperature compared with healthy individuals (17). Correspondingly, mouse models of AD show impairments in the MESOR and amplitude of body temperature and sleep, together with cognitive deficits (43). Although additional studies are needed to uncover the basis of the circadian changes observed in the monkeys of this study, these reports, together with our findings, suggest that the functionality of hypothalamic circadian control is compromised in SIV-infected animals during the progression to CNS disease.
Another interesting observation is that the acrophase of body temperature and gross activity in the terminal phase was not affected in the same way, suggesting that in rhesus macaques, the pacemaker of the circadian timing system for body temperature and locomotor activity differ in susceptibility to the effects of SIV infection. Studies carried out in squirrel monkeys suggest the existence of two separate self-sustained oscillators (44, 45). In these studies, SCN lesions disrupted the rhythm of behavioral activity without affecting the body temperature rhythm, whereas manipulation of the light/dark cycle resulted in desynchronization between the acrophases of the body temperature and locomotor activity. Likewise, studies in rats have shown that the ventrolateral and dorsomedial regions of the SCN independently control the circadian rhythm of activity and body temperature, respectively (46).
The existence of distinct anatomical substrates for the circadian timing system for body temperature and activity is also supported by studies in rats indicating that specific regions in the SPZ, the major target of SCN neurons within the hypothalamus, regulate different circadian rhythms. Thus, the dorsal SPZ regulates the circadian rhythm of body temperature, whereas the ventral SPZ regulates the circadian rhythm of locomotor activity, sleep, and feeding (11). The microglia activation and macrophage accumulation that we found in the SPZ was likely associated with alterations in SPZ function. Both the SCN and the SPZ send projections to the brain structure related with the homeostatic regulation of body temperature, the preoptic area (POA) (11, 47–49). However, because lesions of the POA alter the amplitude of the circadian rhythm of body temperature (50), there could be a bidirectional interaction between the homeostatic and circadian system. In addition, the SIVE neuropathology was not limited to the SPZ. Thus, impairments in SCN, POA, or other hypothalamic control regions cannot be ruled out as an additional factor for circadian dysregulation. Future studies, including microinjection of proinflammatory cytokines and viral products, are needed to determine the role of distinct hypothalamic regions on the circadian alterations caused by the infection with SIV. Although the ventral SPZ regulates sleep, as well as locomotor activity, unfortunately, our studies did not allow a formal examination of alterations in sleep patterns, which have been reported to be disrupted in HIV-infected patients (51).
In conclusion, our results indicate that the circadian rhythm of body temperature and gross locomotor activity are progressively disrupted in SIV-infected monkeys that progress to CNS disease and that the alterations in the amplitude, MESOR, and acrophase are not related to the acute fever response induced by the SIV infection. Although the precise mechanisms associated with these alterations are unknown, we propose that SIV infection of monkeys impairs the control of circadian rhythms, leading to disturbances in neurobehavioral functions, which has important implications not only for pathogenesis but also for treatment strategies of HIV-infected individuals.
Methods
SIV Infection.
Studies were performed with the approval and guidelines of the Institutional Animal Care and Use Committee of The Scripps Research Institute. Rhesus monkeys were kept under a light/dark cycle of 12:12 h (6:00 a.m. to 6:00 p.m.) and a room temperature between 24 and 26°C. For SIV infection, animals were intravenously inoculated with a cell-free stock of SIV, derived from SIVmac251 (52, 53). All infected animals developed rapidly progressive simian AIDS necessitating euthanasia within 6 months after viral inoculation, and neuropathological examination revealed SIVE. For additional animal details, euthanasia criteria, and postmortem analysis methods, see supporting information (SI) Methods.
Telemetry Recordings and Cosinor Analysis.
Body temperature and gross locomotor activity were measured by means of radiotelemetry implants. Signals from individually implanted telemetry transmitters (Physiotel TA10TA-D70; Data Sciences International, St. Paul, MN) were received by individual receivers (model RMC-1, Data Sciences International), which were connected to the data acquisition matrix, and the data were processed by using Dataquest ART 4.0 software (Data Sciences International). Temperature and activity were continuously monitored before and after infection. However, to minimize confounders potentially affecting measured parameters, the values used for this study correspond to the recording periods of 48 h obtained during weekends, when monkeys remained undisturbed except for feeding and drinking water replacement. Examination of the data revealed that these periods were indeed representative of the data preceding and after the weekends. Thus, two consecutive 24-h periods for each monkey, through different stages of the disease, were averaged. For additional radiotelemetry details, see SI Methods.
The analysis of the effects of SIV on the homeostatic regulation of body temperature and activity was performed after averaging their values during the stages, as described above, whereas to determine circadian variations, the temperature and activity data were analyzed by using the Time Series Analysis Serial Cosinor 6.3 software (Expert Soft Technologie, Richelieu, France), and the fitted cosinor curves were calculated with a 24-h rhythm. The cosinor curve is represented by the following function: C(t) = MESOR + amplitude cos[2π(t-acrophase)/24 h], where MESOR (mean of the oscillation), amplitude (half the difference between the highest and the lowest values), and acrophase (timing of the cosine maximum given in degrees, where 360° corresponded to a 24-h cycle). After the single cosinor analyses, the group mean cosinor values were obtained.
Statistical Analysis.
Statistical comparisons by using an ANOVA for repeated measures, with the Dunnett's multiple comparison test done to compare cosinor results from the preinfection stage against the different stages of the disease, as established in this study. StatGraphics Centurion 15.2 (StatPoint, Inc., Herndon, VA), Prism 4.1 (GraphPad, San Diego, CA), and StatView (SAS Institute, Cary, NC) software were used for the analysis.
Supplementary Material
Acknowledgments
We thank the members of the laboratory of H.S.F. and Dr. Bruno Conti for comments on the manuscript and Debbie Watry and Michelle Zandonatti for work on the monkey blood and tissue samples. This work was supported by National Institutes of Health Grants MH062261, NS045534, and MH073490 (to H.S.F.). This is manuscript no. 18817 from The Scripps Research Institute.
Abbreviations
- AD
Alzheimer's disease
- MESOR
midline estimating statistic of rhythm
- POA
preoptic area
- SCN
suprachiasmatic nucleus
- SIV
simian immunodeficiency virus
- SIVE
SIV encephalitis
- SPZ
subparaventricular zone.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707171104/DC1.
References
- 1.Brew BJ. AIDS. 2004;18(Suppl 1):S75–S78. [PubMed] [Google Scholar]
- 2.Gonzalez-Scarano F, Martin-Garcia J. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 3.Jones G, Power C. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 5.Bourin P, Mansour I, Doinel C, Roue R, Rouger P, Levi F. Chronobiol Int. 1993;10:298–305. doi: 10.1080/07420529309059712. [DOI] [PubMed] [Google Scholar]
- 6.Moulignier A, Guiard-Schmid JB, Gbadoe AH, Rozenbaum W. Neurology. 2003;61:418–419. doi: 10.1212/01.wnl.0000073539.21469.b7. [DOI] [PubMed] [Google Scholar]
- 7.Rondanelli M, Solerte SB, Fioravanti M, Scevola D, Locatelli M, Minoli L, Ferrari E. AIDS Res Hum Retroviruses. 1997;13:1243–1249. doi: 10.1089/aid.1997.13.1243. [DOI] [PubMed] [Google Scholar]
- 8.Swoyer J, Rhame F, Hrushesky W, Sackett-Lundeen L, Sothern R, Gale H, Haus E. Prog Clin Biol Res. 1990;341A:437–449. [PubMed] [Google Scholar]
- 9.White JL, Darko DF, Brown SJ, Miller JC, Hayduk R, Kelly T, Mitler MM. AIDS. 1995;9:1043–1050. doi: 10.1097/00002030-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Darko DF, Miller JC, Gallen C, White J, Koziol J, Brown SJ, Hayduk R, Atkinson JH, Assmus J, Munnell DT, et al. Proc Natl Acad Sci USA. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saper CB, Scammell TE, Lu J. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz WJ, Busis NA, Hedley-Whyte ET. J Neurol. 1986;233:1–4. doi: 10.1007/BF00313981. [DOI] [PubMed] [Google Scholar]
- 14.Swaab DF, Fliers E, Partiman TS. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Okamoto N, Ohara K, Morita Y. Brain Res. 1996;717:154–159. doi: 10.1016/0006-8993(96)00086-8. [DOI] [PubMed] [Google Scholar]
- 16.Skene DJ, Swaab DF. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 17.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Am J Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- 18.Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, Gori S, Iudice A, Murri L. J Sleep Res. 2005;14:311–317. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 19.Horn TFW, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Proc Natl Acad Sci USA. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts ES, Burudi EM, Flynn C, Madden LJ, Roinick KL, Watry DD, Zandonatti MA, Taffe MA, Fox HS. J Neuroimmunol. 2004;157:81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson W, Tong YL, Lee JK, Halberg F. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 23.Roberts ES, Masliah E, Fox HS. J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- 24.Lell B, Brandts CH, Graninger W, Kremsner PG. Wien Klin Wochenschr. 2000;112:1014–1015. [PubMed] [Google Scholar]
- 25.Mphahlele NR, Fuller A, Roth J, Kamerman PR. Am J Physiol Regul Integr Comp Physiol. 2004;287:R863–R869. doi: 10.1152/ajpregu.00064.2004. [DOI] [PubMed] [Google Scholar]
- 26.Musher DM, Fainstein V, Young EJ, Pruett TL. Arch Intern Med. 1979;139:1225–1228. doi: 10.1001/archinte.139.11.1225. [DOI] [PubMed] [Google Scholar]
- 27.Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Proc Natl Acad Sci USA. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundkvist GB, Hill RH, Kristensson K. Neurobiol Dis. 2002;11:20–27. doi: 10.1006/nbdi.2002.0536. [DOI] [PubMed] [Google Scholar]
- 30.Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Nat Med. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- 31.Clark JP, III, Sampair CS, Kofuji P, Nath A, Ding JM. Am J Physiol Regul Integr Comp Physiol. 2005;289:R656–R662. doi: 10.1152/ajpregu.00179.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kimes AS, London ED, Szabo G, Raymon L, Tabakoff B. Exp Neurol. 1991;112:224–228. doi: 10.1016/0014-4886(91)90073-l. [DOI] [PubMed] [Google Scholar]
- 33.Roberts ES, Huitron-Resendiz S, Taffe MA, Marcondes MC, Flynn CT, Lanigan CM, Hammond JA, Head SR, Henriksen SJ, Fox HS. J Neurosci. 2006;26:4577–4585. doi: 10.1523/JNEUROSCI.4504-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcondes MC, Phillipson CA, Fox HS. AIDS. 2003;17:1605–1611. doi: 10.1097/00002030-200307250-00004. [DOI] [PubMed] [Google Scholar]
- 35.Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, Flynn C, Zandonatti M, Fox HS. J Immunol. 2007;178:5812–5819. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- 36.Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- 37.Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. AIDS Res Hum Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- 38.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Am J Pathol. 2003;162:2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayoub AE, Salm AK. J Neurosci. 2003;23:7759–7766. doi: 10.1523/JNEUROSCI.23-21-07759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swaab DF, Hofman MA. Brain Res. 1990;537:141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- 41.Purba JS, Hofman MA, Portegies P, Troost D, Swaab DF. Brain. 1993;116(Pt 4):795–809. doi: 10.1093/brain/116.4.795. [DOI] [PubMed] [Google Scholar]
- 42.Little JT, Satlin A, Sunderland T, Volicer L. J Geriatr Psychiatry Neurol. 1995;8:103–106. doi: 10.1177/089198879500800205. [DOI] [PubMed] [Google Scholar]
- 43.Huitron-Resendiz S, Sanchez-Alavez M, Gallegos R, Berg G, Crawford E, Giacchino JL, Games D, Henriksen SJ, Criado JR. Brain Res. 2002;928:126–137. doi: 10.1016/s0006-8993(01)03373-x. [DOI] [PubMed] [Google Scholar]
- 44.Gander PH, Lydic R, Albers HE, Moore-Ede MC. Am J Physiol. 1985;248:R567–R572. doi: 10.1152/ajpregu.1985.248.5.R567. [DOI] [PubMed] [Google Scholar]
- 45.Fuller CA, Lydic R, Sulzman FM, Albers HE, Tepper B, Moore-Ede MC. Am J Physiol. 1981;241:R385–R391. doi: 10.1152/ajpregu.1981.241.5.R385. [DOI] [PubMed] [Google Scholar]
- 46.Cambras T, Weller JR, Angles-Pujoras M, Lee ML, Christopher A, Diez-Noguera A, Krueger JM, de la Iglesia HO. Proc Natl Acad Sci USA. 2007;104:7634–7639. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watts AG, Swanson LW, Sanchez-Watts G. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 48.Boulant JA. Clin Infect Dis. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 49.Saper CB, Lu J, Chou TC, Gooley J. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Osborne AR, Refinetti R. NeuroReport. 1995;6:2187–2192. doi: 10.1097/00001756-199511000-00021. [DOI] [PubMed] [Google Scholar]
- 51.White JL, Darko DF, Brown SJ, Miller JC, Hayduk R, Kelly T, Mitler MM. AIDS. 1995;9:1043–1050. doi: 10.1097/00002030-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS. AIDS. 2005;19:1704–1706. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- 53.Lane TE, Buchmeier MJ, Watry DD, Jakubowski DB, Fox HS. Virology. 1995;212:458–465. doi: 10.1006/viro.1995.1503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.