Abstract
A variety of signals governing early extension, guidance, and connectivity of olfactory receptor neuron (ORN) axons has been identified; however, little is known about axon–mesoderm and forebrain (FB)–mesoderm signals. Using Wnt–βcatenin reporter mice, we identify a novel Wnt-responsive resident cell population, located in a Frizzled7 expression domain at the surface of the embryonic FB, along the trajectory of incoming ORN axons. Organotypic slice cultures that recapitulate olfactory-associated Wnt–βcatenin activation show that the βcatenin response depends on a placode-derived signal(s). Likewise, in Dlx5−/− embryos, in which the primary connections fail to form, Wnt–βcatenin response on the surface of the FB is strongly reduced. The olfactory placode expresses a number of βcatenin-activating Wnt genes, and the Frizzled7 receptor transduces the “canonical” Wnt signal; using Wnt expression plasmids we show that Wnt5a and Wnt7b are sufficient to rescue βcatenin activation in the absence of incoming axons. Finally, blocking the canonical Wnt pathway with the exogenous application of the antagonists Dikkopf-1 or secreted-Frizzled-receptor protein-2 prevents ORN axon contact to the FB. These data reveal a novel function for Wnt signaling in the establishment of periphery–CNS olfactory connections and highlight a complex interplay between cells of different embryonic origin for ORN axon connectivity.
Keywords: Wnt, Frizzled, βcatenin, olfactory, axon, connectivity
Introduction
During development of the olfactory pathway, a number of processes occur that coordinate axon guidance and connectivity (Scott and Brirley, 1999; Bozza et al., 2002; Brunjes and Greer, 2003). Beginning at embryonic day 10 (E10), immature olfactory receptor neurons (ORNs) in the olfactory placode (OP) extend axons toward the forebrain (FB) along with several populations of migratory cells (Gong and Shipley, 1996). This meshwork of neurites and migratory cells form a migratory mass (MM) that remains separated from the FB by the basement membrane (BM). A small number of pioneer axons enter the FB at earlier stages (Fueshko and Wray, 1994; Key, 1998; Astic et al., 2002), but their function is still unclear. At E14.5, the BM reorganizes and ORN axons enter the FB to form the outer sublaminae of the nerve layer (Doucette, 1989; Bailey et al., 1999; Au et al., 2002).
This organization results from sequential interactions between growing axons and their environment (Key, 1998; Lin and Ngai, 1999; Brunjes and Greer, 2003). Numerous signals and cell populations, of both placodal and mesenchymal origin, are known to participate, such as the chemoattractant Netrin1 (Astic et al., 2002), semaphorins/neuropilins (Williams-Hogarth et al., 2000), Eph/Ephrins (Gao et al., 2000), Slit/Robo (Li et al., 1999), neural cell adhesion molecule (NCAM) (Treloar et al., 1997), Galectin1 (Puche et al., 1996), and p75 NGF receptor (Tisay et al., 2000). Retinoic acid, FGF8, sonic hedgehog, and bone morphogenetic proteins have been shown to mediate mesenchymal–OP interactions and initiate neurite outgrowth (LaMantia et al., 2000; Bashin et al., 2003). However, little is known about signals that mediate neurite–mesenchyme and mesenchyma–FB interactions, essential for connectivity. Their importance for axon–mesenchyme–FB interactions is highlighted by the phenotype of Kallmann patients, in which olfactory axons–olfactory bulb (OB) connections and the migration of gonadotropin-releasing hormone (GnRH) cells are compromised (Rugarli et al., 1996; Dode and Hardelin, 2004; Gonzàlez-Martinez et al., 2004). We (Levi et al., 2003) and others (Long et al., 2003) have reported a Kallmann-like phenotype in Dlx5−/− mice: ORNs extend axons, but these fail to contact the OB.
Wnt proteins are a family of morphogens involved in several key developmental processes (Logan and Nusse, 2004) and recently have been shown to have guidance functions for CNS axons (Lyuksyutova et al., 2003; Liu et al., 2005); however, little is known about their role in axonal connectivity. Because of the complexity of the Wnt signaling and its transduction pathways, an in vivo Wnt readout (BATnlacZ mice) has been devised: activation of a Wnt-dependent βcatenin “canonical” pathway (Maretto et al., 2003; Widelitz, 2005; Willert and Jones, 2006) can be visualized by the presence of nuclear βgalactosidase (βgal) in target cells. We have used these reporter mice to examine the role of Wnt signaling in early olfactory assembly. We have identified a Wnt-responsive cell population located at the interface of the BM and the FB, expressing the Frizzled7 receptor gene. Incoming axons are required for βcatenin activation, and we show that Wnt5a and Wnt7b are sufficient to replace them. We also show that βcatenin activation is required for the ability of axons to form connections with the FB, a novel function for this family of morphogens.
Materials and Methods
Mouse strains and breeding.
The Wnt reporter mice denominated BATnlacZ contain the nucleus-targeted lacZ reporter sequence expressed from a promoter containing seven copies of a βcatenin-lymphoid enhancer factor/T cell factor-responsive site (Maretto et al., 2003). These transgenic animals were maintained and used as a heterozygous (+/−) strain. Mice with targeted disruption of Dlx5 have been reported previously (Acampora et al., 1999). BATnlacZ mice were crossed with Dlx5+/− mice to obtain double-heterozygous ones. From crossbreeding these with Dlx5+/− mates, embryos with genotype Dlx5−/−;BATnlacZ were obtained at the expected frequency (12.5%). To visualize the ORN axons in cultured slices, tau-eGFP mice were used (Tucker et al., 2001), in which the enhanced green fluorescent protein (eGFP) cDNA was targeted to the tau locus [a microtubule-associated protein present in all axonal extensions (Binder et al., 1985)]. Fluorescence was readily observed in early olfactory neurites. β-actin-eGFP mice were used as donors in grafting experiments (Okabe et al., 1997). Both eGFP transgenic strains were maintained and used as heterozygous. β-actin-eGFP mice were crossed with Dlx5+/− mates to obtain double heterozygous. By crossing these with Dlx5+/− mates, Dlx5−/−;eGFP+ embryos were obtained at the expected frequency. The Institutional Animal Care Committee and the Ministry of Health approved all the animal procedures used.
Sample collection and histochemistry.
Embryos were collected between 11.5 and 14.5 d of gestation by cesarean section, or at birth (postnatal day 0), fixed in 4% paraformaldehyde (PFA) overnight, washed in PBS, cryoprotected in 30% sucrose, transferred in OCT compound, frozen, and sectioned at 12 μm. Newborns were perfused with 4% PFA, and either the brain was dissected, postfixed, washed in PBS, stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), and processed for cryostatic sections or the entire head was washed in PBS, stained with X-gal, decalcified with 10% EDTA for 8 h, and processed as above. βgal+ nuclei were detected by histoenzymatic staining with X-gal, as described previously (Merlo et al., 2002). Alternatively, βgal+ nuclei were detected by immunostaining with anti-βgal antibody.
Immunohistochemistry was done on cryostatic sections, according to standard protocols. Permeabilization was done with 10% Triton X-100 at room temperature for 10 min, followed by blocking with 10% goat serum and 0.5% Triton X-100 for 1 h at room temperature. The following antibodies were used: rabbit anti-βgal (1:5000; MP Biomedical, Illkirch, France), rabbit anti-S100 (1:200; DakoCytomation, Glostrup, Denmark), goat anti-olfactory marker protein (OMP; 1:2000; Wako Chemicals, Neuss, Germany), rabbit anti-laminin (1:200) (Duband and Thiery, 1987), mouse anti-collagen type IV [monoclonal, 1:200; Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA], mouse anti-heparan sulfate (HSPG) and anti-chondroitin sulfate (CSPG) (monoclonal, 1:200; DSHB), mouse anti-vimentin (monoclonal, 1:500; DSHB), mouse anti-vinculin (monoclonal, 1:500; DSHB), anti-talin (1:100; Abcam, Cambridge, UK), mouse anti-neuronal class-III β-tubulin (monoclonal TuJ1, 1:1000; Sigma, St. Louis, MO), mouse anti-polysialic acid (PSA)–NCAM (monoclonal, 1:2000; AbCys, Paris, France), and goat anti-doublecortin (DCX; polyclonal, 1:500, C18; Santa Cruz Biotechnology, Santa Cruz, CA). Monoclonal antibodies were revealed with the ARK kit (DakoCytomation). Rabbit and goat antisera were revealed either with peroxidase-conjugated goat anti-rabbit (EnVision+; DakoCytomation) and rabbit anti-goat (Rockland, Gilbertsville, PA) antibodies, respectively, or with anti-mouse-Cy2 and anti-rabbit-Cy3 (1:200 and 1:600; Jackson ImmunoResearch, West Grove, PA) antibodies. Peroxidase was developed with DAB.
Bright-field micrographs were taken with a digital photocamera on an Olympus (Tokyo, Japan) AX70 microscope. Fluorescence micrographs were obtained with a confocal microscope (TCS SP2 AOBS; Leica, Nussloch, Germany), using the sequential scanning/stacking function with false colors, digitally merged. For colocalization, single Z-slices, rather than the Z-stack, were used.
Organotypic slice cultures.
Donor and host tissues were obtained from E11.5 and E12.5 embryos, as indicated for each experiment. Embryonic heads were dissected in cold PBS–glucose (0.6%), embedded in warm (42°C) 3% low melting point agarose (Sigma), let harden at room temperature, and transferred on ice. The gel blocks were sectioned by a vibratome (250 μm) and kept 1 h in PBS–glucose on ice. Slices were transferred on Millicell-CM membranes (Millipore, Billerica, MA) floating on DMEM/F-12 medium (Invitrogen, Carlsbad, CA), 6.5 mg/ml glucose, 0.1 m glutamine, and 10% FBS at 37°C for 1 h, changed to Neurobasal medium supplemented with B27 (Invitrogen), and maintained at 100% humidity, 5% CO2 at 37°C for 48–72 h.
The OP or the anterior FB tissues were removed from slices using a fine needle. For the grafting experiments, sections from Dlx5−/−;BATnlacZ mice were used as hosts, whereas β-actin-eGFP+ [wild-type (WT)] embryos were used as donors of normal OPs. The olfactory region of E11.5 embryos was dissected, vibratome sliced (250 μm), and processed as described below. The donor heads were sectioned as for the host tissues, kept in cold PBS–glucose, and further dissected to separate OP fragments. These were transferred onto the host slices using a glass capillary; cultures were changed to Neurobasal/B27 and maintained for 48 h. For the analyses, slices were fixed in 4% PFA and examined by fluorescence microscopy, or stained with X-gal.
Blocking Wnt signal in slice cultures.
Organotypic slice cultures were set up from E11.5 WT embryos, as described above. To visualize the olfactory nerves, we used embryos heterozygous for the tau-eGFP transgene. To test the efficacy of the Wnt inhibition, the same experiments were performed on slices of E11.5 BATnlacZ embryos. To antagonize the Wnt signal, we used heparin-acrylic beads (Sigma) adsorbed either with PBS alone (as negative controls) or with recombinant Dickkopf-1 (Dkk1; 25 μg/ml in PBS/0.1% BSA) or secreted Frizzled-related protein-2 (sFRP2; 10 μg/ml) purified proteins (R & D Systems, Minneapolis, MN). Beads were soaked in the protein solution for 8 h at room temperature and transferred onto the anterior forebrain surface (AFS) or onto the frontonasal process of the host slice.
Transfection and small interfering RNA.
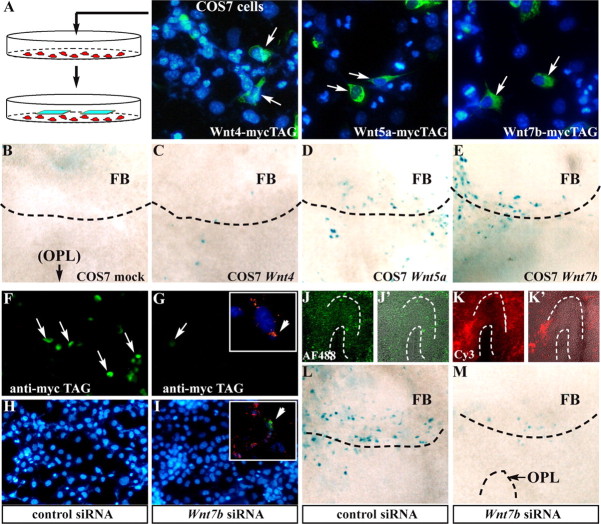
COS7 cells were transfected with plasmids expressing Wnt4-, Wnt5a-, and Wnt7b-mycTAG fusion proteins (obtained from Dr. Y. Zou, University of California, San Diego, La Jolla, CA) (Liu et al., 2005) using Lipofectamine-2000 (Invitrogen) and were tested for expression of Myc-tagged-Wnt fusion proteins by immunostaining with anti-mycTAG (monoclonal 9E10; Sigma). Transfected cells were replated 12 h after transfection, allowed to adhere to the surface, and used as feeder layer for organotypic cultures from E11.5 BATnlacZ embryos. Cultures were continued for 48 h, fixed with 4% PFA and stained with X-gal.
Alexaflour488- and Cy3-labeled Wnt7b and control small interfering RNAs (siRNAs) were synthesized (Qiagen, Hilden, Germany) with the following sequence: Wnt7b (1), forward 5′GCAUGAAGCUGGAAUGUAdTdT, reverse 5′ UUACAUCCAGCUUCAUGCdGdG; Wnt7b (2), forward 5′ CCUGGAUCAUGCACAGAAAdTdT, reverse 5′ UUUCUGUGCAUGAUCCAGGdGdA; control, forward 5′ UUUUCCGAACGUGUCACGUdTdT, reverse 5′ ACGUGACACGUUCGGAGAAdTdT.
Their efficiency was examined in COS7 cells transfected with 0.8 μg of Wnt7b-mycTAG expression plasmids, followed by retransfection with 50 nm siRNA (and control), using Hyperfect reagent (Qiagen) according to instructions. Cells were then immunostained for mycTAG 24 h later and scored. Organotypic cultures from E11.5 BATnlacZ embryos were transfected with a higher concentration (300 nm) of siRNA, maintained for 48 h, and stained with X-gal. Uptake of siRNA oligonucleotides was confirmed by fluorescence microscopy. Depletion of mRNA in the cultured OP was examined by real-time quantitative PCR (qPCR) after siRNA transfection.
Detection of Wnt and Frizzled mRNAs by reverse transcription-PCR, real-time PCR, and in situ hybridization.
For reverse transcription (RT)-PCR and real-time PCR, OB, olfactory epithelium (OE), and AFS tissue fragments were dissected under microscopic examination from 100 μm vibratome slices of E13.5 WT embryos and transferred in Trizol (Invitrogen), and total RNA was isolated according to the manufacturer's instructions and reverse transcribed (1 μg of RNA) with SuperScript II RT (Invitrogen) at 42°C for 50 min, with random hexamers. RT-PCR was performed using standard cycle programs and reagents. Real-time qPCR was performed with LightCycler and FastStart DNA MasterPLUS SYBR-Green I (Roche, Milan, Italy). Each sample was done in triplicate, and experiments were repeated twice. Standard curves were determined using WT cDNA with four calibration points: 1:1; 1:3; 1:9; 1:27. Specificity and absence of primer dimers was controlled by denaturation curves on the PCR products. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was used for normalization, calculated using LightCycler software 3.5.3. Intron-spanning oligonucleotides were designed to amplify the cDNA of all the Wnt and the following Frizzled (Fzd) genes: Fzd1, Fzd3, Fzd4, Fzd5, Fzd7, and Fzd9.
In situ hybridization was performed with digoxigenin-labeled RNA probes obtained by in vitro transcription. Wnt2 and Wnt11 were gifts from Dr. A. McMahon (Harvard University, Boston, MA), Wnt5a and Wnt5b were gifts from Dr. Y. Yang (National Human Genome Research Institute, Bethesda, MD), Fzd4 and Fzd5 were gifts from Dr. S. Pleasure (University of California San Francisco, San Francisco, CA), and Fzd9 was from Dr. L. Muzio (DiBit-San Raffaele, Milan, Italy). Other probes were obtained by RT-PCR cloning and were sequence verified, and the signal obtained was consistent with available data (www.genepaint.org). Hybridization was done on cryostatic sections from E12.5–E14.5 embryos, using published procedures (Merlo et al., 2002), and revealed with BM Purple (Roche). After hybridization, selected sections (from BATnlacZ specimens) were rinsed in PBS and immunostained for βgal.
Results
Wnt–βcatenin-responsive cells and early ORN–FB innervation
To investigate the distribution of Wnt-responsive cells in the olfactory pathway, we examined mice carrying the BATnlacZ reporter. In these mice, cells responding to a Wnt signal through the βcatenin pathway express βgal in their nuclei and can be visualized (Maretto et al., 2003). We stained sections of BATnlacZ heterozygous embryos of several ages. At E12.5, βgal+ nuclei were present on the ventromedial surface of the anterior FB, the region that is the precursor to the OB. This region is referred to as the AFS (Fig. 1A–E, black arrows). The number of βgal+ nuclei increased at E13.5 and persisted at all subsequent embryonic ages (Fig. 1A–E) and in the neonatal OB (Fig. 1N). βgal+ nuclei were absent from the AFS at and before E11.5 (Fig. 1A). With the exception of a few positive nuclei associated with developing blood vessels (late embryonic/postnatal) or displaced by the vibratome sectioning, there was no expression in the OP (early embryo), the OE (late embryonic/postnatal), the MM, and the olfactory and vomeronasal nerves. Intense X-gal staining was observed in the mesenchyme of the frontonasal processes (Fig. 2A), as expected.
Figure 1.
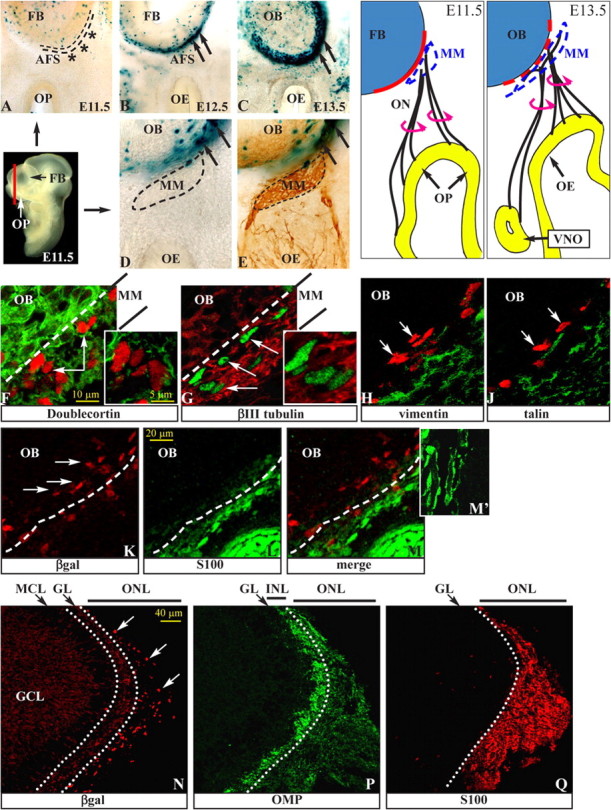
Wnt–βcatenin-responsive cells in the developing olfactory system. A–C, X-gal staining of frontal sections of BATnlacZ embryos at E11.5 (A), E12.5 (B), and E13.5 (C). A section plane is shown (solid red line). βgal+ nuclei are visible around the OB at E12.5 and subsequent stages (black arrows) but not at E11.5 (black asterisks). D, E, X-gal staining of sections from E12.5 BATnlacZ embryos (D), followed by immunostaining with anti-NCAM (E), to show the position of the MM (area within the dashed line) and the βgal+ nuclei. The drawings on the right show the relationship between ORN neurites and the FB at the same stages. F–J, Double immunostaining for βgal (white arrows) and DCX (F, red), βIII-tubulin (G, green), vimentin (H, green), or talin (J, green) on sections of E12.5 BATnlacZ embryos. Insets, Higher magnification of single confocal Z-slices. K–M, Double immunostaining for βgal (K, M, red) and S100 (L, M, green). M′, Higher magnification of ON-associated S100+ cells. N–Q, Immunostaining for βgal (N, red, white arrows), OMP (P, green), and S100 (Q, red) on consecutive sagittal sections of neonatal BATnlacZ;Dlx5+/lacZ OB. In N, anti-βgal also recognizes OB interneurons (weaker cytoplasmic signal). Dotted white lines separate the outer and the inner nerve sublayers. GCL, Granule cell layer; GL, glomerular layer; INL, inner nerve layer; MCL, mitral cell layer; ONL, outer nerve layer.
Figure 2.
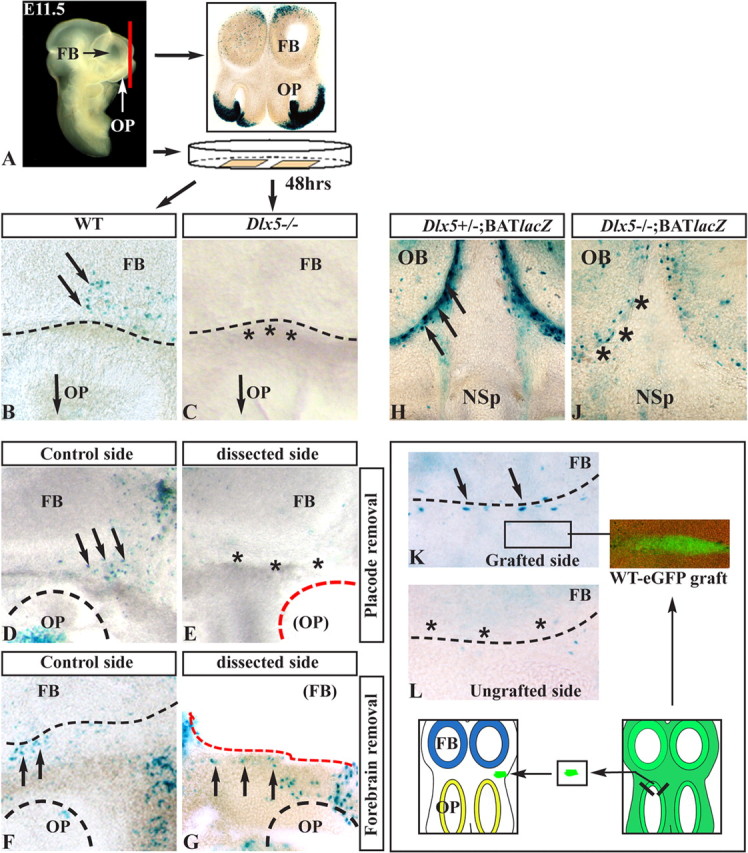
Organotypic cultures to define the origin of Wnt signal. A, Section plane and experimental procedure using organotypic cultures. B, C, Sections of E11.5 BATnlacZ WT (B) and BATnlacZ;Dlx5−/− (C) embryos, cultured for 48 h and stained with X-gal. βgal+ nuclei (black arrows) are visible in the WT but not in the Dlx5−/− (black asterisks). D, E, Removal of one OP (dashed red line) from slices of E11.5 BATnlacZ WT embryos: βgal+ nuclei are detected in the control sections (black arrows) but absent after OP removal (black asterisks). F, G, Same as in D and E but removing the FB (dashed red line). The number of βgal+ nuclei is not greatly reduced compared with the control side. H, J, Coronal sections of E13.5 embryos with genotypes BATnlacZ;Dlx5+/− (H) and BATnlacZ;Dlx5−/− (J), stained with X-gal. The Dlx5−/− sections show a drastic reduction in βgal+ nuclei (black asterisks) compared with the Dlx5 heterozygous ones (black arrows). K, L, Grafting of fragments of OP from E11.5 eGFP+ WT donor embryos onto slices from E11.5 BATnlacZ;Dlx5−/− embryos. The donor tissue was placed anterior to the FB, as illustrated in the drawing (bottom) and indicated in K. After culture, βgal+ nuclei were detected (X-gal staining) in the AFS of grafted slices (arrows in K) but not in the control side (asterisks in L). NSp, Nasal septum.
At E12–E13, ORN axons and OP-derived migratory cells form a cap-like structure, called the MM, against the ventromedial end of the anterior FB. The MM contains axons and migratory cells positive for NCAM (Fig. 1E). The βgal+ nuclei located at the ventromedial edge of the anterior FB were internal with respect to the MM cap and did not express NCAM, suggesting they are not neuronal (Fig. 1D,E). We can also exclude the cells as Cajal-Retzius cell bodies because of the absence of NCAM and βIII-tubulin, normally expressed by this cell type.
The MM contains a population of migrating cells derived from the OP, among which are GnRH+, NCAM+, and DCX neurons (Wray et al., 1989; Tarozzo et al., 1995). The βgal+ nuclei at the surface of the FB could belong to these migratory cells. However, at E12.5, βgal+ nuclei are already present on the surface of the FB, whereas migratory cells are found only along the emerging axon bundles and in the MM; thus, the migratory origin of these cells appears unlikely. However, we performed double immunostaining of sections from E12.5 embryos to detect βgal and either DCX, a marker for migratory and immature neurons, or βIII-tubulin, a general marker for the neuronal lineage (Menezes and Luskin, 1994; Gleeson et al., 1999). At E12.5, βgal+ nuclei were located at the boundary between the MM and the FB and were surrounded by DCX+ and βIII-tubulin+ cell bodies and neurites, but they did not show immunoreactivity for either of these markers in their cytoplasms (Fig. 1F,G). DCX+ cell bodies were abundant in the OB; however, none of their nuclei was βgal+. Staining for PSA-NCAM yielded the same result (data not shown). These data point to both a non-neuronal and nonmigratory nature of the βcatenin-activated cells. Finally, in Dlx5−/− embryos, OP-derived cell migration is essentially eliminated as a consequence of the primary lack of ORN axon innervation (see below). After grafting a WT OP onto slices from Dlx5−/−;BATnlacZ embryos, βcatenin response can be restored, providing another indication that the βcatenin-activated cells are not derived from OP migration.
Mesenchymal-type cells are present in and around the olfactory pathway, and some are tightly associated with the BM of the FB. Vimentin, vinculin, and talin are known markers for mesenchymal cells (Hay, 1989; Treloar et al., 1996). We performed double immunohistochemistry on sections from E12.5 BATnlacZ embryos to determine whether βgal+ cells express any of these mesencymal markers. Expression of all these markers was abundant and widespread in the head mesenchyme, but only low expression of talin, vimentin, and vinculin (data not shown) was observed in the MM and at the interface between the MM and the FB, where βgal+ nuclei are located (Fig. 1H–J). No βgal+ nuclei coexpressed these mesenchymal markers. However, the Wnt receptor gene Fzd7 is expressed at the site where βgal+ nuclei are located (Fig. 3E), and βgal+ nuclei are closely associated with the BM (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Thus, βgal+ cells, if mesodermal in origin, are clearly nontypical mesenchymal cells.
Figure 3.
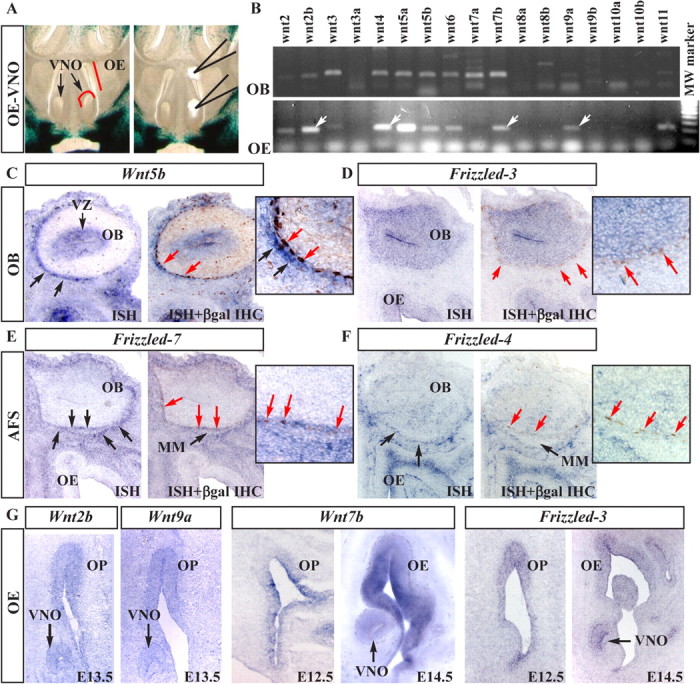
Expression of Wnt and Fzd genes in the embryonic olfactory system. A, Dissection of OE and VNO tissues from slices of E13.5 embryos. OB was obtained from more rostral slices. B, RT-PCR amplification for Wnt mRNAs (as indicated on top) on dissected OB (top) or OE plus VNO (bottom) from E13.5 WT embryos. “Canonical” Wnt genes expressed in the OE–VNO are indicated (white arrows). MW, Molecular weight. C–F, In situ hybridization (ISH) with probes for Wnt5b (C), Fzd3 (D), Fzd7 (E), and Fzd4 (F) on frontal sections of E14.5 embryos (left, blue, black arrows), followed immunostaining for βgal (right, brown nuclei, red arrows). Insets show higher magnifications. βgal+ nuclei are found in the Fzd7 expression domain of the AFS. VZ, Ventricular zone. G, Expression of selected Wnt and Fzd genes in the embryonic OE and VNO. Embyonic stages are indicated at the bottom.
The MM also contains migratory cells that are the precursors of olfactory ensheathing cells (OECs), a population of Schwann-like cells that expresses S100. We therefore examined whether the βgal+ nuclei could belong to early OEC-like cells. Using double immunohistochemistry, we determined that βgal+ nuclei are located in a layer internal with respect to that of the S100+ cell bodies in the MM (Fig. 1K–M′) and did not express S100. By birth, the (OMP+) layers of the OB have reorganized into a glomerular layer and two nerve sublayers: an outer layer, rich in laminin/collagen-IV and containing abundant S100+ cells, and an inner one, low in laminin/collagen-IV and devoid of S100+ cells (Bailey et al., 1999). Because of the position of βgal+ nuclei external to the embryonic FB, we predicted that at birth these nuclei should be absent from the glomerular and inner nerve sublayer and instead be located in the outer sublayer. We immunostained sections of BATnlacZ;Dlx5+/− OB for βgal, OMP, and S100. The Dlx5-lacZ allele provided a marker for OB interneurons (Levi et al., 2003) that can be distinguished for the weak cytoplasmic staining. βgal+ nuclei were located within the OMP+;S100+ layer but were absent from the OMP+;S100− layer (Fig. 1N–Q).
With the exception of a few pioneer axons entering the anterior FB before E10.5 (Key, 1998; Astic et al., 2002), at E12.5 the MM and the FB are separated by a BM, which in late embryonic stages is extensively degraded and fenestrated to allow for the formation of the nerve layer (Doucette, 1989). This BM is composed of laminin, collagen-IV, and proteoglycans of the CSPG and the HSPG classes (Gong and Shipley, 1996; Treloar et al., 1996; Julliard and Hartmann, 1998). The position of βgal+ nuclei with respect to the BM was assessed by double immunostaining for βgal and either collagen-IV or HSPG, on E12.5 and E14.5 BATnlacZ embryonic heads. βgal+ nuclei were often found associated with the internal surface of the BM, and their position often corresponded to areas where the BM appears discontinuous and fenestrated (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Staining with CSPG antibody yielded results identical to HSPG (data not shown). At subsequent stages, the association of the βgal+ nuclei with the fenestrations of the BM is maintained.
ORN to mesenchymal signals induce Wnt–βcatenin response
Neurite-to-mesenchyme signaling is thought to be essential for initial ORN axons pathfinding and connectivity (Calof and Chikaraishi, 1989; LaMantia et al., 2000). The Wnt–βcatenin response on the surface of the FB could be elicited by signals from the OP, from the mesenchyme, or even from the FB. To investigate the source of Wnt signaling to these cells, we developed culture conditions that preserve the OP–mesenchyme–FB organization and maintain Wnt–βcatenin cell activation. We prepared organotypic slice cultures of the olfactory region, using BATnlacZ embryos (Fig. 2A). Because βgal+ nuclei associated with the anterior FB are present at E12.5 but not at earlier stages (Fig. 1), we expect that if slices are cut at E11.5 and recapitulate in vivo development, βgal+ nuclei should appear within 24–48 h in vitro. Indeed, after 48 h, βgal+ nuclei were detected at the edge of the FB, a region corresponding to the AFS (Fig. 2A,B).
Using this culture system on BATnlacZ embryo slices, we then removed either the OP or the FB from one side of the sections, retaining the contralateral side for control. Removal of the OP resulted in a near complete loss of βgal+ nuclei from the AFS in the majority of slices (33 of 40), whereas removal of the FB tissue rarely resulted in loss of βgal+ nuclei (2 of 30) (Fig. 2D–G; Table 1). These data suggest that an OP-derived signal(s) is required to activate the βcatenin pathway in the AFS. Because the OP is at some distance from the responsive cells, this signal molecule(s) is most likely released from extended ORN axons as they approach the FB region. Of note, neither the OP nor the mesenchyme around the olfactory pathway display Wnt–βcatenin-responsive cells, another indication that the activating signal is conveyed by the axons and targeted to the AFS region.
Table 1.
Wnt–βcatenin response in cultured slices from BATnlacZ embryos: removal of OP and FB from WT slices
| OP removala | FB removala | |
|---|---|---|
| Total | 40b | 30 |
| Presence of X-gal+ nucleic | 7 (18%) | 28 (93.3%) |
| Absence of X-gal+ nucleid | 33 (82%) | 2 (6.7%) |
aSlices from E11.5 embryos, maintained in culture for 48 h.
bNumbers of slices showing the indicated response.
cX-gal-stained nuclei (nuclear βgal+) detected in AFS.
dSame as in footnote c; absence of X-gal-stained nuclei.
To complement this observation, and to verify that removal of the OP does not change local expression of Wnt and Fzd in the AFS, we determined the mRNA abundance of Wnt5b and three Fzd genes (Fzd3, Fzd4, and Fzd7) in the AFS (see below) of cultured slices, in which the OP was either removed or left in place. We did not observe any reduction, but rather a modest increase, in the expression of these genes (supplemental Fig. 2A, available at www.jneurosci.org as supplemental material).
Reduced Wnt–βcatenin activation in Dlx5−/− embryos
We also examined the role of olfactory axons approaching the FB for the activation of the βcatenin pathway, by using a genetic model for lack of axon–FB contact, that would not require drastic manipulation such as OP removal. In the absence of Dlx5, ORN neurites elongate and extend toward the FB but inevitably fail to make contact and form connections, as the consequence of cell-autonomous defects in ORN axons (Levi et al., 2003; Long et al., 2003; Merlo et al., 2007). If neurite-to-mesenchyme signals near the FB are critical to activate the Wnt–βcatenin pathway, the disrupted axon growth in Dlx5−/− embryos should result in an absence of βgal+ nuclei. Indeed, in Dlx5−/−;BATnlacZ embryos, the number of βgal+ nuclei is drastically reduced (−75 to −85%) compared with WT or Dlx5+/− controls (Fig. 2H,J). As expected, organotypic slice cultures established from E11.5 Dlx5−/−;BATnlacZ embryos also failed to show βgal+ nuclei in the AFS (Fig. 2C, Table 2).
Table 2.
Wnt–βcatenin response in cultured slices from BATnlacZ embryos: comparison between WT and Dlx5−/− slices
aSlices from E11.5 embryos, maintained in culture for 48 h.
bX-gal-stained nuclei (nuclear βgal+) detected in AFS.
The absence of βgal+ nuclei in the AFS of Dlx5−/− embryos could be attributable to either the absence of the responsive cells or a failure of activation of present cells. If the second case was true, grafting a normal OP in a Dlx5−/− slice should restore Wnt–βcatenin response. However, if the cells are absent, “rescue” with WT OP would be ineffective. We combined the technique of organotypic cultures of BATnlacZ;Dlx5−/− embryos with the grafting of a normal OP obtained from β-actin-eGFP+ embryos of the same age. The donor tissue fragment was positioned near the AFS, on one side of the section, with the contralateral side left intact as a control. Grafting a WT OP near to the AFS resulted in restoration of βgal expression at the boundary of the anterior FB (seven of eight slices) (Fig. 2K,L; Table 3). This indicates that an OP signal induces Wnt–βcatenin response, a function that is absent in Dlx5−/− mice caused only by the absence of Dlx5−/− axons reaching the FB.
Table 3.
Wnt–βcatenin response in cultured slices from BATnlacZ embryos: rescuing the βcatenin response in Dlx5−/− embryos
| Total | 8 |
| Presence of X-gal + nucleia | 7 |
X-gal-stained nuclei (nuclear βgal +) detected in AFS.
To better characterize the Dlx5 mutant model, we examined expression of some of the relevant Wnt and Fzd genes (see below) in the OP and mesenchyme by in situ hybridization and real-time qPCR, comparing WT and Dlx5−/− samples. Wnt2b, Wnt7b, and Fzd3 (OP expressed) were expressed at an equal level. Wnt5b expression (in the mesenchyme around the OB) was reduced, whereas Fzd7 expression (mesenchyme) was not significantly changed (supplemental Fig. 2A–H, available at www.jneurosci.org as supplemental material, and data not shown). Finally, grafting experiments indicate that the olfactory connectivity defect in Dlx5 null animals is linked to cell-autonomous properties of ORNs and does not depend on the OB (Levi et al., 2003; Merlo et al., 2007).
Expression of Wnt and Fzd genes in the olfactory system
Obvious candidates for OP-derived signals inducing Wnt–βcatenin response are the Wnt genes themselves. First, we screened for expression of all Wnts by RT-PCR on RNA from dissected the OE and vomeronasal organ (VNO) from E13.5 WT embryos (Fig. 3A). As control for the efficiency of the RT-PCRs, a mix of total RNA from embryos at various stages was used with each primer pair generating an appropriate positive control amplicon (data not shown). We detected expression of Wnt2, Wnt2b, Wnt3, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7b, Wnt9a, and Wnt11 (Fig. 3B; Table 4). However, the dissected OE will inevitably contain some of the associated mesenchyme. To better localize the expression of Wnt genes, we performed in situ hybridization on sections of E12.5–E14.5 WT heads. Wnt2b, Wnt7b, and Wnt9a are expressed in the OE–VNO (Fig. 3G, Table 4; supplemental Fig. 2, available at www.jneurosci.org as supplemental material), Wnt5a is expressed in the submucosal mesenchyme and not the OE, and Wnt4 is detected in the non-OE of the nasal cavity (data not shown).
Table 4.
Expression of Wnt and Fzd genes in the embryonic olfactory system
| RT-PCR |
In situ hybridization |
RT-PCR |
In situ |
||
|---|---|---|---|---|---|
| WT OB | WT OB | WT AFS | WT OE | WT OE–VNO | |
| Wnt2a | +/− | − | − | + | +/− |
| Wnt2b | + | − | + | + | + |
| Wnt3 | ++ | +b | − | +/− | +/−b |
| Wnt4 | ++ | ++ | +/− | + | + |
| Wnt5a | ++ | ++c | − | + | − |
| Wnt5b | ++ | ++c | ++ | + | + |
| Wnt6 | + | ++b | − | + | + |
| Wnt7a | +++ | nd | nd | − | nd |
| Wnt7b | +++ | +c | − | + | + |
| Wnt9a | + | ++b | − | + | + |
| Wnt11 | +/− | − | ++b | + | +/−b |
| Fzd1a | ++c | − | − | ||
| Fzd3 | +b | − | + | ||
| Fzd4 | +/− | +/− | +/− | ||
| Fzd7 | − | ++ | − | ||
| Fzd9 | +c | − | − | ||
+++, High; ++, present; +, low; −, absent; nd, not done.
aAdditional Wnt and Fzd genes examined but not included in this list because expression was undetectable: Wnt1, Wnt3a, Wnt8a, Wnt8b, Wnt9b, Wnt10a, Wnt10b, and Fzd5.
bWidespread expression.
cVentricular zone of the embryonic OB.
Then, we screened for expression of Wnt genes on RNA from dissected WT OB at E13.5. By RT-PCR, we detected expression of Wnt2b, Wnt3, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt9a, and Wnt11 (Fig. 3B; Table 4). By in situ hybridization, we found that Wnt4, Wnt5a, Wnt5b, and Wnt7b are expressed in the ventricular zone of the OB, whereas Wnt2b and Wnt9a are expressed throughout the OB (supplemental Fig. 2, available at www.jneurosci.org as supplemental material, and data not shown). Wnt5b is expressed in the mesenchyme surrounding the OB (AFS), near the βgal+ nuclei (Fig. 3C; supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
We examined expression of Fzd genes in the olfactory system of E12.5–E14.5 WT embryos (Fig. 3D–G; Table 4). Fzd3 is expressed throughout the OE–VNO and OB; Fzd4, Fzd7, and Fzd9 are expressed in the mesenchyme surrounding the OB, near the βgal+ nuclei. We decided to determine the position of the βgal+ nuclei relative to the expression of Wnt and Fzd genes. We hybridized sections from E14.5 BATnlacZ embryos for Wnt4, Wnt5b, Wnt7b, Fzd1, Fzd3, Fzd4, Fzd7, and Fzd9 and immunostained the sections with anti-βgal. Stained nuclei were found within the Fzd7 expression domain around the OB (Fig. 3E), adjacent (but internally) with respect to the Wnt5b domain (Fig. 3C) but distant from the Fzd1, Fzd4, and Fzd9 (mesenchymal) domains (Fig. 3F). Furthermore, βgal+ nuclei were found externally to the Fzd3 (OB) domain (Fig. 3D), as expected. No other Fzd gene expression colocalized with the position of the βgal+ nuclei. Thus, the βcatenin-activated cells on the surface of the OB most likely respond to the Wnt signal(s) via Fzd7. A modest increase in Fzd7 expression is observed after OP removal (supplemental Fig. 2A, available at www.jneurosci.org as supplemental material), possibly reflecting the absence of the activating signal.
Wnt7b and Wnt5a are sufficient to activate βcatenin response.
Of the OP-expressed Wnt genes, Wnt2b, Wnt4, Wnt7b, and Wnt9a are known activators of the canonical pathway. Expression of Wnt5b, in the mesenchyme around the FB, could also play a role. Wnt5b displays the highest sequence identity (>95%) with Wnt5a, known to activate the βcatenin pathway when interacting specifically with Fzd7 (Zou, 2004). Wnt4, expressed only in the respiratory epithelium, is unlikely to activate Fzd receptors in the AFS. We transfected Wnt4-, Wnt5a-, and Wnt7b-mycTAG expression plasmids into COS7 cells (Liu et al., 2005) and used these cells as the feeder layer for slice cultures of E11.5 BATnlaZ embryos (Fig. 4A). The OP was removed from one side of each slice, leaving the counterlateral OP for control. Wnt7b- and Wnt5a-expressing COS7 cells restored βgal+ nuclei in, respectively, 8 of 10 and 12 of 14 slices, whereas the control Wnt4-expressing or mock-transfected COS7 cells did not (Fig. 4B–E). On intact slices, we did not observe any significant increase in X-gal+ cells, suggesting that all potentially responsive cells are normally activated by the OP (data not shown). These data show that Wnt7b or Wnt5a alone are sufficient to activate the βcatenin pathway in the olfactory-associated, Wnt-responsive cells. However, a potential role for Wnt2b or other Wnts expressed by the OP or the mesenchyme around the OB cannot be excluded.
Figure 4.
Wnt7b and Wnt5a are sufficient to activate βcatenin response on the surface of the FB. A, Detection of Wnt-mycTAG proteins in COS7 transfected with the indicated expression plasmids. mycTAG+ cells are indicated (white arrows). 4′,6-Diamidino-2-phenylindole (DAPI) was used for counterstaining. B–E, Effect of Wnt4-, Wnt5a-, and Wnt7b-expressing feeder layers (C–E) on transfected E11.5 BATnlacZ embryos, from which the OP has been removed, compared with slices maintained with mock-transfected cells (B). X-gal+ nuclei are rescued in the presence of Wnt5a and Wnt7b but not Wnt4. F–I, Depletion of Wnt-mycTAG protein in transfected COS7 cells after treatment with the control (F, H) and the Wnt7b siRNA (G, I). mycTAG+ cells are indicated (white arrows). Insets, Uptake of the fluorescent siRNA duplex and DAPI counterstaining. J–K′, Detection of the Alexafluor488-labeled (control; J, J′) and Cy3-labeled (Wnt7b; K, K′) siRNA duplex in transfected slices. J′ and K′ are overlays of bright-field and fluorescence images. L, M, Effect of control (L) and Wnt7b (M) siRNA on slices from E11.5 BATnlacZ embryos. The number of X-gal+ nuclei is significantly reduced. OPL, olfactory placode.
Wnt7b is partly required to activate βcatenin response
At early stages of development, Wnt7b is expressed in the OP and in the FB (Fig. 3G; Suppl. Fig. 2C). We hypothesized that if exogenous Wnt7b can activate βgal cells in BATnlacZ organotypic slices in the absence of an OP, then inhibition of the Wnt7b signal should abolish βgal expression when an OP is present. To inhibit Wnt7b, we used an siRNA approach to reduce endogenous Wnt7b production. First, we tested Wnt7b and control siRNA on COS7 cells transfected with a Wnt7b-mycTAG expression plasmid: a >85% reduction in the number of mycTAG+ cells was observed after transfection of Wnt7b siRNA compared with control siRNA (Fig. 4F–I). Next, we transfected slices of E11.5 BATnlacZ embryos and maintained them in culture for 48 h. Wnt7b siRNA resulted in a clear reduction in the number of X-gal+ cells in the AFS (−60 to −80%; five of six slices), whereas the control siRNA had no effect (eight of eight) (Fig. 4L,M). siRNA duplexes were tagged with Alexafluor488 and Cy3, and we could verify the uptake of the siRNA duplexes both in COS7 cells and in cultured slices by fluorescence microscopy (Fig. 4J,K′ and insets). Real-time qPCR on RNA extracted from pools of dissected OP showed a modest 15–20% reduction in the Wnt7b mRNA in samples treated with Wnt7b siRNA compared with control siRNA (data not shown). However, even this modest reduction in mRNA resulted in a pronounced reduction in the activation the βcatenin cells in the AFS. Together, these results show that Wnt7b is a major component of the activation of the βcatenin pathway on the surface of the OB. The presence of residual X-gal+ cells after treatment with Wnt7b siRNA is an indication of the role of other Wnts expressed by the OE and by the mesenchyme surrounding the OB.
Canonical Wnt signals are required to activate βcatenin response
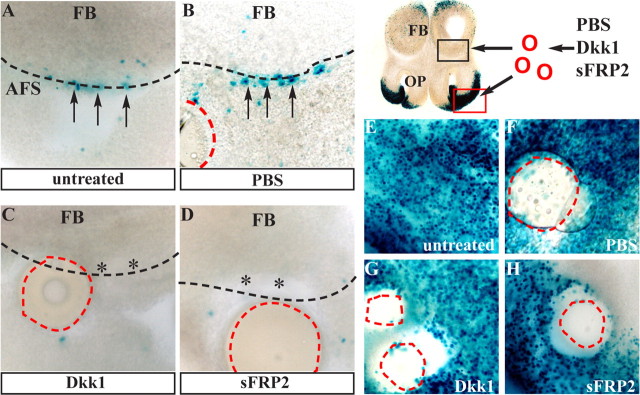
If canonical Wnt signals are essential for the observed activation of the βcatenin response in the AFS, then antagonists to this pathway should also abolish this response. We used recombinant Dkk1 and sFRP2 as antagonists. These two naturally occurring polypeptides are expressed during embryonic development and can inhibit the canonical Wnt–Fzd pathway extracellularly. Dkk1 blocks the interaction of Fzd receptors with LDL (low-density lipoprotein) receptor-related protein (Kawano and Kypta, 2003), whereas sFRPs resemble membrane-bound Fzd and competitively bind to Wnts to reduce the level of ligand available to interact with the receptors. Importantly, both Dkk1 and sFRP2 are known or proposed to specifically interfere with signaling by Wnt7a and Wnt7b (Kim et al., 2001; Caricasole et al., 2003; Kawano and Kypta, 2003), which are >95% identical. We therefore assayed the efficacy of these inhibitors on slice cultures of BATnlacZ embryos by placing beads soaked with Dkk1 or sFRP2, either on the AFS (Fig. 5A–D) or on the frontonasal processes (Fig. 5E–H). Dkk1 and sFRP2 were able to drastically reduce the number of X-gal+ nuclei in the AFS (Fig. 4C,D; Table 5). In the frontonasal processes, in which the Wnt–βcatenin response is very abundant, X-gal+ nuclei were reduced only in the proximity of the beads (Fig. 4G,H). Control slices untreated or treated with PBS-soaked beads showed no alteration in X-gal staining. Thus, both inhibitors are effective in blocking the Wnt canonical signal in cultured slices.
Figure 5.
A–D, Blocking canonical Wnt signal in the AFS of E11.5 BATnlacZ embryos, with beads incubated with Dkk1 (C) or sFRP2 (D); as control, the untreated side (A) or PBS-soaked bead (B) are shown. E–H, Same as before (A–D), except that beads were placed onto the frontonasal processes. The number of X-gal+ nuclei is reduced near the bead (G, H). The control side (untreated or PBS) is shown (E, F). Black arrows and asterisks indicate, respectively, presence and absence of X-gal-stained nuclei. The position of the bead is shown with dashed red lines.
Table 5.
Effect of Wnt/Fzd inhibition on olfactory connection: inhibition of βcatenin activation
aNumber of slices in which X-gal+ nuclei were significantly reduced in number (at least −70%), after the application of the Wnt inhibitor.
bNumber of slices in which X-gal+ nuclei were absent.
Wnt/Fzd antagonists Dkk and sFRP inhibit olfactory connectivity
To examine the role of Wnt–βcatenin signaling at the surface of the FB in ORN axons connectivity, the trajectory of ORN axons was visualized using transgenic embryos expressing the fluorescent marker eGFP under control of the tau promoter (Tucker et al., 2001). We readily detected eGFP fluorescence in early olfactory axons in slice cultures (Fig. 6A,A′). Beads soaked in Wnt/Fzd inhibitors were placed near the AFS of E11.5 tau-eGFP slices and maintained for 24 h in culture.
Figure 6.
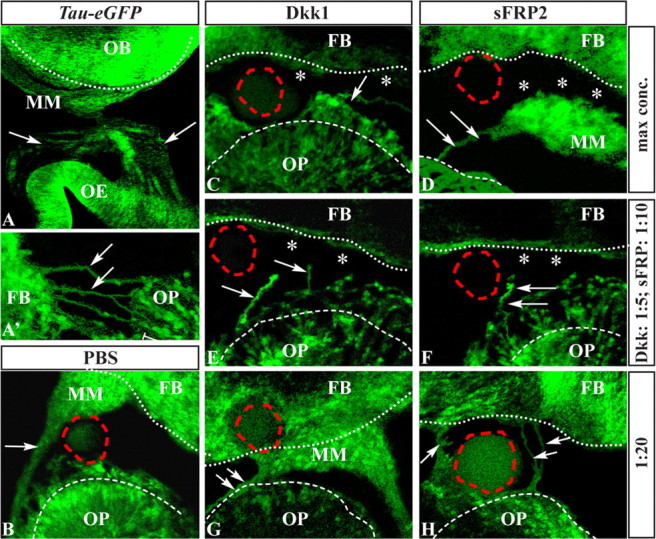
Wnt signaling on the surface of the OB is required for axon connectivity. Experimental design are as in Figure 4. A, A′, Coronal and sagittal sections of tau-eGFP embryos at E14.5, to show the olfactory nerves (white arrows) and the MM. B–H, Effect of Wnt/Fzd inhibition on the trajectory and connectivity of ORN axons, in slices of E11.5 tau-eGFP embryos, using beads soaked with PBS (B), Dkk1 (C, E, G), or sFPR2 (D, F, H) at different concentrations (top row, maximum; middle row, intermediate; bottom row, lower). Note the altered nerve trajectory (white arrows) and the lack of nerve-to-FB contact (white asterisks) using the maximum concentration and the 1:10 dilution of the inhibitors. Beads soaked with Dkk1 and sFRP2 at the dilution 1:20 or with PBS had little or no effect (B, G, H).
When Dkk1 or sFRP was used, frequently the axon bundles failed to reach the FB, and most processes remained near the OP or adopted an abnormal trajectory (Dkk1 maximum concentration, 14 of 16; sFRP2 maximum concentration,19 of 19) (Fig. 6C–H; Table 6). In contrast, when PBS or highly diluted (1:20) Dkk1 or sFRP2 was used, alterations of axon trajectory were rarely observed; axon bundles coursed around the beads to reach the FB (PBS, 28 of 34; Dkk1, 8 of 9; sFRP2, 14 of 16).
Table 6.
Effect of Wnt/Fzd inhibition on olfactory connection: effect on ORN axons, at maximal concentration
| PBS | Dkk1 | sFRP2 | |
|---|---|---|---|
| Totala | 34 | 16 | 19 |
| Normal trajectory and connectionb | 28 (82%) | 2 (12%) | 0 (0%) |
| Lack of connection onlyc | 6 (18%) | 11 (68%) | 15 (79%) |
| Lack of connection and altered trajectoryd | 0 | 3 | 4 |
aNumber of slices in which ORN axons could be visualized.
bNumber of slices in which ORN axons were normally oriented and contacted the FB.
cNumber of slices in which ORN axons were normally oriented but failed to contact the FB.
dNumber of slices in which ORN axons show abnormal trajectory and (consequently) lack of connection.
Misguided axons may fail to contact the FB as a consequence of altered trajectory or have intrinsic problems of connectivity independent of the trajectory. To begin to distinguish “guidance” versus “connectivity” defects, we soaked the beads with an intermediate concentration of Dkk1 or sFRP2 (1:5 and 1:10, respectively): this condition rarely elicited drastic changes on axon trajectory, whereas the effect on connectivity was prominent (Fig. 6E,F; Table 7). Thus, blocking of the Wnt-βcatenin response in the AFS can affect connectivity with the FB even when axons adopt a “normal” trajectory.
Table 7.
Effect of Wnt/Fzd inhibition on olfactory connection: effect on ORN axons, at reduced concentration
| Dkk1 |
sFRP2 |
|||
|---|---|---|---|---|
| 1:5 | 1:20 | 1:10 | 1:20 | |
| Totala | 6 | 9 | 7 | 16 |
| Normal trajectory and connectionb | 1 (17%) | 8 (88%) | 1 (14%) | 14 (87%) |
| Lack of connection onlyc | 4 (67%) | 1 (11%) | 5 (71%) | 2 (12%) |
| Lack of connection and altered trajectoryd | 1 | 0 | 1 | 0 |
aNumber of slices in which ORN axons could be visualized.
bNumber of slices in which ORN axons were normally oriented and contacted the FB.
cNumber of slices in which ORN axons were normally oriented but failed to contact the FB.
dNumber of slices in which ORN axons show abnormal trajectory and (consequently) lack of connection.
Discussion
Morphogenesis and wiring of the embryonic olfactory system requires interactions between cells and signals of different origin, including the OP, migratory cells, nasal mesenchyme, and FB (Bailey et al., 1999; Lin and Ngai, 1999; St. John et al., 2002; Bashin et al., 2003; Brunjes and Greer, 2003; Nedelec et al., 2005). Here we describe a novel Wnt-responsive cell population, closely associated with the surface of the FB at the location of the initial ORN axon-to-FB contact. Activation of the βcatenin pathway in these cells is associated with expression of the Fzd7 receptor gene in the same region but critically depends on initiating signals from the OP, among which Wnt7b is sufficient to perform this function. Blocking Wnt/Fzd activation in the region of axon contact results in a severely reduced number of ORN axons reaching the FB. These data suggest that neurite-induced Wnt–βcatenin activation is needed for the establishment of the early olfactory pathway, a novel function for this pleiotropic family of morphogens. We propose that the OP-derived Wnt signal is released and/or active during axon–FB contact and can recruit responsive cells of mesodermal origin to participate in tissue remodeling of the primitive BM that, in turn, is essential for the formation of the nerve layer of the OB.
The appearance of the olfactory Wnt-responsive cells corresponds with early ORN axon-to-FB contact (E11–E12). The location of these cells at the interface of the FB and mesenchyme suggest several potential origins, such as migratory cells from the OP or mesenchymal cells of the BM. We have characterized these cells and have established (1) the absence of typical markers for migratory neurons (DCX and PSA-NCAM), (2) the absence of the OEC marker S100, and (3) the absence of mesenchymal markers vimentin, vinculin, and talin. βcatenin response can be restored in Dlx5−/− embryos, in which OP-derived cell migration is necessarily interrupted (Levi et al., 2003; Merlo et al., 2007), excluding a placodal migration origin of these cells. Importantly, the receptor Fzd7 is expressed in the same mesenchymal location as the βcatenin-activated cells but is excluded from the MM. Together, the data suggest that the Wnt–βcatenin-responsive cells are a population of nontypical resident mesoderm-derived cells, distinct from more typical ones.
Wnt molecules have been implicated as key morphogens in several developmental processes (Logan and Nusse, 2004). We assessed the function of “canonical” Wnt signaling in the olfactory region using assays based on cultures of BATnlacZ slices, in which axon growth and Wnt signaling is preserved. The BATnlacZ model used in this study visualizes only the canonical Wnt response (Maretto et al., 2003), which involves stabilization and nuclear translocation of βcatenin (Wang and Wynshaw-Boris, 2004; Widelitz, 2005). In general, members of the Wnt1 subclass (including Wnt1, Wnt2, Wnt3, Wnt7, and Wnt8) are potent activators of this pathway, whereas members of the Wnt5a subclass (including Wnt4, Wnt5, Wnt8, and Wnt11) are poor activators or, in some cases, inhibitors (Torres et al., 1996; Yamaguchi, 2001; Church et al., 2002; Oishi et al., 2003; Topol et al., 2003; Maye et al., 2004; Tian et al., 2005). However, signaling pathway specificity exerted by distinct Wnt molecules is highly dependent on the Fzd receptor repertoire, their coreceptors, and subcellular localization, rather than Wnt ligand (Wu et al., 2004; Mikels and Nusse, 2006). Of the first subclass, only Wnt2b, Wnt7b, and Wnt9a are expressed in the OP: here we show that exogenous Wnt7b is sufficient, and partly necessary, to rescue the βcatenin signaling after OP removal. Therefore, we hypothesize that Wnt7b is the main physiological βcatenin activator, although the contribution of other Wnts of either placodal or mesencymal origin cannot be excluded.
We also found that exogenous expression of Wnt5a is able to restore βcatenin activation on the surface of the FB. This Wnt does not usually activate the βcatenin pathway, whereas it is known to promote βcatenin degradation (Topol et al., 2003). Wnt5b (the closest homolog to Wnt5a) is expressed in the AFS, the location where ORN axons/MM contact the FB, adjacent to the βgal+ nuclei. Wnt5b is endogenously expressed around the OB and might also contribute to the activation of βcatenin in the AFS, possibly secondary to the arrival of the ORN in proximity of the OB. Our observation on the activity of exogenously added Wnt5a is in accord with the fact that the Fzd7 gene, the only Fzd receptor expressed in the AFS, is known to mediate both βcatenin-dependent (Linker et al., 2005; Abu-Elmaqd et al., 2006) and βcatenin-independent (DeCalisto et al., 2005) signaling. Furthermore, Wnt5a can specifically activate the βcatenin signal in the presence of the Fzd7 (Zou, 2004) or the Fzd4 (Mikels and Nusse, 2006) receptors. We have detected expression of Fzd7, but not Fzd4, in the Wnt–βcatenin-responsive cell location, therefore the Wnt5b–Fzd7 pathway can potentially contribute to βcatenin activation on the FB surface. Thus, at least two Wnt molecules can activate the βcatenin pathway on the FB surface, suggesting some degree of redundancy. Conversely, depletion of Wnt7b reduces βcatenin activation in the responsive cells, thus Wnt7b is likely to be the primary signal. Finally, the role of “noncanonical” Wnt genes expressed by the OP cells is unclear. As shown for Wnt5a (Topol et al., 2003), noncanonical Wnt can modulate the βcatenin-activating function of the canonical ones, or alternatively perform as yet unknown functions.
Our data indicate that the initial signal for the activation of Wnt–βcatenin response is derived from the OP. In fact, grafting of a WT OP onto slices from Dlx5−/− embryos restores the βcatenin response in this region. Thus, we must postulate that the ORN axon-derived signal(s) is initially required for activating the βcatenin response in cells adjacent to the surface of the FB. Together with the observation that Wnt-βcatenin signaling is required for ORN axons to target the FB, these data show a novel function of specific Wnt genes. This class of morphogens is required for (centripetal-type) connectivity of olfactory axons with the CNS.
Blocking Wnt/Fzd activation with exogenous Dkk1 and sFRP2 results in reduced growth, abnormal orientation, and lack of axon–FB connectivity. In this experimental setup, however, both connectivity and axon guidance seem to be affected. It is important to note that axon-guidance functions for Wnt molecules have been demonstrated recently: in vivo Wnt4/Fzd3 signaling guide dorsal commissural axons in the embryonic spinal chord, and in vitro Wnt1, Wnt4, Wnt5a, Wnt6, and Wnt7b polypeptides can stimulate axon extension (Lyuksyutova et al., 2003; Liu et al., 2005). Interestingly, the anterior FB expresses the Wnt4 gene, and the embryonic OP expresses the Fzd3 receptor. Therefore, Wnt signaling might logically contribute to elongation and guidance of newly sprouted ORN neuritis during development of the olfactory system, expanding the guidance functions of Wnt molecules to the peripheral neurites (Zou, 2004; Bovolenta, 2005; Ille and Sommer 2005). This interesting possibility will have to be investigated with appropriate models and tools.
OB innervation is thought to be coordinated by local interactions between the ORN axons, migratory cells, and the BM that surrounds the FB, whereas the FB itself does not seem to play a major role (Levi et al., 2003; Hirata et al., 2006). The molecular events leading to BM fenestration and formation of the nerve layer are primarily unknown. ORN axons arrive near the BM of the anterior FB and organize in the MM, much before the neurites are able to contact the FB, with the exception of the pioneer fibers, the function for connectivity of which remains elusive. Abundant innervation of the presumptive OB is visible around E13–E14, when the BM separating the MM from the FB parenchyma fenestrates extensively and allows the MM to wrap onto the surface of the OB (Doucette, 1989). The position of Wnt-responsive cells, at the interface of the BM and FB at early stages, suggests that these cells may play a role in cell–cell and cell–extracellular matrix (ECM) interactions that coordinate ECM degradation and remodeling necessary for the formation of the nerve layers and synaptic contact. In Dlx5−/− embryos, the BM remains unfenestrated (our unpublished results), and the Wnt–βcatenin response in the same region is strongly reduced, despite the fact that the mutant ORN axons do come to a close proximity with the FB (Merlo et al., 2007). Interestingly, fibronectin has been reported to be a direct target of Wnt/Wg signaling in Xenopus fibroblasts (Gradl et al., 1999), and βcatenin is known to be associated with cell adhesion complexes and to regulate cell–substrate adhesion (Gottardi and Gumbriner, 2004). One possibility is that Wnt signaling on the surface of the OB activates the ECM remodeling functions of resident mesenchymal cells.
Our data suggest a model (Fig. 7) in which early olfactory axons extend through the nasal mesenchyme and approach the FB conveying OP-derived Wnt molecules to the mesenchyme surrounding the OB. This activates a Wnt-dependent βcatenin response pathway in Fzd7-expressing resident cells, before the formation of the nerve layer. Among the OP-expressed Wnt molecules, Wnt7b is sufficient and partly necessary for the activation of the pathway, but Wnt2b and Wnt9a may complement Wnt7b function. The OP is also likely to convey signals that activate or induce the release of mesenchymal Wnt5b, able to activate βcatenin on the surface of the FB through Fzd7. Subsequently, βcatenin-activated cells are possibly linked to the fenestration of the BM and OB innervation. This newly revealed role of Wnt molecules in olfactory connectivity contributes to clarify how distinct cell types interact on the surface of the anterior FB to coordinate this complex developmental process.
Figure 7.
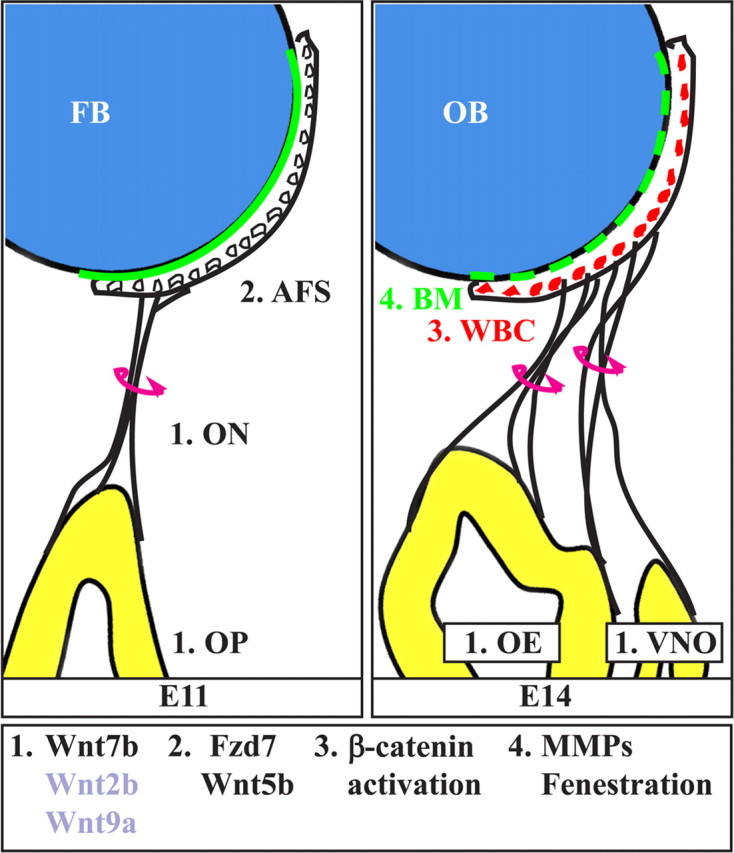
Model of Wnt signaling in olfactory connectivity. The stages depicted are E11–E14. The AFS is indicated with a white crescent, and the Wnt–βcatenin cells (WBC) are depicted in red, near the surface of the FB (blue). The BM is shown with a solid (E11) or a dashed (E14) green line on the surface of the FB. Initial axon sprouting and elongation is controlled cell-autonomously by transcription factors (such as Emx2, Dlx5, and Fez1) and locally by early patterning cues. ORN cells express canonical Wnt genes; among these, Wnt7b is sufficient for the subsequent functions (step 1). Axons elongate and contact the FB within the AFS region (step 2); this contact is required for delivery and/or release of βcatenin-activating Wnt molecules. Cells resident on the surface of the OB express Fzd7 and activate the βcatenin pathway in response to Wnts (step 3). We hypothesize that these cells are involved in the subsequent remodeling and fenestration of the BM (step 4).
Footnotes
G.R.M. is the recipient of a Telethon Career Award (TCP99003) and was supported by Fondazione Cariplo (2005–2007) and Fondazione San Paolo (2005, 2007 (Dulbecco Telethan Institute). A.A.Z. is in the Doctorate Program of the Department of Comparative Anatomy at the University of Milano. S.Pa. was supported by Ingenio (Italy). S.Pi. was supported by Telethon-Italy. A.P. was supported by National Institutes of Health–National Institute on Deafness and Other Communication Disorders Grant DC05739. P.P. was supported by Fondazione San Paolo (2004, 2019) and CIPE Reg. Piemonte (2004, A14). We thank Dr. A. McMahon (Harvard University, Boston, MA), Dr. S. Pleasure (University of California San Francisco, San Francisco, CA), Dr. R. Nusse (Stanford University, Stanford, CA), and Dr. L. Muzio (DiBit-San Raffaele, Milan, Italy) for probes. We thank Dr. Y. Zou (University of California San Diego, La Jolla, CA) for the Wnt-myc expression plasmids. We are indebted to Dr. K. Tucker (Center of Neuroscience, University of Heidelberg, Heidelberg, Germany) for the tau-eGFP mice, to Prof. U. Fascio (CIMA, University of Milano, Milano, Italy) for confocal microscopy, and to Drs. S. Bertuzzi and H. Arnheiter (National Institute of Neurological Disorders and Stroke, Bethesda, MD) for helpful criticism.
References
- Abu-Elmaqd M, Garcia-Morales C, Wheeler GN. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev Biol. 2006;298:285–298. doi: 10.1016/j.ydbio.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Acampora D, Merlo GR, Paleari L, Zerega B, Mantero S, Barbieri O, Postiglione MP, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the distal-less-related gene Dlx5. Development. 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- Astic L, Pellier-Monnin V, Saucier D, Charrier C, Mehlen P. Expression of netrin-1 and netrin-1 receptor, DCC, in the rat olfactory nerve pathway during development and axonal regeneration. Neuroscience. 2002;109:643–656. doi: 10.1016/s0306-4522(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Au WW, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. J Comp Neurol. 2002;446:68–80. doi: 10.1002/cne.10182. [DOI] [PubMed] [Google Scholar]
- Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb: evidence for glia-neuron interactions in the glomerular formation. J Comp Neurol. 1999;415:423–448. [PubMed] [Google Scholar]
- Bashin N, Maynard TM, Gallagher PA, LaMantia AS. Mesenchymal-epithelial regulation of retinoic acid signaling in the olfactory placode. Dev Biol. 2003;261:82–98. doi: 10.1016/s0012-1606(03)00295-1. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P. Morphogen signaling at the vertebrate growth cone: a few case or a general strategy? J Neurobiol. 2005;64:405–416. doi: 10.1002/neu.20161. [DOI] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunjes PC, Greer CA. Progress and directions in olfactory development. Neuron. 2003;38:371–374. doi: 10.1016/s0896-6273(03)00263-0. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraischi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Ferraro T, Iacovelli T, Barletta E, Caruso A, Melchiorri D, Terstappen G, Nicoletti F. Functional characterization of Wnt7a signaling in PC12 cells: interaction with a FZD5 × LRP6 receptor complex and modulation by Dickkopf proteins. J Biol Chem. 2003;278:37024–37031. doi: 10.1074/jbc.M300191200. [DOI] [PubMed] [Google Scholar]
- Church V, Tsutomu N, Linker C, Marcell C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- DeCalisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signaling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- Dode C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med. 2004;82:725–734. doi: 10.1007/s00109-004-0571-y. [DOI] [PubMed] [Google Scholar]
- Doucette R. Development of the nerve fiber layer in the olfactory bulb of mouse embryos. J Comp Neurol. 1989;285:514–527. doi: 10.1002/cne.902850407. [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. Distribution of laminin and collagens during avian neural crest development. Development. 1987;101:461–478. doi: 10.1242/dev.101.3.461. [DOI] [PubMed] [Google Scholar]
- Fueshko S, Wray S. LHRH cells migrate on peripherin fibers I embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166:331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- Gao PP, Sun CH, Zhou XF, DiCicco-Bloom E, Zhou R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J Neurosci Res. 2000;60:427–436. doi: 10.1002/(SICI)1097-4547(20000515)60:4<427::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gong Q, Shipley MT. Expression of extracellular matrix molecules and cell surface molecules in the olfactory nerve pathway during early development. J Comp Neurol. 1996;366:1–14. doi: 10.1002/(SICI)1096-9861(19960226)366:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gonzàlez-Martinez D, Hu Y, Bouloux PM. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann's syndrome. Front Neuroendocrinol. 2004;25:108–130. doi: 10.1016/j.yfrne.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix, cell skeletons, and embryonic development. Am J Med Genet. 1989;34:14–29. doi: 10.1002/ajmg.1320340107. [DOI] [PubMed] [Google Scholar]
- Hirata T, Nazakawa M, Yoshihara S, Miyachi H, Kitamusa K, Yoshihara Y, Hibi M. Zinc-finger gene Fez in the olfactory sensory neurons regulates development of the olfactory bulb non-cell-autonomously. Development. 2006;133:1433–1443. doi: 10.1242/dev.02329. [DOI] [PubMed] [Google Scholar]
- Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard AK, Hartmann DJ. Spatiotemporal patterns of expression of extracellular matrix molecules in the developing and adult rat olfactory system. Neuroscience. 1998;84:1135–1150. doi: 10.1016/s0306-4522(97)00544-7. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Key B. Molecular development of the olfactory nerve pathway. Ann NY Acad Sci. 1998;855:76–82. doi: 10.1111/j.1749-6632.1998.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and WNT-7b in the developing CNS. J Neurosci. 2001;21:RC312. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Bashin N, Rhodes K, Heemskerk J. Mesenchymal-epithelial induction mediates olfactory pathway formation. Neuron. 2000;28:411–425. doi: 10.1016/s0896-6273(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Levi G, Puche AC, Mantero S, Barbieri O, Trombino S, Paleari L, Egeo A, Merlo GR. The Dlx5 homeodomain gene is essential for olfactory development and connectivity in the mouse. Mol Cell Neurosci. 2003;22:530–543. doi: 10.1016/s1044-7431(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;9:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Lin DM, Ngai J. Development of the vertebrate main olfactory system. Curr Opin Neurobiol. 1999;9:4–8. doi: 10.1016/s0959-4388(99)80009-9. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. β-catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132:3895–3905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu C, Wang Z, Lyuksyutova AI, Song X, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Depew MJ, Tobet S, Rubenstein JLR. DLX5 regulates development of peripheral and central components of the olfactory system. J Neurosci. 2003;23:568–578. doi: 10.1523/JNEUROSCI.23-02-00568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance af commissural axons by Wnt-frizzled signalling. Science. 2003;302:1903–1904. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan G, Piccolo S. Mapping the wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11 mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo GR, Paleari L, Mantero S, Zerega B, Adamska M, Rinkwitz S, Bober E, Levi G. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-dependent pathway. Dev Biol. 2002;248:157–169. doi: 10.1006/dbio.2002.0713. [DOI] [PubMed] [Google Scholar]
- Merlo GR, Mantero S, Zaghetto A, Peretto P, Paina S, Gozzo M. The role of Dlx homeogenes in early development of the olfactory pathway. J Mol Histol. 2007 doi: 10.1007/s10735-007-9109-2. in press. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits b-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:570–582. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec S, Dubacq C, Trembleau A. Morphological and molecular features of the mammalian olfactory sensory neuron axons: what makes these axons so special? J Neurocytol. 2005;34:49–64. doi: 10.1007/s11068-005-5047-7. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuchi H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase ROR2 is involved in non-canonical Wnt5a/JNK signallng pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Puche AC, Poirier F, Hair M, Bartlett PF, Key B. Role of galectin-1 in the developing mouse olfactory system. Dev Biol. 1996;179:274–287. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- Rugarli E, Ghezzi C, Valsecchi V, Ballabio A. The Kallmann sindrome gene product expressed in COS cells is cleaved on the cell surface to yield a diffusible component. Hum Mol Genet. 1996;5:1109–1115. doi: 10.1093/hmg/5.8.1109. [DOI] [PubMed] [Google Scholar]
- Scott JW, Brirley T. A functional map in rat olfactory epithelium. Chem Senses. 1999;24:679–690. doi: 10.1093/chemse/24.6.679. [DOI] [PubMed] [Google Scholar]
- St John JA, Clarris HJ, Key B. Multiple axon guidance cues establish the olfactory topographic map: how do these cues interact? Int J Dev Biol. 2002;46:639–647. [PubMed] [Google Scholar]
- Tarozzo G, Peretto P, Fasolo A. Cell migration from the olfactory placode and the ontogeny of the neuroendocrine compartment. Zool Sci. 1995;12:367–383. doi: 10.2108/zsj.12.367. [DOI] [PubMed] [Google Scholar]
- Tian Q, Jin H, Cui Y, Guo C, Lu X. Regulation of Wnt gene expression. Development. 2005;47:273–281. doi: 10.1111/j.1440-169X.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- Tisay KT, Bartlett PF, Key B. Primary olfactory axons form ectopic glomeruli in mice lacking p75-NTR. J Comp Neurol. 2000;428:656–670. doi: 10.1002/1096-9861(20001225)428:4<656::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garret-Beal L, Carolan PJ, Yang Y. Wnt5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, De Marais AA, McGrew LL, Moon RT. Activities of Wnt-1 class of secreted signalling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HB, Nurcombe V, Key B. Expression of extracellular matrix molecules in the embryonic rat olfactory pathway. J Neurobiol. 1996;31:41–55. doi: 10.1002/(SICI)1097-4695(199609)31:1<41::AID-NEU4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Tomasiewicz H, Magnuson T, Key B. The central pathway of primary olfactory axons is abnormal in mice lacking the NCAM-180 isoform. J Neurobiol. 1997;32:643–658. doi: 10.1002/(sici)1097-4695(19970620)32:7<643::aid-neu1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–116. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Williams-Hogarth LC, Puche AC, Torrey C, Cai X, Song I, Kolodkin AL, Shipley MT, Ronnett GV. Expression of semaphorins in developing and regenerating olfactory epithelium. J Comp Neurosci. 2000;423:565–578. [PubMed] [Google Scholar]
- Wray S, Grant P, Gaine H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Klein TJ, Mlodzik M. Subcellular localization of Frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:1004–1014. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]