Abstract
Transgenic plants with reduced poly(ADP-ribose) polymerase (PARP) levels have broad-spectrum stress-resistant phenotypes. Both Arabidopsis thaliana and oilseed rape (Brassica napus) lines overexpressing RNA interference-PARP constructs were more resistant to various abiotic stress treatments in laboratory and greenhouse experiments without negative effects on growth, development, and seed production. This outperforming stress tolerance was initially attributed solely to a maintained energy homeostasis due to reduced NAD+ consumption. We show that in PARP2-deficient Arabidopsis plants, the observed abiotic stress resistance can also be explained by alterations in abscisic acid levels that facilitate the induction of a wide set of defense-related genes.
Keywords: gene expression, protection, transcriptome analysis
Abiotic stresses, such as drought, salinity, and heat, are the major causes of yield loss in cultivated crops. These losses result from a combination of different stresses during the growing season as well as from periodically occurring extreme weather conditions (1). The gap between the attainable and the actual yields in modern agriculture is estimated to be 40–50% (www.isaaa.org). Therefore, breeding crop varieties with a sustainably improved performance under suboptimal growing conditions is one of the ambitious, but crucial, objectives in modern agriculture. Varieties with enhanced tolerance to abiotic stresses will broaden the window of optimal growth conditions for cultivated crops, thereby increasing yield stability, average yield, and productive acreage. In the past years, the expression levels of individual plant genes were increased in Arabidopsis thaliana as a convenient testing procedure to assess the potential of these genes to improve yield stability in crops (2–4). These efforts have led to the identification of genes with a direct protective mode of action (e.g., antioxidants and osmoprotectants) and transcription factors with significantly improved stress tolerance that could be realistic targets for further evaluation in various crops. Overexpression of the stress-responsive transcription factor SNAC1 has recently been shown to significantly enhance drought resistance in transgenic rice (Oryza sativa) (5).
Currently, modulation of cellular energy homeostasis is an attractive alternative to improve plant performance and yield stability during environmental stress conditions. In the mitochondrial CMSII mutant of Nicotiana sylvestris, increased pools of NAD and NADH are concomitant with increased resistance to ozone and tobacco mosaic virus (6, 7). The biotic and abiotic stress-resistance phenotypes of transgenic rice overexpressing an NADPH-dependent Helminthosporium carbonum (HC) toxin reductase-like gene has also been assigned to elevated pools of NAD(P)H (8). Previously, in poly(ADP-ribose) polymerase (PARP)-deficient transgenic Arabidopsis and Brassica napus (oilseed rape) plants, reduced NAD+ depletion and ATP consumption have been reported to increase tolerance to a broad range of abiotic stresses, such as high light, drought, and heat (9).
Poly(ADP-ribosyl)ation (PAR) is a unique posttranslational protein modification mediated by the PARP enzyme that tags long-branched poly(ADP-ribose) polymers to nuclear target proteins (10, 11). PAR plays an important role within the cellular response to genotoxic stress and modulates DNA synthesis and repair, chromatin structure, transcription, and cell cycle activities (11, 12). The extent of PAR is directly proportional to the severity of the stress and determines the type of cellular response, ranging from cellular defense under mild stress to DNA repair under moderate stress and to cell death under severe stress (13). PARP uses NAD+ as a substrate and, therefore, poly(ADP-ribose) polymer formation requires a constant supply and resynthesis of NAD+. The latter can occur through the NAD+ salvage and de novo synthesis pathway, both consuming ATP (14). Hence, PAR is an energy-consuming process and, in mammals, one of the main causes of energy depletion leading to cell death (15).
In plants, PARP genes are stress-inducible and structurally and functionally homologous to their mammalian counterparts (16–19). The increased broad-spectrum abiotic stress tolerance in transgenic PARP-deficient plants has initially been attributed to a high energy-use efficiency under stress conditions. A reduced ATP consumption avoids extensive mitochondrial respiration and prevents the formation of deleterious reactive oxygen species (ROS) (9, 20). Here, we present another interpretation for stress tolerance that depends on PARP deficiency. Genome-wide transcript analysis of stressed PARP2-deficient transgenic Arabidopsis (hpAtPARP2) revealed the induction of specific ABA signaling pathways that might be steered through increased levels of the cyclic nucleotide cyclic ADP-ribose (cADPR).
Results
Genome-Wide Transcriptome Analysis of Stress-Tolerant hpAtPARP2 Plants.
Transgenic Arabidopsis plants, transformed with a double-stranded RNA construct containing the 5′ end of the Arabidopsis PARP2 gene in the stem structure (hpAtPARP2), were shown to have reduced PAR activity that correlates with a higher energy-use efficiency and abiotic stress tolerance (9). This enhanced energy-use efficiency could be rapidly assessed with an in vitro stress assay that allows us to monitor vigor. In this assay, total NAD(P)H and ATP contents and the capacity of plants to reduce 2,3,5-triphenyltetrazolium chloride after a few hours of high-light (HL) stress are quantified (9, 21). To obtain a better understanding of the molecular mechanisms driving the stress tolerance in hpAtPARP2 plants, we followed the gene expression dynamics in both wild-type and transgenic plants during this HL stress-dependent assay.
Two-week-old in vitro grown wild-type and hpAtPARP2 Arabidopsis plants were stressed by increasing the light intensity from 50 to 300 μmol·m−2·s−1 for 0, 2, 4, and 6 h. RNA from two biological repeats was hybridized to 16 ATH1 Genechips (Affymetrix, Santa Clara, CA). After data processing, a two-factor ANOVA was performed to assess the significance of the three major sources of variability affecting mRNA levels: the HL stress time course, the genotype (wild-type versus transgenic), and the combined effect of HL stress and genotype. Multiple comparisons were corrected by controlling the false discovery rate (22).
Differential expression of 3,882 transcripts was steered exclusively by HL stress, whereas 593 transcripts responded differentially through a combined effect of reduced PAR activity and HL stress and 293 transcripts were differentially expressed between wild-type and hpAtPARP2, independently of the HL stress [supporting information (SI) Fig. 4]. Because HL-induced transcriptomic changes are already extensively documented (23–25), we will focus on the differences between wild-type and hpAtPARP2 plants before and during HL stress.
Independently from HL stress, the steady-state levels of 106 transcripts were increased in the hpAtPARP2 plants of which almost one-third encode proteins with unknown function (SI Table 2). On the other hand, the steady-state levels of 187 transcripts were decreased in hpAtPARP2, independently of HL stress (SI Table 3). Remarkably, ≈30% of these down-regulated genes were involved in protein metabolism (e.g., ribosomal proteins and translation initiation factors). Furthermore, genes involved in cellular transport (e.g., ABC transporters and mitochondrial inner membrane translocases), electron transport, and DNA metabolism processes, such as chromatin remodeling and DNA repair, were repressed in the hpAtPARP2 plants.
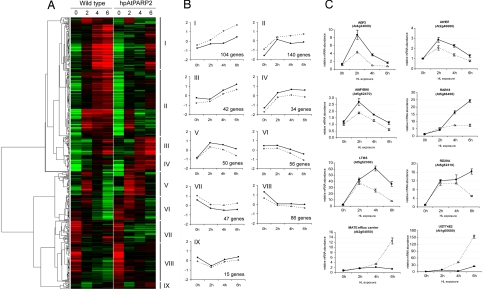
For 593 transcripts, the expression was significantly affected by a combination of HL and PARP deficiency. Hierarchical average linkage clustering identified nine clusters with distinct expression characteristics (Fig. 1 A and B). Clusters I and II group transcripts induced by HL stress in wild-type plants but whose induction is seriously delayed or impaired in the hpAtPARP2 plants. In clusters III and IV, genes have a more pronounced HL induction in the hpAtPARP2 plants. Clusters V and VI represent transcripts with a transient HL induction (peaking at 2 h) and HL-induced repression, respectively. For a complete overview of the individual genes present within clusters I to VI, grouped according to their functional category, see SI Tables 4–9. In contrast to clusters I–VI, no apparent overrepresentation of specific functional categories was found in clusters VII, VIII, and IX. Reproducibility of the microarray results was validated by quantitative RT-PCR of eight individual genes (Fig. 1C).
Fig. 1.
Hierarchical average linkage clustering of 593 differentially expressed genes by the combined effect of reduced PAR activity and HL stress. (A) Temporal expression patterns in wild-type and hpAtPARP2 plants during 0, 2, 4, and 6 h of HL stress. Red and green correspond to up- and down-regulation, respectively. Nine clusters of transcriptional changes (I–IX) are indicated. (B) Trend line graphs and number of genes for each cluster, summarizing the average transcript profiles in wild-type (dashed line) and hpAtPARP2 (solid line) plants during HL stress. (C) Verification of microarray results with quantitative RT-PCR. For wild-type (○) and hpAtPARP2 (●) plants; each line connects the four time points during the HL treatment at which the samples were collected. Relative transcript levels were normalized to the actin-related protein 7 and represent the mean ± SE of at least three quantitative PCR repeats.
Oxidative Stress-Dependent Gene Expression Is Attenuated in HL-Stressed hpAtPARP2 Plants.
The HL stress induction of ≈250 genes was impaired or delayed in hpAtPARP2 plants (Fig. 1 A and B; clusters I and II). Assessing overrepresented gene ontology terms within these two clusters with the Biological Networks Gene Ontology tool (BiNGO) (26), revealed that most genes were associated with stress responses (SI Tables 10 and 11). The most significant gene ontology term is the “temperature stimulus response.” Twenty-two transcripts encoding HSP70, HSP80, DNAJ proteins, small HSPs, and the heat shock factor AtHsf2A were strongly and rapidly induced by HL in the wild-type plants but did not change in the hpAtPARP2 plants (SI Table 5). Other overrepresented functional categories were “metabolism,” “protein metabolism,” “generation of precursor metabolites and energy,” and “transport” (SI Tables 10 and 11). Furthermore, we noticed that approximately one-third of the genes in clusters I and II were at least threefold induced by H2O2 in catalase-deficient Arabidopsis (25). Therefore, we also assessed the expression characteristics of the genes in clusters I and II in other publicly available microarray data sets with Genevestigator (27). This analysis revealed that most genes in both clusters were also induced by oxidative stress-causing treatments, such as H2O2 spray, syringolin treatment, and ozone fumigation (SI Tables 12 and 13). The expression characteristics of the 20 most deregulated genes in clusters I and II during a HL treatment in catalase-deficient plants and during different oxidative stress treatments are presented in Table 1.
Table 1.
Expression characteristics of genes in cluster I and II during different oxidative stress experiments
| Affymetrix probe set name | AGI code | Description | hpAtPARP2 + 6 h HL | CAT2HP1 + 3-h HL | CAT2HP1 + 8-h HL | H2O2 spray | Ozone fumigation | Syringolin treatment | Cluster |
|---|---|---|---|---|---|---|---|---|---|
| 248486_at | At5g51060 | NADPH respiratory burst oxidase protein C (RbohC) | −24.64 | A | A | −2.75 | 2.56 | 1.13 | I |
| 263210_at | At1g10585 | bHLH transcription factor | −17.49 | 85.12 | 16.03 | 3.42 | 102.65 | −2.15 | I |
| 262518_at | At1g17170 | Glutathione S-transferase AtGSTU24 | −16.20 | 78.53 | 107.72 | 31.63 | 6.09 | 22.35 | I |
| 262911_s_at | At1g59860 | 17.6-kDa class I heat shock protein (HSP17.6A-CI) | −14.58 | 8.76 | 118.84 | 59.00 | 28.70 | 114.67 | II |
| 253316_s_at | At4g34300 | Glycine-rich protein | −12.47 | A | A | 1.29 | 1.33 | 25.62 | I |
| 260248_at | At1g74310 | Heat shock protein 101 (HSP101) | −12.07 | 22.17 | 27.07 | 127.79 | 23.03 | 15.35 | II |
| 263403_at | At2g04040 | MATE efflux family protein | −11.45 | 2.30 | 9.57 | 2.55 | 9.29 | 5.57 | I |
| 260522_x_at | At2g41730 | Expressed protein | −11.39 | 27.26 | 32.93 | 2.50 | 10.66 | 25.24 | I |
| 263823_s_at | At2g40350 | DREB2-like transcription factor | −10.58 | 4.03 | 6.55 | 10.23 | 8.25 | 22.92 | I |
| 248434_at | At5g51440 | 23.5-kDa mitochondrial small heat shock protein (HSP23.5-M) | −9.70 | 12.83 | 53.64 | 20.30 | 9.29 | 117.44 | II |
| 250296_at | At5g12020 | 17.6-kDa class II heat shock protein (HSP17.6-CII) | −9.58 | 7.49 | 65.26 | 104.97 | 5.48 | 4.31 | II |
| 266752_at | At2g47000 | ATP-binding cassette (ABC) transport protein | −8.86 | 5.19 | 14.17 | 1.33 | 3.07 | 72.21 | I |
| 266841_at | At2g26150 | Heat shock transcription factor AtHsfA2 | −7.66 | 27.64 | 112.82 | 55.23 | 43.64 | 14.58 | II |
| 245392_at | At4g15680 | Glutaredoxin family protein | −6.63 | 1.34 | 3.14 | −1.43 | −1.23 | 1.05 | I |
| 259037_at | At3g09350 | Armadillo/β-catenin repeat family protein | −5.95 | 15.21 | 18.46 | 6.87 | 6.92 | 16.71 | II |
| 253046_at | At4g37370 | Cytochrome P450, putative | −5.75 | 31.20 | 27.06 | 28.21 | 25.62 | 15.67 | II |
| 259445_at | At1g02400 | Gibberellin 2-oxidase, putative | −5.50 | 5.95 | 4.94 | 6.25 | 2.28 | −2.72 | II |
| 263231_at | At1g05680 | UDP-glucoronosyl/UDP-glucosyl transferase | −5.38 | 338.78 | 195.80 | 8.64 | 13.27 | 7.80 | I |
| 263402_at | At2g04050 | MATE efflux family protein | −5.21 | 2.76 | 5.42 | 1.76 | 29.41 | 14.71 | I |
| 259297_at | At3g05360 | Disease resistance family protein/LRR family protein | −4.92 | A | A | 12.31 | 8.34 | 20.83 | I |
Relative expression data (transgenic to wild-type or treatment to control) for the 20 most deregulated genes of clusters I and II are presented. The induction/suppression of each gene by HL treatment in the hpAtPARP2 plants or catalase-deficient plants (CAT2HP1) or by the different oxidative stress-causing treatments (H2O2 spray, ozone fumigation, or syringolin treatment) is indicated. Expression data of transcripts with calls absent in both control and transgenic plants are indicated with A. Data were partly obtained with Genevestigator (27).
In addition to genes with a delayed or impaired HL induction, many genes were superinduced by HL in the hpAtPARP2 plants compared with wild-type plants. Different kinetics of HL induction could be distinguished in clusters III, IV, and V (Fig. 1 A and B). Within cluster III, transcripts involved in starch metabolism were significantly overrepresented and encoded two and eight starch biosynthesis and starch-degrading enzymes, respectively (SI Table 6). These transcripts, together with genes involved in flavonoid biosynthesis, have been shown previously to be negatively regulated by H2O2 during HL stress (25). Accordingly, in HL-stressed hpAtPARP2, two flavonoid biosynthetic genes (dihydroflavonol reductase and leucoanthocyanidin dioxygenase), and one regulatory gene (MYB transcription factor PAP1) were also superinduced (SI Table 7), indicating decreased H2O2 accumulation or perception in hpAtPARP2.
ABA-Related Gene Expression Is Up-Regulated in hpAtPARP2 Plants.
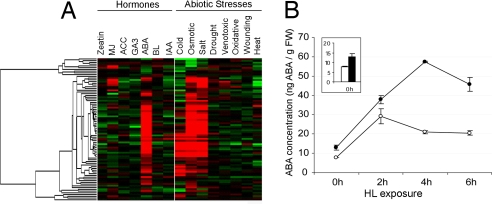
Within the set of superinduced genes in hpAtPARP2 plants, genes that have been reported to be responsive to ABA, dehydration, and cold were clearly overrepresented. Among these, we recognized transcriptional regulators, such as ABA-responsive element-binding factor 3 (ABF3), homeobox transcription factor 7 (AtHB7), and AtMYB96 that have been shown to be involved in stress-responsive ABA signaling (28–30). These transcription factors were grouped in cluster V, whose transcript levels rapidly increased and peaked after 2 h of HL stress (Fig. 1 B and C and SI Table 8). Such expression characteristics suggest that they steer the subsequent induction of ABA/stress-responsive genes, such as RAB18, LTI65, ERD4, ERD7, ERD10, KIN1, RD29A, Cor15a, and ABI2 [Fig. 1 B and C (cluster IV) and SI Table 7]. These transcripts gradually increased during the HL stress, with higher levels in the hpAtPARP2 plants. With Genevestigator, the expression characteristics of all transcripts in clusters IV and V were assessed during various abiotic stresses and hormonal treatments (27). Most genes were also induced by cold, salt, and osmotic stress treatment. Strikingly, almost all genes were responsive to ABA (Fig. 2A). Therefore, we measured the endogenous ABA content in HL-stressed (0, 2, 4, and 6 h) wild-type and hpAtPARP2 plants. In both genotypes, ABA levels increased approximately three- to fourfold within 2 h of HL exposure. However, in hpAtPARP2 plants, the accumulation of ABA levels was higher, ranging from a minor, but significant, increase prior the HL stress to a threefold increase after 4 h of HL (Fig. 2B).
Fig. 2.
ABA-dependent signaling in hpAtPARP2 plants. (A) Expression analysis of cluster IV and V transcripts in response to various environmental stimuli. Hierarchical average linkage clustering of the response profiles of 84 transcripts to various abiotic stress conditions and treatment with plant hormones is presented. Data were obtained with Genevestigator (27). MJ, methyl jasmonate; ACC, 1-aminocyclopropane-1-carboxylic acid; GA3, gibberellic acid 3; ABA, abscisic acid; BL, brassinolide; IAA, indole-3-acetic acid. (B) Quantification of the endogenous ABA content in wild-type (○) and hpAtPARP2 (●) plants exposed to 0, 2, 4, and 6 h of HL. Initial levels of ABA in wild-type (white bar) and hpAtPARP2 (black bar) plants are enlarged. Results are expressed as ng ABA per g of fresh weight (FW) and represent the mean ± SE of two repeat experiments.
Furthermore, we assessed the expression characteristics of ABA-independent stress-responsive genes, such as ERD1, Cor47, RD19, and RD21 (31). None of these genes was significantly differentially expressed in wild-type and hpAtPARP2 plants.
Discussion
Oxidative Stress Response Decreases in hpAtPARP2 Plants.
When the transcriptomes of hpAtPARP2 and wild-type Arabidopsis plants were compared before and during an HL-dependent in vitro stress assay, we found that ≈900 transcripts were differentially expressed. The largest functional category of genes repressed in the hpAtPARP2 plants were those involved in protein metabolism processes, such as ribosome biogenesis and protein translation (SI Table 3). Active repression of protein synthesis is known to protect cells against protein misfolding during stress, which might indicate that hpAtPARP2 plants are less susceptible to stress than the wild-type plants. Accordingly, upon HL stress, wild-type plants induce many oxidative stress-related genes, whose expression was delayed or even completely abolished in the hpAtPARP2 plants (SI Tables 4 and 5). This impaired oxidative stress-dependent response, together with the previously reported decreased superoxide levels during stress (9) might be caused by a more efficient scavenging of ROS in the hpAtPARP2 plants. Alternatively, fewer ROS-dependent signals are produced or transduced in these transgenic plants. Noteworthy is the strongly abolished induction of the superoxide-producing NADPH respiratory burst oxidase homolog C (Table 1) that, besides being an important superoxide-producing enzyme, also functions as a positive amplification factor of ROS signal transduction and activation of defense responses (32). Furthermore, genes involved in cellular transport and energy metabolism were repressed in the hpAtPARP2 plants, reflecting the previously reported reduced energy consumption that allows a normal mitochondrial respiration and low ROS production (9) (SI Tables 2, 4, and 5).
HpAtPARP2 Plants Superinduce Three Classes of Genes Involved in Stress Protection.
As importantly, we noticed in the PARP-deficient plants, increased transcript levels of three classes of genes involved in stress protection: starch metabolism, flavonoid biosynthesis, and ABA-responsive genes. Enhanced starch metabolism can lead to increased soluble sugar levels that positively correlate with cold and freezing tolerance (33), whereas anthocyanins are well-known protectants against HL stress because of their ability to reduce oxidative damage by means of light attenuation (34). Fig. 2 clearly indicates elevated ABA levels and the overrepresentation of ABA-responsive genes in the hpAtPARP2 plants. Increased ABA levels can mitigate stress-induced damage through the activation of stress-responsive genes, which collectively increase plant stress tolerance (35–37). Overexpression of the ABA-responsive transcription factor ABF3 provides, in addition to drought tolerance, also tolerance to chilling, freezing, high temperature, and oxidative stress (10, 28). However, stress tolerance often comes at the expense of plant growth and productivity (38). In contrast, broad-spectrum abiotic stress resistance of hpAtPARP2 plants is not associated with any negative effects on growth, development, or seed production (9), possibly because of the moderate increase of ABA and associated ABA/dehydration/cold-responsive downstream gene expression. Besides being an important stress-related hormone, ABA is also required for normal plant growth and development. Very high levels of ABA can inhibit plant growth capacities (39, 40), explaining why unleashed induction of ABA-responsive genes due to overexpression of transcription factors involved in ABA signaling lead to the reported growth-related aberrations. The more moderate perturbation of ABA signal transduction in hpAtPARP2 plants seemingly keeps plant stress signaling and developmental cues in pace and leads to stress-tolerant plants that do not suffer from yield penalties. Field trials over the last 3 years (2004–2006) showed consistently that corn (Zea mays) and oilseed rape hpPARP lines have similar yields to those of the azygous control plants under nonstressed conditions (M.D.B. and M.M., unpublished results).
Along the same lines, enhanced abiotic stress tolerance often impairs pathogen resistance because of the interference of ABA with salicylic acid, jasmonate, and ethylene signaling during biotic stresses (41). However, hpAtPARP2 plants have no increased susceptibility toward several biotrophic and necrotrophic pathogens (M.D.B., unpublished results). These findings are corroborated by the above-mentioned field trials in which significant differences in yield (≈20–40%) were recorded in favor of both corn and oilseed rape hpPARP lines under drought conditions (M.D.B. and M.M., unpublished results).
Improved Abiotic Stress Resistance Through cADPR Signaling?
The mutual up-regulation of abiotic stress defense-related genes sheds another light on the previously reported stress-tolerant phenotype of PARP-deficient transgenic plants. Until now, the improved performance of PARP-deficient plants under stressful conditions was mainly explained by a more proficient energy-use efficiency during stress (9). Molecular phenotyping of the Arabidopsis hpAtPARP2 plants clearly shows that, in parallel or likely as a consequence of the preserved energy homeostasis, ABA levels increase and a large set of stress-protective genes is induced that could be responsible for the stress-resistant phenotypes. Because hpAtPARP2 plants have no higher mutation frequency upon ethyl methane sulfonate treatment, it is unlikely that they experience increased DNA damage during abiotic stresses that could lead to the release of stress signaling cues (9).
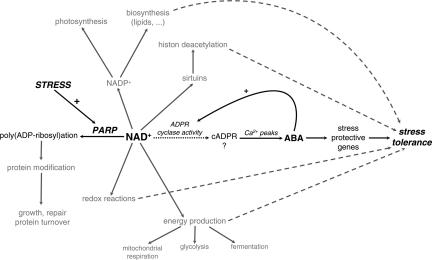
Because of the obvious up-regulation of ABA-responsive genes, an ABA-mediated stress tolerance is more likely. The most prominent question is then how increased NAD+ levels are connected to this ABA-dependent stress signaling. In addition to a key position within the energy metabolism, NAD can mediate signaling events as a precursor of the intracellular Ca2+-mobilizing molecule, cADPR (42). cADPR is synthesized from NAD+ by ADP-ribosyl cyclase that has been cloned and biochemically characterized in human and rat and in the snail Aplysia (19, 43). Although no genes with significant homologies to the animal ADP-ribosyl cyclase are present in plant genomes, several studies have demonstrated the action of cADPR in plants (19, 44–46). Moreover, cADPR has been established as a key player in ABA signal transduction pathways in plants, and increased cADPR levels in Arabidopsis induced >100 ABA-responsive genes (47). Therefore, we attempted to measure cellular cADPR levels with the cADPR cycling assay (48). Although this assay reproducibly quantifies cADPR in animal tissues and has partly been used to determine cADPR levels in transgenic plants overproducing the Aplysia ADPR cyclase (47), we failed to reduce the high background signals that impeded accurate monitoring of endogenous cADPR levels in hpAtPARP2 plants. This apparent discrepancy in successful measurement of cADPR might be attributed to the fact that ectopic overproduction of the ADPR cyclase allows enough cADPR to exceed the noise signals within the currently available assay. Nevertheless, we hypothesize that during stress, hpAtPARP2 plants consume less NAD+, hereby increasing their NAD+ pool and facilitating the production of cADPR. cADPR can mobilize internal Ca2+ stores, leading to repetitive Ca2+ peaks that trigger ABA biosynthesis (40). A consequence of the subsequent cellular rise in ABA is the early induction of ABA-responsive transcription factors that could steer the expression of more downstream ABA-responsive and stress-protective genes. In addition, ABA can positively regulate the activity of ADP-ribosyl cyclase (47), providing a relay mechanism that might amplify the original cADPR signal (Fig. 3). Very recently, reduced NAD(H) content has been associated with impaired ABA signaling in Arabidopsis (49).
Fig. 3.
Model for the functionality of PARP and NAD in stress signaling. Stress provokes an increase of NAD+ in hpAtPARP2 plants. By the action of the ADP-ribosyl cyclase, NAD+ might be converted to cADPR. Repetitive Ca2+ peaks trigger the biosynthesis of ABA, activating ABA-responsive and stress-protective genes. In addition, ABA could positively regulate the activity of ADP-ribosyl cyclase, providing a relay mechanism that might amplify the original cADPR signal. Other pathways possibly involved in mediating stress tolerance are indicated in gray. The relative contribution of each pathway to the final stress tolerance is not known and might depend on the nature and severity of the stress.
As mentioned above, improved performance of PARP-deficient plants under various abiotic stresses has previously been explained mainly by an enhanced energy-use efficiency during stress (9). We show that, likely as a consequence of the preserved NAD content, abiotic stress tolerance of hpAtPARP2 plants can also be attributed to a signaling role of NAD+ that facilitates the induction of an ABA-related defense response. However, it cannot be excluded that other NAD+-dependent pathways are also involved in mediating the stress tolerance or that the effect of PARP down-regulation is due to perturbed posttranslational modifications of proteins that function within growth, protein turnover, repair, and transcriptional control of pathways that respond to stress signals (Fig. 3). The relative contribution of each pathway to the final stress tolerance is not known and might depend on the nature and severity of the stress.
Materials and Methods
Plant Material, Growth Conditions, and in Vitro HL Stress.
Transgenic hpAtPARP2 plants of Arabidopsis thaliana (L.) Heyhn. (ecotype C24) were obtained as described (9). Seeds were sterilized with bleach containing 6% active chlorine, pregerminated for 1 day in sterile tap water and grown on solid agar medium (Murashige and Skoog salts and 0.7% Difco agar [BD, Franklin Lakes, NJ]) for 18 days under controlled conditions (22°C–23°C, 16-h day at 50 μmol·m−2·s−1 and 8-h night). HL stress was applied by increasing the light intensity to 250–300 μmol·m−2·s−1 while all other parameters remained constant. Shoot material of ≈30 plants was harvested after 0, 2, 4, and 6 h of HL, frozen in liquid nitrogen, and stored for further analysis at −80°C. All experiments were performed in triplicate.
Microarray Analysis.
Details of microarray data processing and analysis are provided in SI Methods.
Quantitative RT-PCR.
Details on the quantitative RT-PCR analysis are provided in SI Methods.
Microarray Metaanalysis.
Our transcriptomic data sets were compared with those obtained by the AtGenExpress consortium by means of the MetaAnalyzer tool of Genevestigator (27): the abiotic stress time series (50) and hormone treatments (Yoshida's Laboratory, RIKEN, Wako, Japan).
ABA Measurement.
ABA levels were quantified with the Phytodetek ABA Immunoassay kit (Agdia, Elkhart, IN). Frozen samples were pulverized under liquid nitrogen. Of the extraction solution (80% methanol, 100 mg/liter butylated hydroxytoluene, 0.5 g/liter citric acid monohydrate), 8 ml was added to ≈100 mg of powdered tissue, stirred overnight at 4°C in the dark, and subsequently centrifuged at 1,000 × g for 20 min at 4°C. The supernatant was transferred to a new tube and dried under vacuum. The dry residue was dissolved with 100 μl of 100% methanol and 900 μl of Tris-buffered saline [TBS; 50 mM Tris/0.1 mM MgCl2·6H2O/0.15 M NaCl (pH 7.8)] and analyzed directly or diluted with TBS to bring ABA levels within the linear range of the ABA standard curve of the assay.
Supplementary Material
Acknowledgments
We thank Drs. Steven Vandenabeele and Frank Hoeberichts for helpful contribution and discussions; Dr. Steven Maere for assistance with BiNGO; Ina Faché for technical assistance, Karel Spruyt for artwork, and Dr. Martine De Cock for help in preparing the manuscript. This work was supported by the Research Fund of the Ghent University (Geconcerteerde Onderzoeksacties no. 12051403), the Institute for the Promotion of Innovation by Science and Technology in Flanders (Grant 040134/Bayer), and the Research Foundation-Flanders (Grant G.0350.04).
Abbreviations
- ABA
abscisic acid
- ADPR
ADP-ribosyl
- cADPR
cyclic ADP-ribose
- HL
high light
- hpAtPARP2
transgenic PARP2-deficient Arabidopsis
- PAR
poly(ADP-ribosyl)ation
- PARP
poly(ADP-ribose) polymerase
- ROS
reactive oxygen species.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706668104/DC1.
References
- 1.Mittler R. Trends Plant Sci. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bohnert HJ, Gong Q, Li P, Ma S. Curr Opin Plant Biol. 2006;9:180–188. doi: 10.1016/j.pbi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Vinocur B, Altman A. Curr Opin Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Valliyodan B, Nguyen HT. Curr Opin Plant Biol. 2006;9:189–195. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. Proc Natl Acad Sci USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R. Plant Cell. 2003;15:1212–1226. doi: 10.1105/tpc.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noctor G, Queval G, Gakière B. J Exp Bot. 2006;57:1603–1620. doi: 10.1093/jxb/erj202. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi M, Takahashi H, Tamura K, Huang J, Yu L-H, Kawai-Yamada M, Tezuka T, Uchimiya H. Proc Natl Acad Sci USA. 2005;102:7020–7025. doi: 10.1073/pnas.0502556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Block M, Verduyn C, De Brouwer D, Cornelissen M. Plant J. 2005;41:95–106. doi: 10.1111/j.1365-313X.2004.02277.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim MY, Zhang T, Kraus WL. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber V, Dantzer F, Amé J-C, de Murcia G. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 12.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Biochem J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 13.Bürkle A. BioEssays. 2001;23:795–806. doi: 10.1002/bies.1115. [DOI] [PubMed] [Google Scholar]
- 14.Rongvaux A, Andris F, Van Gool F, Leo O. BioEssays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 15.Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 16.Amor Y, Babiychuk E, Inzé D, Levine A. FEBS Lett. 1998;440:1–7. doi: 10.1016/s0014-5793(98)01408-2. [DOI] [PubMed] [Google Scholar]
- 17.Babiychuk E, Cottrill PB, Storozhenko S, Fuangthong M, Chen Y, O'Farrell MK, Van Montagu M, Inzé D, Kushnir S. Plant J. 1998;15:635–645. doi: 10.1046/j.1365-313x.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- 18.Doucet-Chabeaud G, Godon C, Brutesco C, de Murcia G, Kazmaier M. Mol Genet Genom. 2001;265:954–963. doi: 10.1007/s004380100506. [DOI] [PubMed] [Google Scholar]
- 19.Hunt L, Lerner F, Ziegler M. New Phytol. 2004;163:31–44. doi: 10.1111/j.1469-8137.2004.01087.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Breusegem F, Dat JF. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Block M, De Brouwer D. Plant Physiol Biochem. 2002;40:845–852. [Google Scholar]
- 22.Storey JD, Tibshirani R. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossel JB, Wilson IW, Pogson BJ. Plant Physiol. 2002;130:1109–1120. doi: 10.1104/pp.005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto YY, Shimada Y, Kimura M, Manabe K, Sekine Y, Matsui M, Ryuto H, Fukunishi N, Abe T, Yoshida S. Endocytobiosis Cell Res. 2004;15:438–452. [Google Scholar]
- 25.Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inzé D, Van Breusegem F. Plant Physiol. 2005;139:806–821. doi: 10.1104/pp.105.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maere S, Heymans K, Kuiper M. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang J-Y, Choi H-i, Im M-y, Kim SY. Plant Cell. 2002;14:343–357. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Söderman E, Mattsson J, Engström P. Plant J. 1996;10:375–381. doi: 10.1046/j.1365-313x.1996.10020375.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Yang X, He K, Liu M, Li J, Gao Z, Lin Z, Zhang Y, Wang X, Qiu X, et al. Plant Mol Biol. 2006;60:107–124. [Google Scholar]
- 31.Shinozaki K, Yamaguchi-Shinozaki K. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Yano R, Nakamura M, Yoneyama T, Nishida I. Plant Physiol. 2005;138:837–846. doi: 10.1104/pp.104.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. New Phytol. 2002;155:349–361. doi: 10.1046/j.1469-8137.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 35.Bray EA, Bailey-Serres J, Weretilnyk E. In: Biochemistry and Molecular Biology of Plants. Buchanan BB, Gruissem W, Jones RL, editors. Rockville, MD: Am Soc Plant Physiol; 2000. pp. 1158–1203. [Google Scholar]
- 36.Finkelstein RR, Gampala SSL, Rock CD. Plant Cell. 2002;14:S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong L, Schumaker KS, Zhu J-K. Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denby K, Gehring C. Trends Biotechnol. 2005;23:547–552. doi: 10.1016/j.tibtech.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Šwiątek A, Lenjou M, Van Bockstaele D, Inzé D, Van Onckelen H. Plant Physiol. 2002;128:201–211. [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong L, Zhu J-K. Plant Physiol. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauch-Mani B, Mauch F. Curr Opin Plant Biol. 2005;8:409–414. doi: 10.1016/j.pbi.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Berger F, Ramírez-Hernández MH, Ziegler M. Trends Biochem Sci. 2004;29:111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Lee HC. Biol Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- 44.Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua N-H. Science. 1997;278:2126–2129. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 46.Durner J, Wendehenne D, Klessig DF. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez J-P, Duque P, Chua N-H. Plant J. 2004;38:381–395. doi: 10.1111/j.1365-313X.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- 48.Graeff R, Lee HC. Biochem J. 2002;361:379–384. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G, Pichersky E. Plant J. 2007;49:1020–1029. doi: 10.1111/j.1365-313X.2006.03013.x. [DOI] [PubMed] [Google Scholar]
- 50.Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D'Angelo C, Bornberg-Bauer E, Kudla J, Harter K. Plant J. 2007;50:347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.