Abstract
Objective
With behavior genetic analyses of data from young adult twins, we evaluated theoretical perspectives that differentially emphasize biological dispositions, social/cultural factors, or universal pathways to explain individual differences in sexual behaviors.
Design
We fit biometric sex limitation models to three aspects of sexual behavior reported by 4,925 Finnish twins aged 23–27. The sample, from five consecutive birth cohorts, of 2,448 twin pairs (886 sister-sister, 862 brother-sister, 700 brother-brother), included 785 monozygotic and 801 same-sex dizygotic pairs. In addition to model fitting data from twin pairs, we conducted analyses on twins as individuals to test for independence of initiation of sexual behavior from onset age and number of partners.
Main Outcome Measures
From a postal questionnaire, we obtained self-report information on initiation/abstinence of sexual intercourse, onset age, and number of sexual partners.
Results
Genetic and non-shared environmental influences were significant for all three measures. There were trends for common environmental influences on initiation and, in females, age at first intercourse. Some differential effects in males and females were found. Results comparing onset age and number of partners among experienced twins from pairs concordant and discordant for initiation found genetic and environmental influences on initiation/abstinence overlapped those found for the other aspects of sexual behavior.
Conclusions
These results document genetic variation in individual differences in sexual behavior of young adults. Incorporating genetic dispositions into integrated models of sexual behavior will facilitate more effective health promotion and risk taking intervention.
The Surgeon General's Call to Action to Promote Sexual Health and Responsible Sexual Behavior (Satcher, 2001) underscores the challenge in promoting sexual health: five of the ten most commonly reported infectious diseases in the U.S. are sexually transmitted; 40,000 new cases of HIV infection occur annually; nearly one-half of all pregnancies are unintended. Challenges to global sexual health are documented by international health organizations (UNAIDS/WHO, 2003).
Sexual health has been studied from several perspectives, but without an integrated conceptual framework (Kotchick, Shaffe, Forehand, & Miller, 2001). One perspective, illustrated by the Health Belief Model (Rosenstock, Stretcher, & Becker, 1994), Social Cognitive Theory (Bandura, 1994), and the Theory of Reasoned Action (Fishbein, Middlestradt, & Hitchcock, 1994), emphasizes the role of attitudes and knowledge about sex and sexually transmitted diseases. That perspective has been criticized for emphasizing a universal pathway from rational appraisal of risk to conscious commitment to risk reduction; dispositional and social differences that make some individuals more likely to take sexual risks or less likely to respond to health promotion interventions (Hoyle, Fejfar, & Miller, 2000; Kalichman, Weinhardt, DiFonzo, Austin, & Luke, 2002; Marks, Bingman, & Duval, 1998) are largely ignored. To accommodate individual differences in social factors, an alternate perspective focuses on familial and sociocultural factors that modulate sexual risk-taking, factors such as family differences in structure and status, religiosity, parenting, and parental monitoring (Kotchick, Shaffer, Forehand, & Miller, 2001; Scaramella, Conger, Simons, & Whitbeck, 1998). But this perspective fails to account for the fact that, within any social context, individual differences remain in behavioral choices and the attitudes that support them (Bancroft, 2000). A third perspective emphasizes such individual differences by implicating genetic dispositions and biological differences. Illustrating this perspective, individual differences in sexual excitability (Bancroft, 2000), mood-sex relationships (Bancroft et al., 2003), personality (Hoyle, Fejfar, & Miller, 2000), pubertal timing (Mezzich et al., 1997), and risk-relevant genes (Miller et al., 1999) have been associated with sexual risk behaviors.
How can we evaluate the adequacy with which these three theoretical perspectives explain existing data? Twin data that partition observed behavioral variation into genetic and environmental components can be informative. We illustrated the utility of twin data in testing predictions about the role of genetic or environmental effects in substance use (Mustanski, Viken, Kaprio, & Rose, 2003) and pubertal development (Mustanski, Viken, Kaprio, Pulkkinen, & Rose, 2004). Applied to sexual behavior, twin data are useful in evaluating the second and third perspectives, because each offers hypotheses about the importance of genetic (dispositional differences perspective) and common environmental influences (sociocultural perspective). The attitude/knowledge perspective is difficult to test with behavior genetic methods, because it assumes a universal pathway with little attention to individual differences. But convincing documentation of sociocultural or genetic factors would require their inclusion and, accordingly, some revision of this universal pathway perspective.
We evaluated genetic and environmental influences in explaining variation in sexual behavior in a large, representative sample of young-adult Finnish twins. Three inter-related sexual health variables were investigated: abstinence/initiation of sexual intercourse (Initiation), and, among the majority of twins who had initiated sexual experience, number of sexual partners (N Partners) and age at first sexual intercourse (Age). Although not direct measures of high risk sexual behavior, individual differences in these measures relate to inconsistent condom use, precocious pregnancy, infection with STDs, and other negative sexual health outcomes (DiClemente et al., 1992; Edgardh, 2002; Melchert & Burnett, 1990; Smith, 1997; Svare, Kjaer, Thomsen, & Bock, 2002).
We distinguish initiation of sexual intercourse from number of partners, because individual differences in the two variables may result from different genetic and environmental effects. Familial environmental factors might influence initiation — e.g., higher parental monitoring is associated with a later age of initiation (Capaldi, Crosby, & Stoolmiller, 1996; Luster & Small, 1994; Small & Luster, 1994) — but once initiation occurs, variation in number of partners could be determined largely by genetic factors. Alternatively, whether and when a young adult initiates sexual experience may be largely determined by heritable differences in dispositional temperament, but after that initial experience, environmental factors may dominantly influence the number of partners. Most theories on sexual risk-taking ignore such alternative possibilities and assume, without evidence, that the same causal factors are involved in different aspects of early sexual experience. We explicitly test that assumption. We also explore sex differences in the magnitude of genetic and environmental effects and the extent to which each of these effects is sex-specific.
Earlier twin studies of sexual experience provide the context for our analyses. Studies of adult twins offer evidence of genetic influences on age at first sexual intercourse (Dunne et al., 1997; (Martin, Eaves, & Eysenck, 1977). Two studies of sexual experience among adolescent twins found sex differences in the magnitude of genetic and common environmental effects, and one reported modulation of those effects by sibling interactions. Analysis of data from same- and opposite-sex twins, full siblings, half-siblings, adoptive siblings, and cousins, in the National Longitudinal Survey of Youth (Rodgers, Rowe, & Buster, 1999) found significant genetic influences (accounting for half the variance) on age at first sexual intercourse only among brother-brother dyads; in contrast, common environmental effects were significant in sister-sister pairs. A report on>1,400 adolescent sibling pairs in different degrees of genetic relatedness also found greater genetic effects for brothers, adding evidence that contact between siblings significantly modulated the magnitude of genetic effects (Hunt & Rowe, 2003). Genetic effects were greater (and shared environmental influences weaker) in sibling pairs in limited social contact; in contrast, among adolescent siblings in high social contact, genetic influences were negligible (and common environmental effects substantial). Finally, another recent report, of >6,000 adult twin brothers, found genetic effects on both age of initiation and experience with multiple partners within one year, and suggested that the correlation between earlier age of initiation and having multiple partners was, itself, due to common genetic influences (Lyons et al., 2004).
What can our analyses add to this research literature? Our data are from a large, population-based sample of twins born within a 60-month window of time, a sample of five consecutive, complete birth cohorts. Response rates were high, and self-selection biases were modest. The data were collected in a 30-month time frame, to restrict age-variation in the sample. Because a third of our twins are brother-sister pairs, as expected with population-based ascertainment, we can robustly test for sex-limitation of genetic and environmental effects in our data. And, in innovative contrasts, we report analyses of age of initiation and number of partners among sexually experienced twins from pairs concordant and discordant for initiation of sexual experience; these analyses empirically test the assumption that Initiation is etiologically independent from N partners and onset Age.
METHODS
Sample
Our sample consists of young adult twin pairs from the longitudinal FinnTwin16-25 study (Kaprio, Pulkkinen, & Rose, 2002; Keski-Rahkonen, Bulik, Neale, Rose, Rissanen & Kaprio, 2005): consecutive birth cohorts of Finnish twins born 1975–1979. Ascertained from Finland’s Population Register Centre, the sample was effectively exhaustive of all twin pairs born in the five birth cohorts with both co-twins alive and resident within the country at the age 16 baseline assessment. Here, we use data from the fourth wave of data collection, conducted during 2000 –2002, when the mean age of participant twins was 24.4. At this age, participation remained high among female twins (92.6%), but declined somewhat among twin brothers (83.3%) from response rates realized from these twins during their adolescence. We have data from 4,925 twins, including 2,448 twin pairs of known zygosity. Finnish twins showed no reluctance to complete the questionnaire’s two sexual experience items, and >99% of all respondent twins completed the items on Age and N.
Measures
Zygosity was determined by validated questionnaires separately completed by both co-twins and their parents, supplemented by school photographs and DNA markers (Rose, Dick, Viken, & Kaprio, 2001) . Pending DNA confirmation, zygosity remains uncertain for some (< 5 %) same-sex twins in the full sample, and data from these pairs were excluded from our analyses.
Number of sexual partners was assessed with the question, “How many sexual partners have you had?” The questionnaire provided five options for a twin’s response: “zero”, “one”, “two”, “three or four”, and “five or more.” From replies to this question, two variables were created: abstinence versus initiation, a dichotomous variable we refer to as Initiation; and, among those who had initiated sexual behavior, number of sexual partners, which we refer to as N Partners. Age at first sexual intercourse (Age) was assessed by a follow-up question with an open-ended response, “What age were you when you first engaged in sexual intercourse?” Participants filled in the response space with their age in years.
Our questionnaire item did not define sexual intercourse as heterosexual, nor did our question on number of sexual partners define its meaning or exclude partners of the same sex as that of the respondent twin. We assume that participants interpreted the question on number of sexual partners to refer to partners in sexual intercourse, but, as in a recent report based on telephone interviews of adult twins (Lyons et al 2004), we cannot establish that assumption. If ambiguity in questionnaire meaning enhanced measurement error in our data, genetic and/or shared environmental influences would be underestimated in our analyses.
Statistical Analyses
In a quantitative genetic study, variation in the studied variables is assumed to result from three sources: an additive genetic effect (A), a common environmental effect (C), and an unshared environmental effect (E). Additive genetic effects reflect actions of multiple genes that combine in additive fashion to produce differences observed in the variable under study. Common environmental effects include all environmental factors shared by siblings that make them similar ---- e.g., the socioeconomic status of their parents, and the household, school, neighborhood, and community environments they shared while growing up. Unshared environmental influences make twins different from each other, including experience unique to each individual, including, e.g., different peers and partners. It is important to add that our models allocate measurement error as an unshared environmental influence, and unreliable measurement inflates estimates of E.
Estimation of A, C, and E effects is accomplished by fitting a biometric model to the twin correlations (Neale & Cardon, 1992). As shown in Figure 1, constraints are placed on the model based on differences in genetic relatedness of the two kinds of twins and in the two types of environmental influence. Because MZ twins share all of their genes in common, while DZ twins, on average share one-half, effects of A are modeled with a correlation of 1.0 for MZ twins and 0.5 for DZ twins. Because all co-twins in our sample were reared together and were cohabiting at age 16, C effects are modeled as a correlation of 1.0 in both MZ and DZ twins. Unshared environmental effects (E) are influences not shared by co-twins and are modeled as uncorrelated. When these parameter estimates are standardized within a structural equations model, such as the one presented here, the squared parameter values sum to 100%. To facilitate interpretation, squared parameter values will be reported; thus, A2 corresponds to an estimate of genetic variance or heritability.
Figure 1.
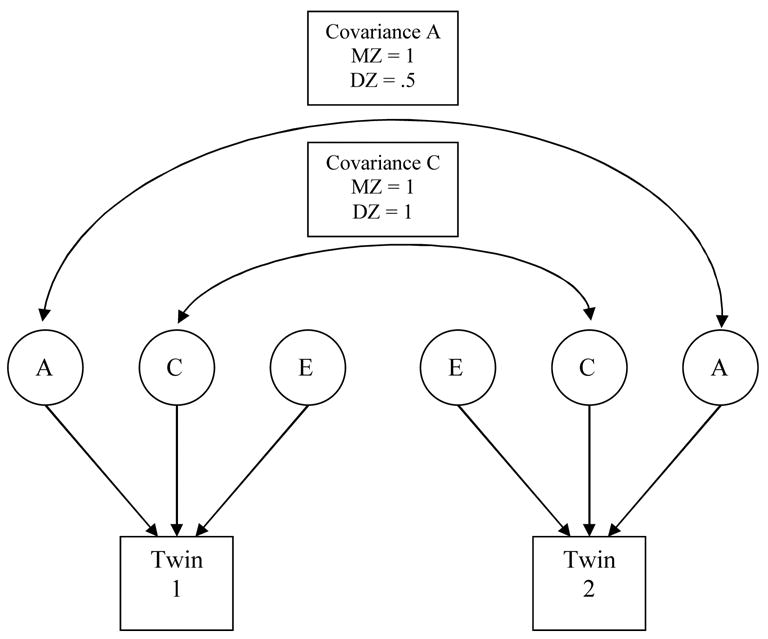
Biometric model fit to twin data to estimate A, C, and E effects.
Sex-limitation models were fit to the data using the Mx (Neale, Boker, Xie, & Maes, 1999) software package. These are sex-limitation models that test two ways that biological sex can play a role in the genetic and environmental architecture (Neale & Cardon, 1992). First, if correlations between opposite-sex twins are substantially less than those found among same-sex pairs, sex-specificity of some portion of either genetic or common environmental influences is suggested. Sex-specificity can occur if two traits with distinct genetic influences, for example experience seeking (Mustanski, Viken, Kaprio, & Rose, 2003) and pubertal timing (Mustanski, Viken, Kaprio, Pulkkinen, & Rose, 2004), differentially relate to a sexual experience outcome in men and women. Pubertal timing, for example, might be a more important trait for age of first intercourse among women. This effect is calculated by freely estimating, instead of fixing, the covariance paths A or C among DZO (brother-sister) twins and comparing it to the value expected in the absence of any sex limitation (i.e. 0.5 for covariance path A and 1 for C). A second type of sex-limitation is evident when the magnitude of either genetic or environmental effects significantly differs between the sexes. For example, genetic factors could have a greater impact on N Partners among men than among women. The full sex-limitation model permits the estimation of different magnitudes of parameters for males and females, as well as either sex-specific common environmental effects or sex-specific additive genetic effects. This test constrains the magnitudes of A, C, and E effects to be equal across sex, asking whether that constraint significantly degrades model fit.
To use all available data, we performed raw data analyses. Data from incomplete twin pairs contribute information on the variance in each trait (but not to the covariance between co-twins). These analyses are implemented in Mx by calculating twice the negative log-likelihood (−LL) of the raw data of each twin pair and summing these over all pairs. Doubling the −LL allows comparisons between nested models, as twice the difference in −LL between models is distributed asymptotically as chi-square. Model comparisons are made with a likelihood ratio chi-square test: a significant change in chi-square, based on the difference of the degrees of freedom between the nested models, indicates that the model with the fewer degrees of freedom should be adopted. For the Age and N Partners variables, we fit a series of nested sub-models of the full sex-limitation model to test the significance of sex-specific effects and sex-differences in the magnitudes of the genetic and environmental parameters. For the Initiation variable, in contrast, the full model was fit, due to the limited power for this variable. When assessed at ages 23 – 27, the vast majority of our twin participants had already initiated sexual experience; few remained abstinent. The very low prevalence of abstinence, combined with the limited power of the basic twin model to distinguish between A and C effects, is magnified for measures scored dichotomously, and leaves little power to distinguish between competing models. Accordingly, instead of fitting a series of sub-models for the initiation/abstinence variable, we report parameter estimates obtained from the full sex-limitation model. Threshold models were used to take into account the non-interval scales for Initiation and N Partners.
In addition to model fitting data from twin pairs, we conducted analyses on twins as individuals to test independence of Initiation from Age and N Partners. Analyzing Initiation separately implicitly assumes that the genetic and environmental factors influencing initiation are independent from those associated with other aspects of sexual behavior. That assumption may be false, and to investigate possible connections between Initiation and the other variables, we compared age of onset and number of partners for initiated twins whose co-twins had not initiated sexual activity (pairs discordant for initiation) to initiated twins whose co-twins had initiated sexual activity (pairs concordant for initiation). That is, we used the data from all twins who had initiated sexual experience; for most, the co-twins had concordantly initiated, but others had co-twins who were discordant for Initiation. This design is a type of family history study, in which some of the subjects have a same-age sibling who has initiated sexual activity, but some have a same-age sibling who has not. Differences in Age of onset or N Partners for subjects who differ in this family history variable suggest a link between the processes influencing sexual initiation and those influencing variation in the other variables. Because pairs who are concordant for initiation of sexual behavior are clustered within families, and therefore violate the standard statistical assumption of independent observations, we used appropriate methods for complex data structures in the software program Stata (Stata, 2000).
RESULTS
Descriptive Results
The mean age of respondent twins was 24.4 years (St. Dev. = .84); 95% were ages 23.1 to 25.8. Descriptive statistics from their reports of sexual behavior are given in Table 1. From responses to the question on N Partners, 88.8% of the males and 92.0% of the females reported Initiation. Given the constricted age (and birth cohort) variation of our twins, their reports on initiation/abstinence correspond well to those from a population survey conducted by Statistics Finland (Haavio-Mannila, Kontula, & Kuusi, 2001). The non-overlapping 95% confidence intervals, shown in Table 1, reveal a significant sex difference in Age of initiation, with the mean Age for women about 4 months earlier than for men; that again is consistent with results from the Statistics Finland survey (Haavio-Mannila, Kontula, & Kuusi, 2001). There is, as well, a significant sex difference in the distribution of N Partners, X2 (4) = 30.89, p < .001, with proportionately more males at both extremes. Due to unavoidable, albeit modest variability in ages of our respondent twins, correlations were computed between age at response and each sexual experience variable to investigate whether, for example, older respondents reported more sexual partners. These correlations were small; the largest between age-at-response and any of the measured sexual experience variables was r < .06.
Table 1.
Descriptive statistics for Age and N Partners Measures
| Males
|
Females
|
|
|---|---|---|
| Age at first sexual intercourse | ||
| Mean (95% CI) | 17.59 (17.49 – 17.70) | 17.31 (17.22 – 17.40) |
| SD | 2.31 | 2.26 |
|
| ||
| Number of sexual partners | ||
|
| ||
| % endorsing (95% CI) | ||
| 0 | 11.2 (9.9 – 12.5) | 8.0 (7.0 – 9.0) |
| 1 | 18.2 (16.6 – 19.8) | 21.9 (20.3 – 23.5) |
| 2 | 10.8 (9.5 – 12.1) | 10.5 (9.3 – 11.7) |
| 3 or 4 | 18.4 (16.8 – 20.0) | 21.5 (19.9 – 23.1) |
| 5 or more | 41.5 (39.4 – 43.6) | 38.0 (36.2 – 39.8) |
Analyses of Twins
Initiation/abstinence was scored dichotomously and, as noted above, 10.2% of all twins reported abstinence. Prevalence of abstinence among individual twins differed by zygosity, in the ordered pattern consistent with a model of cooperative sibling interaction effects: 12.1% of individual twins from MZ pairs reported abstinence, compared to 11.4% among twins from SSDZ pairs and 7.2% from twins from OSDZ twin pairs. Although not significant, these ordered differences suggest that social influence co-twins have on each other may contribute to their similarity.
Pairwise rates of discordance for abstinence also varied by zygosity, as proportionately fewer MZ pairs (7.59%) were discordant for abstinence/initiation than were DZ twin pairs whether same-sex (11.89%) or opposite-sex (10.82%). That result suggests a genetic influence on abstinence/initiation, and there is evidence of common environmental effects on Initiation, as well: Table 2 shows DZ twin correlations more than half the MZ twin correlations, especially among twin sisters. Given this suggestive evidence of effects from shared genes and common experience for Initiation, we fit the full sex-limitation model to these data (−2LL = 2786.316, df = 4889). This model included A, C, and E effects, with minor variation in their magnitudes across sex. As shown in the top section of Table 3, the A and E effects were significant, but the shared environmental (C) effects suggested by the MZ/DZ correlations were not significant, and the confidence intervals include zero.
Table 2.
Twin correlations and number of respondent twin pairs by sex and zygosity
| Variable | MZF | DZF | MZM | DZM | DZO |
|---|---|---|---|---|---|
| Age1 | .671 | .392 | .625 | .252 | .292 |
| N Partners2 | .483 | .282 | .552 | .254 | .279 |
| Initiation2 | .864 | .613 | .907 | .564 | .384 |
| N twin pairs | 472 | 414 | 313 | 387 | 862 |
Note: Age = Age at first sexual intercourse. N Partners = Number of sexual partners excluding abstainers. Initiation = dichotomous scoring of initiation or abstinence (zero versus one or more partners). Superscripts indicate the type of correlation computed:
Pearson Product Moment Correlation;
Polychoric Correlation.
Table 3.
Proportions of total variance in Initiation, N Partners, and AGE. Estimates include additive genetic effects (A2), shared environmental effects (C2), and nonshared environmental effects (E2); 95% confidence intervals are given under the parameter estimates in parentheses.
| Additive Genetic Effects (A2) | Common Environmental Effects (C2) | Nonshared Environmental Effects (E2) | |
|---|---|---|---|
| Initiation | |||
| Males | .668 (.28 – .95) | .229 (.00 – .51) | .104 (.05 – .20) |
| Female | .492 (.10 – .90) | .362 (.00 – .71) | .146 (.08 – .25) |
| AGE* | |||
| Males | .61 (.54 – .68) | .00 (.00 – .11) | .39 (.32 – .46) |
| Female | .54 (.36 – .71) | .14 (.00 – .31) | .31 (.27 – .37) |
| N Partners | |||
| Male | .55 (.28 – .67) | .01 (.00 – .22) | .43 (.34 – .56) |
| Female | .42 (.11 – .58) | .06 (.00 – .31) | .51 (.42 – .61) |
Note: = magnitudes significantly different in males and females. AGE = Age at first sexual intercourse. N Partners = Number of sexual partners excluding abstainers. Initiation = dichotomous initiation versus abstinence.
This model also included an estimate of the extent to which genetic influences were sex-specific. The estimate of the opposite-sex genetic association, not shown in the table, was .27 (95% CI: 0 – .50). Relative to the expected value of .5, this result suggests that approximately 47% of the genetic influences on Initiation were sex-specific. But we emphasize again that our models have limited power for dichotomously scored outcomes, especially given the low prevalence of abstinence; accordingly, the model parameters for abstinence/initiation must be interpreted with caution, and the large confidence intervals of these parameter estimates (Table 3) reflect this imprecision in our model-fitting for abstinence/initiation.
Age and N Partners.
Table 2 reports Pearson twin correlations for the Age measure and polychoric twin correlations for N Partners, as well as the number of respondent pairs in each of the five twin types. For both Age and N Partners, DZ twin correlations were approximately half the magnitude of corresponding MZ correlations, consistent with an inference of genetic effects. For Age, the variable we treated continuously, this differential similarity of MZ and DZ twin pairs can be illustrated in their absolute mean difference: that difference was 1.34 years for MZ co-twins but 2.03 years for all DZ co-twins. And for Age and N Partners, opposite-sex DZ twin correlations approximated the same magnitude as the same-sex DZ twin correlations, suggesting no evidence of sex-specific genetic or environmental influences
Nested models specifying no sex-specific genetic or common environmental effects were compared to the full sex-limitation model. For both Age (full model: −2LL = 19366.998 df = 4414) and N Partners (full model: −2LL = 11175.602 df = 4422) there was no evidence of sex specific effects (p values > .9 for tests of change in model fit). Table 3 contains the parameter estimates from the final models. For N Partners and Age, all of the variation was explained by additive genetic and nonshared environmental influences, as shown by confidence intervals that do not include zero. Common environmental effects did not contribute significantly to individual variation in either measure, although there was a suggestion of common environmental effects for Age in females. For N Partners, the parameter magnitudes were not significantly different by sex (change in −2LL = 1.235, change in df = 3, p = .745); for the onset Age measure, in contrast, they were significantly different (change in −2LL = 11.596, change in df = 3, p < .01), with males having larger genetic influences, smaller common environmental influences, and larger nonshared environmental influences.
Analyses of Pairs Concordant and Discordant for Initiation
In novel analyses, we compared Age and N Partners for sexually experienced twins from pairs concordant and discordant for initiation. Table 4 compares the means (and 95% confidence intervals) for Age at first sexual experience reported by both co-twins in all concordantly experienced twin pairs to the experienced individual co-twin from pairs discordant for Initiation. Among MZ twins, the age of onset for twins whose co-twin had not initiated was 2.6 years later than for twins whose co-twin had initiated. This difference was significant as tested by the adjusted Wald test, F(1,675)=61.18, p<.0001. The difference for same-sex DZ twins was smaller, with the age of onset for members of discordant pairs 1.69 years later, but the difference remained significant, F(1,693)=34.07, p<.001. The difference for twins from opposite-sex twin pairs was much smaller yet, only 0.48 years, a difference which was not significant, F(1,763)=2.81, p<.09. The significant differences for both types of same-sex twins suggest that, at least within-sex, the determinants of Initiation and Age of onset are partially overlapping. The fact that the MZ difference was nearly one year greater than that for same-sex DZ twins suggests that at least part of this shared variation is genetic in origin. Sibling cooperation effects (Rose, Dick, Viken, Pulkkinen, & Kaprio, 2001) on both variables offer another explanation, and that interpretation is consistent with evidence that frequency of contact among adolescent siblings modulates genetic effects on their sexual experience.
Table 4.
Mean age of first intercourse (95% CI) for sexually experienced twins whose co-twins have abstained from sexual intercourse (Discordant pairs) and for twins whose co-twins have initiated sexual activity (Concordant pairs).
| MZ Twins | Same-sex DZ Twins | Opposite-sex DZ Twins | |
|---|---|---|---|
| Discordant | 19.88 (19.25–20.51) | 19.07 (18.52–19.62) | 17.97 (17.42–18.51) |
| Concordant | 17.28 (17.12–17.44) | 17.38 (17.24–17.52) | 17.48 (17.34–17.62) |
Differences between twins from concordant and discordant pairs on the ordinal measure of N Partners were assessed by a clustering-adjusted F test that provides a design-based alternative to the χ2 test. For MZ twins from concordant and discordant pairs, the pattern of responses significantly differed F(3,2024)=17.13, p<.0001. Among MZ twins who had initiated sexual experience and whose co-twin also had initiated, 79% reported having had more than one sexual partner, and 44% reported having five or more. Among initiated twins whose MZ co-twin had not initiated, only 41% reported having more than one partner, and only 9% reported having five or more. Put another way, the probability of having five or more partners was 4.9 times greater among MZ twins whose co-twin had concordantly initiated sexual experience than for MZ twins whose co-twin had not initiated. Again, for same-sex DZ twins, the difference was significant F(3,2087)=6.22, p<.001, but as in the age of onset measure, the difference between concordant and discordant pairs was smaller than for MZ twins. For the concordant pairs, 77% reported having more than one partner and 43% five or more. Among initiated twins from discordant pairs, 65% reported more than one partner and 21% five or more. Thus, the risk of five or more partners was about twice as high for DZ twins whose co-twin had initiated as for those whose co-twin had not, a substantially smaller increment in risk than was found for MZ twins. For opposite sex DZ twins, the difference did not quite reach statistical significance F(3,2291)=2.58, p=.05, and it was quite small. Among opposite sex DZ twins whose co-twin had initiated, 79% reported more than one partner and 46% reported five or more. For discordant pairs, where the co-twin had not initiated, 68% reported more than one partner and 32% reported five or more.
For both variables, differences between the concordant and discordant pairs suggest that there is some overlap in the determinants of Initiation and those of Age of onset and N Partners. The larger effects among MZ twins suggest that genetic influences are responsible for at least part of this overlap. The smaller effects found for opposite-sex DZ twins, compared to effects found for same-sex DZ twins, suggests that these shared influences are to some degree sex-specific.
DISCUSSION
Our results show that shared genes make a significant contribution to variation in initiation of sexual experience in both men and women. There was a suggestion of shared environmental contributions to initiation, particularly for females, but those effects did not reach significance in model fitting. For abstinence/initiation, the influence of non-shared environmental effects was significant, but very modest. In contrast, our results are quite different for variation in the age at which initiation occurs, and for number of sexual partners, where about equal contributions from genetic and non-shared environmental influences explain individual differences, with no evidence of significant effects from shared environments. This pattern of results underscores the important point that the relative contribution of genetic and environmental influences on initiation/abstinence is somewhat different than for the age at which initiation occurs and the number of partners subsequent to initiation. This distinction has not been emphasized in earlier research on sexual health behaviors, but it is well documented for other health behaviors; e.g., drinking/abstaining versus the quantity of alcohol consumed (Viken, Kaprio, Koskenvou, & Rose, 1999) or smoking/abstaining versus number of cigarettes smoked (Heath, Kirk, Meyer, & Martin, 1999). We found apparent sex differences in the genetic architecture of the several aspects of sexual behavior; for Age, genetic effects were significantly larger in males (consistent with earlier research with younger twin samples (Hunt & Rowe, 2003; Rodgers, Rowe, & Buster, 1999), and for Initiation, we found some evidence that genetic influences may be at least partially sex-specific, with higher heritability among males. And in novel analyses of the Age and N Partner measures contrasting sexually experienced twins from pairs concordant and discordant for initiation, we found evidence that the causal determinants of Initiation may not be fully independent of the Age and N Partner outcomes. Our findings suggest that genetic and non-shared environmental factors influence multiple aspects of sexual behavior, with common environmental factors specific to Initiation, and at least some of the same genetic influences act across different behaviors.
These results are broadly consistent with earlier studies of retrospective reports by older adult twins (Dunne et al., 1997; Lyons et al., 2004) and with results from two studies of adolescent samples in the United States (Hunt & Rowe, 2003; Rodgers, Rowe, & Buster, 1999). Together, these results importantly suggest that genetic influences on sexual behavior are significant across age, culture, and gender. But to interpret this evidence of genetic effects on sexual behavior, we must consider the multiple mechanisms that might link genes to behavior, for those mechanisms are likely indirect. Hormonal pathways are one. Initiation of sexual behavior has been linked to pubertal timing, (Kotchick, Shaffer, Forehand, & Miller, 2001), which, in turn, is under significant genetic influence (Mustanski, Viken, Kaprio, Pulkkinen, & Rose, 2004). Individual differences in the number of sexual partners have been linked to testosterone level and cortisol reactivity in males (Halpern, Campbell, Agnew, Thompson, & Udry, 2002). Another pathway may lead from genetically influenced personality; sensation seeking, impulsivity, and social deviance proneness have been linked by theory and data to sexual risk taking (Hoyle, Fejfar, & Miller, 2000). Individual differences in personality traits may explain the observation (Costa, Jessor, Donovan, & Fortenberry, 1995) that sexual activity in adolescence often occurs in a cluster of rebellious, externalizing behaviors, such as negative interactions with parents and peers, drug use, and delinquent behavior of all kinds. Another plausible pathway could be individual differences in strength of sexual interest or arousability (Bancroft & Janssen, 2000). An association reported between genes encoding the dopamine receptors and age at first sexual intercourse suggests dispositional differences in sexual motivation (Miller et al., 1999). Finally, physical attractiveness and other morphological features offer another pathway linking genes to sexual behavior. Across each of these putative pathways, psychological and sociocultural factors likely interact with genetic influences.
Our results are relevant to evaluating the theoretical perspectives of sexual behaviors described earlier. The sociocultural perspective posits that common environmental influences are important sources of individual differences in sexual behaviors. In contrast, a dispositional perspective highlights the importance of traits that are genetically influenced. Our results offer only limited support for the sociocultural perspective: only for initiation/abstinence of sexual behavior at age 25 were effects of common environmental factors suggested, albeit not significant. At average age 25, most Finnish twins had initiated sexual intercourse, and, for them, we found no evidence of common environmental factors influencing either their age at initiation or their number of subsequent sexual partners. Our results support a dispositional-genetic perspective, for genetic influences were significant sources of variation across all three aspects of sexual behavior. Our analyses do not address directly the utility/validity of the information/attitudinal perspective, because it is not a model that includes individual differences. However, Trobst and colleagues (2000) proposed that by considering these perspectives as different levels of analysis, their integration is feasible, enhancing an understanding of previously unexplained variation. Examples of this integration are recently reported links between personality and motivation for sexual risk taking (Cooper, Agocha, & Sheldon, 2000) and risk perception (Trobst et al., 2000). It is important to incorporate dispositional characteristics that predispose to sexual risk-taking into models that focus on information and motivation. Our evidence of genetic influences on sexual health behaviors suggests that an individual differences approach constitutes a theoretically useful framework to help explain why some individuals are more likely to take sexual risks than others and why some are more resistant to health promotion messages.
These data also carry practical implications for sexual health research. Consider how to treat abstinent adolescents when measuring sexual risk taking variables; should they be treated as missing data or included in the lowest risk category? The answer largely depends on whether the causes of initiation are essentially the same as for other sexual behaviors. Our results suggest that the magnitude of genetic effects are similar, and analyses of age of initiation and number of partners among sexually experienced twins from pairs concordant and discordant for initiation of sexual experience suggest Initiation shares common causation with N partners and onset Age. Accordingly, our data do not support the necessity of excluding abstinent youth from analyses focused on predicting sexual behaviors.
That conclusion, and others suggested by our data, must be interpreted within limitations intrinsic to twin studies and those specific to our report. We made the “equal environments” assumption that outcome-relevant experiences of MZ and DZ twins are comparable. We further assumed assortative mating is negligible — that parents of twins are not correlated for the outcomes we studied in their twin children. If false, estimates of common environmental effects would be inflated, but we found little evidence of common environmental effects for these behaviors. Finally, limitations in our simple questionnaire assessment of sexual behaviors constrained our analyses. The questionnaire item measuring N Partners was limited in range of response options, and our analysis is based on but two questions.
While acknowledging these limitations, data from our population-based sample of young adult twins, consistent with other reports, suggest moderate genetic effects on sexual behavior. Novel analyses identified significantly larger genetic influences in males and etiological links between initiation and other aspects of sexual behavior. Further research is necessary to incorporate genetic dispositions into pathways, potentially mediated by individual differences in personality, pubertal timing and assortative pairing with peers (Rose, 2002), predictive of sexual risk taking or response to HIV/STD prevention efforts. The realization of such models may facilitate the development of more specific, and potentially more effective, interventions.
Acknowledgments
Data collection in FinnTwin16-25 was supported by National Institute on Alcohol Abuse and Alcoholism (grants AA-08315, AA-00145 and AA-12502), awarded to Richard J. Rose, with supplementary support from the Academy of Finland (Grant 44069) given to Jaakko Kaprio. Data analysis for this report was supported by a National Science Foundation Graduate Fellowship awarded to Brian Mustanski. We thank Osmo Kontula for his helpful advice, and we extend our gratitude to the Finnish twins who have participated in our ongoing research.
References
- Bancroft J. Individual differences in sexual risk taking: A biopsychosocial theoretical approach. In: Bancroft J, editor. The role of theory in sex research. Bloomington, IN: Indiana University Press; 2000. pp. 177–212. [Google Scholar]
- Bancroft J, Janssen E. The dual control model of male sexual response: A theoretical approach to centrally mediated erectile dysfunction. Neuroscience and Biobehavioral Reviews. 2000;24:571–579. doi: 10.1016/s0149-7634(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Janssen E, Strong D, Carnes L, Vukadinovic Z, Long JS. The relationship between mood and sexuality in heterosexual men. Archives of Sexual Behavior. 2003;32:217–230. doi: 10.1023/a:1023409516739. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social cognitive theory and exercise control of HIV infection. In: DiClemente RJ, Peterson JL, editors. Preventing AIDS: Theories and methods of behavioral interventions. New York: Plenum Press; 1994. [Google Scholar]
- Capaldi DM, Crosby L, Stoolmiller M. Predicting the timing of first sexual intercourse for at-risk adolescent males. Child Development. 1996;67:344–359. [PubMed] [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: The role of personality and affect regulatory processes. Journal of Personality. 2000;68:1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Costa FM, Jessor R, Donovan JE, Fortenberry JD. Early initiation of sexual intercourse: The influence of psychosocial unconventionality. Journal of Research on Adolescence. 1995;5:93–121. [Google Scholar]
- DiClemente RJ, Durbin M, Siegel D, Krasnovsky F, Lazarus N, Comacho T. Determinants of condom use among junior high school students in a minority, inner-city school district. Pediatrics. 1992;89:197–202. [PubMed] [Google Scholar]
- Dunne MP, Martin NG, Statham DJ, Slutske WS, Dinwiddie SH, Bucholz KK, et al. Genetic and environmental contributions to variance in age at first sexual intercourse. Psychological Science. 1997;8:211–216. [Google Scholar]
- Edgardh K. Sexual behaviour and early coitarche in a national sample of 17-year-old Swedish boys. Acta Paediatr. 2002;91:985–991. doi: 10.1080/080352502760272704. [DOI] [PubMed] [Google Scholar]
- Fishbein M, Middlestradt SE, Hitchcock PJ. Using information to change sexually transmitted disease-related behaviors. In: DiClemente RJ, Peterson JL, editors. Preventing AIDS: Theories and methods of behavioral interventions. New York: Plenum Press; 1994. [Google Scholar]
- Haavio-Mannila E, Kontula O, Kuusi E. Trends in Sexual Life: Measured by national sex surveys in Finland in 1971, 1992, and 1999, and a comparison to a sex survey in St. Petersburg in 1996. Väestöliitto: The Family Federation of Finland; 2001. [Google Scholar]
- Halpern CT, Campbell B, Agnew CR, Thompson V, Udry JR. Associations between stress reactivity and sexual and nonsexual risk taking in young adult human males. Hormones and Behavior. 2002;42:387–398. doi: 10.1006/hbeh.2002.1831. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kirk KM, Meyer JM, Martin NG. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behavior Genetics. 1999;29:395–407. doi: 10.1023/a:1021670703806. [DOI] [PubMed] [Google Scholar]
- Hoyle RH, Fejfar MC, Miller JD. Personality and sexual risk taking: A quantitative review. Journal of Personality. 2000;68:1203–1231. doi: 10.1111/1467-6494.00132. [DOI] [PubMed] [Google Scholar]
- Hunt CB, Rowe DC. Genetic and shared environmental influences on adolescents' timing of first sexual intercourse: The moderating effect of time spent with a sibling. In: Rodgers JL, Kohler HP, editors. The Biodemography of Human Reproduction and Fertility. Boston: Kluwer Academic; 2003. pp. 161–185. [Google Scholar]
- Kalichman SC, Weinhardt L, DiFonzo K, Austin J, Luke W. Sensation seeking and alcohol use as markers of sexual transmission risk behavior in HIV-positive men. Annals of Behavioral Medicine. 2002;24:229–235. doi: 10.1207/S15324796ABM2403_08. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Bulik CM, Neale B, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. International Journal of Eating Disorders. 2005;37:188–199. doi: 10.1002/eat.20138. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: Studies on Finnish twins and twin families. Twin Research. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kotchick BA, Shaffer A, Forehand R, Miller KS. Adolescent sexual risk behavior: A multi-system perspective. Clinical Psychology Review. 2001;21:493–519. doi: 10.1016/s0272-7358(99)00070-7. [DOI] [PubMed] [Google Scholar]
- Luster T, Small SA. Factors associated with sexual risk taking behaviors among adolescents. Journal of Marriage and the Family. 1994;56:622–632. [Google Scholar]
- Lyons MJ, Koenen KC, Buchting F, Meyer JM, Eaves L, Toomey R, et al. A twin study of sexual behavior in men. Archives of Sexual Behavior. 2004;33:129–136. doi: 10.1023/b:aseb.0000014327.11433.66. [DOI] [PubMed] [Google Scholar]
- Marks G, Bingman CR, Duval TS. Negative affect and unsafe sex in HIV positive men. AIDS and Behavior. 1998;6:131–134. [Google Scholar]
- Martin NE, Eaves LJ, Eysenck HJ. Genetic, environmental and personality factors influencing the age of first intercourse in twins. Biosocial Science. 1977;9:91–97. doi: 10.1017/s0021932000000493. [DOI] [PubMed] [Google Scholar]
- Melchert T, Burnett KF. Attitudes, knowledge, and sexual behavior of high-risk adolescents: Implications for counseling and education. Journal of Counseling and Development. 1990;68:293–298. [Google Scholar]
- Mezzich TE, Tarter RE, Giancola PR, Lu S, Kirisci L, Parks S. Substance use and risky sexual behavior in female adolescents. Drug and Alcohol Dependence. 1997;44:157–166. doi: 10.1016/s0376-8716(96)01333-6. [DOI] [PubMed] [Google Scholar]
- Miller WB, Pasta DJ, MacMurray J, Chiu C, Wu H, Comings DE. Dopamine receptor genes are associated with age at first sexual intercourse. Journal of Biosocial Science. 1999;31:43–54. doi: 10.1017/s0021932099000437. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and Environmental Influences on Pubertal Development: Longitudinal Data from 12–14 year-old Twins. Developmental Psychology. 2004;40:1188–1198. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Rose RJ. Genetic influences on the association between personality risk factors and alcohol use and abuse. Journal of Abnormal Psychology. 2003;112:282–289. doi: 10.1037/0021-843x.112.2.282. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 5. Richmond, VA: Department of Psychiatry, Medical College of Virginia, Box 126 MCV; 1999. [Google Scholar]
- Neale MC, Cardon LR. 1992. Methodology for genetic studies of twins and families. Boston: Kluwer Academic; [Google Scholar]
- Rodgers JL, Rowe DC, Buster M. Nature, nurture and first sexual intercourse in the USA: Fitting behavioural genetic models to NLSY kinship data. Journal of Biosocial Science. 1999;31:29–41. doi: 10.1017/s0021932099000292. [DOI] [PubMed] [Google Scholar]
- Rose RJ. How do adolescents select their friends? A behavior-genetic perspective. In: Pulkkinen L, Caspi A, editors. Paths To Successful Development: Personality in the Life Course. Cambridge University Press; 2002. pp. 106–125. [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism, Clinical and Experimental Research. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Rosenstock IM, Stretcher VJ, Becker MH. The health belief model and HIV risk behavior change. In: DiClemente RJ, Peterson JL, editors. Preventing AIDS: Theories and methods of behavioral interventions. New York: Plenum Press; 1994. [Google Scholar]
- Satcher D. 2001. The Surgeon General's call to action to promote sexual health and responsible sexual behavior. Office of the US Surgeon General., US. Department of Health and Human Services; [Google Scholar]
- Scaramella LV, Conger RD, Simons RL, Whitbeck LB. Predicting risk for pregnancy by late adolescence: A social contextual perspective. Developmental Psychology. 1998;34:1233–1245. doi: 10.1037//0012-1649.34.6.1233. [DOI] [PubMed] [Google Scholar]
- Small SA, Luster T. Adolescent sexual activity: An ecological, risk-factor approach. Journal of Marriage and Family. 1994;52:181–192. [Google Scholar]
- Smith CA. Factors associated with early sexual activity among urban adolescents. Social Work. 1997;42:334–346. doi: 10.1093/sw/42.4.334. [DOI] [PubMed] [Google Scholar]
- Stata. College Station, TX: Stata Corporation; 2000. (Version 7.0) [Google Scholar]
- Svare EI, Kjaer SK, Thomsen BL, Bock JE. Determinants for non-use of contraception at first intercourse; A study of 10841 young Danish women from the general population. Contraception. 2002;66:345–350. doi: 10.1016/s0010-7824(02)00333-5. [DOI] [PubMed] [Google Scholar]
- Trobst KK, Herbst JH, Masters HL, 3rd, Costa PT., Jr Personality pathways to unsafe sex: Personality, condom use, and HIV risk behaviors. Journal of Research in Personality. 2002;36:117–133. [Google Scholar]
- UNAIDS/WHO. AIDS epidemic update. 2003 December 2003; Retrieved 2/11/2004, from www.UNAIDS.org.
- Viken RJ, Kaprio J, Koskenvou M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]


