Abstract
Background
Pregnancy outcomes following in utero exposure to antiepileptic drugs (AEDs) are uncertain, limiting an evidenced-based approach.
Objective
To determine if fetal outcomes vary as a function of different in utero AED exposures.
Methods
This ongoing prospective observational study across 25 epilepsy centers in the USA and UK enrolled pregnant women with epilepsy from October 1999 to February 2004 to determine if differential long-term cognitive and behavioral neurodevelopmental effects exist across the four most commonly used AEDs. This initial report focuses on the incidence of serious adverse outcomes including major congenital malformations (which could be attributable to AEDs) or fetal death. A total of 333 mother/child pairs were analyzed for monotherapy exposures: carbamazepine (n = 110), lamotrigine (n = 98), phenytoin (n = 56), and valproate (n = 69).
Results
Response frequencies of pregnancies resulting in serious adverse outcomes for each AED were as follows: carbamazepine 8.2%, lamotrigine 1.0%, phenytoin 10.7%, and valproate 20.3%. Distribution of serious adverse outcomes differed significantly across AEDs and was not explained by factors other than in utero AED exposure. Valproate exhibited a dose-dependent effect.
Conclusions
More adverse outcomes were observed in pregnancies with in utero valproate exposure vs the other antiepileptic drugs (AEDs). These results combined with several recent studies provide strong evidence that valproate poses the highest risk to the fetus. For women who fail other AEDs and require valproate, the dose should be limited if possible.
Treatment decisions for women with epilepsy are difficult due to conflicting risks. Although the majority of children born to women with epilepsy are normal, these women are at increased risk for complications during pregnancy, and their children are at increased risk for poor outcomes.1–2 Risks include prematurity, low birth weight, increased fetal and neonatal death rates, congenital malformations, and developmental delay. Congenital malformations are more likely in children exposed in utero to antiepileptic drugs (AEDs) and are increased with higher AED dosages, higher AED serum levels, or polytherapy.3 The most common major anatomic abnormalities associated with AEDs are heart malformations (e.g., ventricular septal defect), orofacial defects (e.g., cleft lip with or without cleft palate), urologic defects (e.g., hypospadius), skeletal abnormalities (e.g., radial ray defects, phalangeal hypoplasia), and neural tube defects (e.g., spina bifida).3 In addition to anatomic defects, in utero AED exposure has been associated with behavioral/cognitive defects.4–6 The risk of AED teratogenesis must be balanced against potentially grave risks posed by seizures to both the mother and the child. Maternal deaths during pregnancy in women with epilepsy are 10 times more common than in women without epilepsy; this increase appears to be due to seizures, which are often related to discontinuing AED therapy or poor compliance.7
Treatment decisions for women with epilepsy are further complicated by the lack of evidence-based data on the comparative teratogenic potential of different AEDs. The most recent consensus guidelines by the American Academy of Neurology,8 the American College of Obstetricians and Gynecologists,9 and the International League Against Epilepsy10 could not delineate if differential risks of teratogenesis exist across AEDs. The guidelines might be summarized as follows: 1) optimize treatment prior to conception, 2) use monotherapy if possible, 3) choose the most effective AED for seizure type and syndrome, 4) use the lowest effective dose, 5) supplement with folate, 6) treat the child with vitamin K at birth and possibly the mother late in pregnancy for AEDs that interfere with vitamin K. However, no recommendations related to differential AED teratogenetic risk are offered. The current report provides data from a prospective study demonstrating a differential incidence of serious adverse outcomes (i.e., major congenital malformations and fetal deaths) across the four most commonly used AEDs.
Methods
Design
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) Study is a prospective observational investigation that has enrolled mother/child pairs during pregnancy across 25 centers in the USA and UK. The primary aim of the study is to determine the long-term effects of AED monotherapy on the children’s neurodevelopmental outcomes. The study is designed to follow the children to 6 years of age to determine if behavioral and cognitive effects from in utero AED exposure differ across the four most commonly used AEDs. Separate studies with similar design were begun in the USA and UK and later were combined. As part of the prospective data collection, adverse events occurring in the child or mother were recorded. This report involves the incidence of serious adverse outcomes early in the course of the investigation. Serious adverse outcomes were defined as major congenital abnormalities (which could be attributable to AEDs) or fetal death. Evaluation of cognitive/behavioral outcomes is still ongoing and not included in this report.
Patients
The study was approved by the institutional review board of each participating center. Informed consent was obtained from each mother. The subjects included pregnant women with epilepsy treated with AED monotherapy. Enrollment was limited to mothers on one of the four most commonly used AEDs in pregnant women with epilepsy: carbamazepine (CBZ), lamotrigine (LTG), phenytoin (PHT), and valproate (VPA). The number of women using other AEDs in monotherapy was insufficient to assess outcomes statistically. Exclusion criteria included mothers with IQ < 70, positive syphilis serology, positive HIV, progressive cerebral disease, presence of other major medical illness (e.g., diabetes, cancer), exposure to known teratogens during pregnancy other than AEDs, poor compliance with prenatal care, history of drug abuse (including alcohol) in the year prior to pregnancy, or presence of sequelae to drug abuse.
Procedures
Pregnancy outcomes were monitored. All reports of adverse events were reviewed and classified by the principal investigator, and written reports of physical abnormalities were assessed by a board-certified medical geneticist to determine if they should be classified as major malformations that could be attributed to AEDs. A major malformation is considered to be an inherent alteration in embryogenesis that results in significant impairment of health, longevity, or functional capacity.11 This includes structural abnormalities with surgical, medical, or cosmetic importance. Those physical variations not considered to be major malformations are minor anomalies not requiring surgical intervention, deformations, and birth marks. Malformations secondary to known genetic disorders and chromosomal abnormalities are not considered within the major malformation group for analytical purposes.
A variety of potentially confounding maternal variables were collected to ensure that systematic group differences did not produce or obscure differences in outcomes related to AED exposure. The factors included maternal IQ, education, race, seizure type and frequency, AED dosages and compliance, socioeconomic status, use of folate preconceptionally, use of alcohol, tobacco, or other drugs during pregnancy, history of prior pregnancy abnormalities, complications during current pregnancy, gestational age at enrollment, and birth weight.
Statistical analyses
The primary outcome used in these analyses is a binary indicator of the presence of either a congenital malformation or a pregnancy ending with a fetal death. The total sample size is 333 infants. The sample includes 10 sets of twins. Statistical methods included asymptotic and exact contingency table, logistic regression, and analysis of variance. Logistic regression was used to model the probability of being a case controlling for an AED group and in combination, one at a time, with each of the following covariates: mother’s age, IQ, education, race, alcohol use, tobacco use, preconceptional folate supplementation, seizure type, convulsive and nonconvulsive seizure frequency during pregnancy, socioeconomic status, history of prior pregnancy abnormalities, AED dose, child’s gestational age at enrollment, and child’s birth weight. AED dose was analyzed as a “standard dose” by first transforming the results within each AED group so dose would have mean = 0 and variance = 1. Alternative methods were considered and gave similar results. These models were also used to estimate the profile likelihood CI for the adjusted odds ratio of being a case for each AED group relative to the VPA group. All computations were conducted in SAS versions 8.2 or 9.1 and StatXact version 6.0. No attempt was made to impute missing data.
The data were obtained from the USA and UK. Prior to combining the datasets, they were reviewed for compatibility. Analyses determined that there were no significant differences for the primary comparisons between the US and UK results. Therefore, the data were pooled, but country effects were still examined to determine if inclusion of country in the model significantly impacted the interpretation of the results.
Results
There were 354 mother/child pairs enrolled in the study; 21 women (5.9%) were dropped out prior to completing their pregnancy. The number (%) of dropouts for each AED were as follows: CBZ = 6 (5.2%), LTG = 7 (6.7%), PHT = 4 (6.7%), VPA = 4 (5.5%). Thus, a total of 323 mothers and 333 children were included in the analyses. The US data included 199 mothers with seven sets of twins. In the UK, there were 124 mothers with three sets of twins. Maternal demographics are listed in table 1. Overall, maternal seizure types included 58% partial, 26% generalized (absence, myoclonic, tonic-clonic, or tonic seizures with initial bilateral cerebral involvement as indicated or suggested by EEG or clinical syndrome), 12% generalized tonic-clonic (uncertain if partial or primary generalized), and 4% other or uncertain. Most (81%) of the mothers were seizure-free during their pregnancy, and only 3% had more than five convulsions during their pregnancy. Maternal racial distributions were 80% Caucasian, 10% Hispanic, 4% black, and 5% other as classified by patient report. Pre-enrollment ultrasound was not an exclusion criterion. Overall, 46% of the children had pre-enrollment ultrasounds; most of these were at about 12 weeks’ estimated gestational age. The percentage of pre-enrollment ultrasounds for each AED was CBZ = 50%, LTG = 39%, PHT = 35%, and VPA = 50%.
Table 1.
Maternal demographics for carbamazepine (CBZ), lamotrigine (LTG), phenytoin (PHT), and valproate (VPA) groups
| CBZ | LTG | PHT | VPA | |
|---|---|---|---|---|
| Mean (SD) age, y | 30 (0.5) | 29 (0.6) | 30 (0.8) | 28 (0.7) |
| Mean (SD) IQ | 100 (17) | 101 (16) | 94 (16) | 98 (12) |
| Mean (SD) dose, mg/d, first trimester | 724 (39) | 393 (23) | 345 (15) | 930 (59) |
| Seizure types,* n (%) | ||||
| Partial | 91 (83) | 48 (49) | 39 (70) | 14 (20) |
| Generalized | 5 (5) | 32 (33) | 11 (20) | 39 (56) |
| GTCS | 7 (6) | 14 (14) | 4 (7) | 15 (22) |
| Other | 7 (6) | 4 (4) | 2 (4) | 1 (1) |
| Convulsions, † n (%) | ||||
| None | 82 (85) | 69 (80) | 40 (80) | 51 (76) |
| >5 | 2 (2) | 2 (2) | 4 (8) | 1 (1) |
Seizure types: partial = all partial seizure types including simple, complex, and secondary generalized; generalized = seizures with initial bilateral cerebral involvement as indicated or suggested by EEG or clinical syndrome (includes absence, myoclonic, tonic-clonic, and tonic seizures); GTCS = generalized tonic-clonic seizures (unknown if generalized or secondary generalized); other = other types or uncertain.
Convulsions = n (%) of mothers with convulsions during pregnancy.
The distribution of the 333 children across the four AED monotherapy groups was CBZ (n = 110), LTG (n = 98), PHT (n = 56), and VPA (n = 69). The differences in sample sizes reflect relative differences in AED usage across the tertiary medical centers of the NEAD group. Mean gestational ages at the time of enrollment for each AED group were CBZ = 19, LTG = 18, PHT = 19, and VPA = 17 weeks. Mean ages of the children at the time of analysis for each AED group were CBZ = 3.5, LTG = 2.7, PHT = 2.8, and VPA = 3.5 years old. Diagnosis of malformations was made by ultrasound in 2 children (9% of all malformations), 14 (64%) at birth, 5 (23%) between birth and 1 year old, and 1 (4%) at 73 weeks. Note that no therapeutic abortion was conducted in any mother.
Table 2 compares the percentage of cases with serious adverse outcomes (i.e., major congenital malformation or fetal death) in the four AED groups. In increasing order (exact 95% CI), they are as follows: LTG = 1.02% (0.03, 5.6), CBZ = 8.18% (3.8, 15.0), PHT = 10.71% (4.0, 21.9), and VPA = 20.29% (11.6, 31.7); the incidence of serious adverse outcomes differed across AED groups (exact Mantel–Haenszel [MH] χ2, p < 0.001). This difference is due to the poor outcomes for VPA compared with the other groups and the relatively better outcomes for LTG. The primary analysis was rerun controlling for twins and remained significant. Over 20% of the pregnancies exposed to VPA resulted in serious adverse outcome. The VPA rate is approximately twice the rates for CBZ and PHT; however, if LTG is removed from the analysis, CBZ, PHT, and VPA do not differ in a three-group comparison (exact MH, p = 0.10). In contrast, LTG, CBZ, and PHT do differ in a three-group comparison (exact MH, p = 0.04). Based on a model containing only AED group and AED dose, the estimated relative risks (95% confidence limits) for VPA relative to each AED were as follows: VPA vs CBZ = 3.03 (1.22, 7.92), VPA vs LTG = 27.11 (5.12, 502.00), and VPA vs PHT = 2.66 (0.92, 8.94).
Table 2.
Summary of serious adverse outcomes (SAOs) for carbamazepine (CBZ), lamotrigine (LTG), phenytoin (PHT), and valproate (VPA)
| CBZ | LTG | PHT | VPA | |
|---|---|---|---|---|
| Total n | 110 | 98 | 56 | 69 |
| Fetal death | 4 (3.6) | 0 (0) | 2 (3.6) | 2 (2.9) |
| Congenital malformations | 5 (4.5) | 1 (1.0) | 4 (7.1) | 12 (17.4) |
| Total SAOs | 9 (8.2) | 1 (1.0) | 6 (10.7) | 14 (20.3) |
Values are n (%).
Table 3 summarizes the results for a number of logistic regression models. The first column lists the variables of interest, the second column has the p values for the effect of the variable alone without statistical adjustment, the third column has the effect of each variable adjusted for AED added to the model, and the fourth has the effect of the AED adjusted for each specified variable. The only covariates that were significant after controlling for AED group were AED doses for both first trimester and entire pregnancy. As illustrated in the figure, the dose effect was primarily due to VPA, which is the only AED exhibiting a dose effect (median split, Fisher exact, p < 0.04). Analysis of the individual adverse outcomes revealed that fetal deaths did not differ across AEDs (p ≤ 0.20). Major congenital malformations were more common for VPA (p ≤ 0.0003). The estimated relative risks (95% confidence limits) of congenital malformation for VPA relative to each AED adjusted for standardized AED dose were as follows: VPA vs CBZ = 4.59 (1.58, 15.34), VPA vs LTG = 22.82 (4.25, 424.20), and VPA vs PHT = 2.87 (0.91, 11.02). The specific types of congenital malformations for each AED are listed in table 4.
Table 3.
Summary of logistic regressions
| Variable | p Value for variable alone, unadjusted | p Value for variable adjusted for AED in model | p Value for AED effect adjusted for variable in model |
|---|---|---|---|
| Baseline, AED only | 0.005 | ||
| Maternal age | 0.61 | 0.36 | 0.004 |
| Maternal IQ | 0.73 | 0.46 | 0.02 |
| Education (5 levels) | 0.83 | 0.65 | 0.005 |
| Post–high school education (yes/no) | 0.21 | 0.14 | 0.005 |
| Alcohol use (yes/no) | 0.76 | 0.68 | 0.005 |
| Tobacco use (yes/no) | 0.62 | 0.45 | 0.004 |
| Race(5 categories) | 0.50 | 0.54 | 0.02 |
| Race (2 categories) | 0.75 | 0.78 | 0.01 |
| Gestational age at enrollment | 0.32 | 0.26 | 0.002 |
| Birth weight | 0.71 | 0.87 | 0.003 |
| Convulsive seizures | 0.26 | 0.18 | 0.003 |
| Nonconvulsiveseizures | 0.64 | 0.29 | 0.005 |
| Folate (yes/no) | 0.40 | 0.59 | 0.005 |
| Seizure type | 0.21 | 0.42 | 0.02 |
| AED dose1st trimester | 0.004 | 0.003 | 0.004 |
| AED doseentire pregnancy | 0.003 | 0.002 | 0.004 |
| History of pregnancy abnormality | 0.74 | 0.80 | 0.005 |
| Socioeconomic status27 | 0.56 | 0.65 | 0.01 |
AED = antiepileptic drug.
Figure.
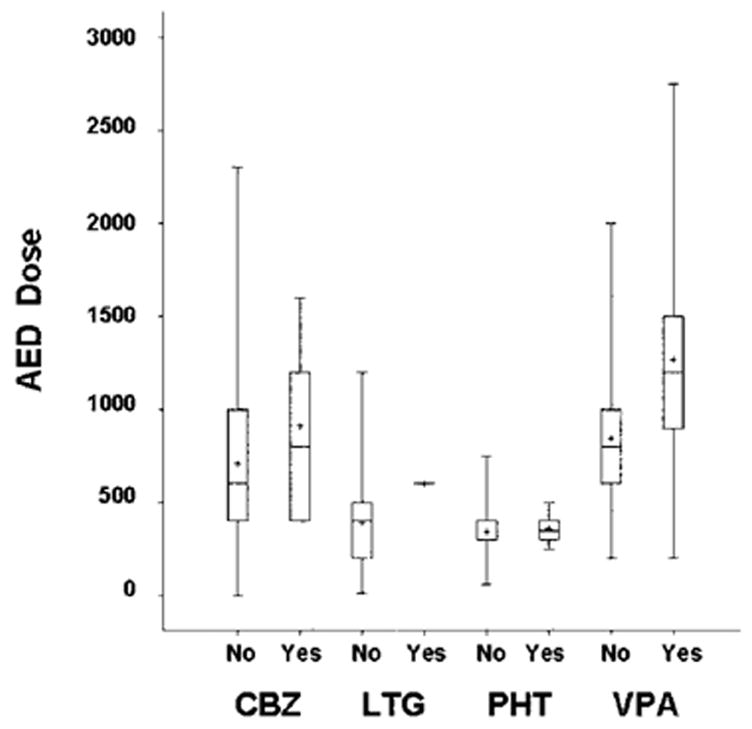
Antiepileptic drug (AED) dose and serious adverse outcomes. First trimester AED dosages (mg/day) by absence (no) or presence (yes) of serious adverse outcomes (i.e., fetal death or major malformations). Boxes = 25th through 75th percentiles; horizontal lines = medians; crosses = means; brackets = ranges.
Table 4.
Summary of congenital malformations for each AED
| AED | Brain | Cardiac | Genitourinary | Other |
|---|---|---|---|---|
| CBZ, n = 5 | Absent kidney; duplicate renal pelvis;×2 hypospadius* | Inguinal hernia* | ||
| LTG, n = 1 | Ventricular septal defect | |||
| PHT, n = 4 | Agenesis of corpus callosum | Ventricular septal defect | Hydronephrosis and extra renal pelvis;undescended testicle* | |
| VPA, n = 12 | Brachycephaly | Coarctation of aorta;hypoplastic right heart;×2 atrial septal defect†‡ | ×2 hydronephrosis;undescended testes*;×3 hypospadius*‡ | Cleft palate*; dysplastic ribs; two thumbs right hand† & third nipple†;pulmonary stenosis‡ |
Required surgical repair.
Both children with atrial septal defects had multiple malformations:
one with two thumbs on right hand and third nipple;
one with pulmonary stenosis and hypospadius.
n = no. of children with malformations for each antiepileptic drug (AED); CBA = carbamazepine; LTG = lamotrigine; PHT = phenytoin; VPA = valproate.
Discussion
This study demonstrates a differential risk for AEDs to produce serious adverse outcomes (i.e., fetal death or major malformations) from in utero exposure in children of mothers with epilepsy. The best pregnancy outcomes in the current study were seen for LTG; however, it is not clear from other studies if LTG clearly possesses a lower risk. Thus, additional studies are needed to confirm this finding. The greatest risk for adverse outcomes in the current study was associated with VPA, which was due to an increased occurrence of major congenital malformations. Additional support that the observed adverse outcomes were drug related is the finding of a dose-dependent effect for VPA and similar adverse results for VPA from other studies discussed below. Strengths of our study include prospective design and monitoring of multiple potential confounding factors. Further, unlike most prior studies, it controlled for type of seizure. Weaknesses of our study include the relatively small sample size and the lack of AED randomization. The current study by itself is not definitive, but combined with several other recent studies, it contributes to a considerable body of evidence that VPA poses a special risk to the unborn child.
Several studies examining AED teratogenesis have recently been published. The North American Pregnancy Registry has prospectively enrolled more than 3,000 women on AEDs during pregnancy. They have reported a 6.5% risk of congenital malformations with phenobarbital monotherapy12 and a 10.7% risk of major malformations in children exposed in utero to VPA monotherapy.13 The risk for VPA was statistically greater than the combined mean rate for all other AEDs (2.9%) and for the general population (1.62%). The Australian Pregnancy Registry has assessed over 500 AED fetal outcomes and noted a significantly greater risk of malformations for children exposed in utero to VPA monotherapy (17.1%) compared with other AEDs (2.4%).14 The International Lamotrigine Pregnancy Registry enrolled 314 women taking LTG during pregnancy15; they found a 2.9% risk for LTG monotherapy and a significantly greater risk of major malformations for children of women on polytherapy that included VPA (12.5%) vs polytherapy without VPA (2.7%). The Swedish Medical Birth Registry survey revealed that the malformation rate in 1,256 AED monotherapy exposures was higher for VPA exposure (9.7%) compared with CBZ (4.0%). 16 A study using the Finnish National Medical Birth Registry examined 2,350 births in women with epilepsy and showed an increase risk of malformations for VPA compared with nonexposed and children exposed in utero to other AEDs; the rate of malformations for VPA monotherapy was 10.7%.17 The United Kingdom Pregnancy Registry, which included 3,607 pregnancies, found a malformation rate of 6.2% for VPA monotherapy, which was significantly greater than CBZ (2.2%).18 Four other prospective studies all exhibited a trend toward higher malformations for VPA despite their relatively low power.19–22 In addition, two retrospective cohorts from the UK and one prospective cohort from Finland have demonstrated greater behavioral teratogenesis for VPA vs other AEDs.7,23–25 Children exposed in utero to VPA had increased incidence of developmental delay in a cohort less than 6 years old.7 In those cohorts examined after 6 years old, in utero VPA exposure was associated with an increased need for special education and a 10- to 14-point reduction in verbal IQ compared with CBZ.7,24–25 In summary, the addition of the current NEAD Study findings brings the total to 14 different cohorts (10 significant) in which children exposed to VPA have had worse outcomes than children exposed to other AEDs or nonexposed children. The risk in children of women without epilepsy is less clear, but at least one study suggests that their risk for congenital malformations due to in utero AED exposure is similar to children of women with epilepsy.26
Our study demonstrated a dose-dependent effect for VPA on total serious adverse outcomes. The rate of serious adverse outcomes was 24.2% for VPA when doses were at or above the median first trimester dose (i.e., 900 mg/day) and was 9.1% for doses below the median. The mean (range) dose for those with serious adverse outcomes was 1,268 mg/day (200 to 2,750 mg/day) and for those without malformations was 844 mg/day (200 to 2,000 mg/day). Significant dose effects for VPA monotherapy have also been reported by the Australian Pregnancy Registry,14 the Finnish Birth Registry,17 the UK Behavioral Study,23 and the Finnish Behavioral Study.25 The Australian Pregnancy Registry had a malformation rate of 34.5% for doses >1,400 mg/day and 5.5% for doses ≤1,400 mg/day.14 The Finnish Birth Registry had a risk of 23.8% for doses >1,500 mg/day and 9.5% for doses ≤1,500 mg/day.17 The UK Behavioral Study found a reduction of 15 points in verbal IQ for doses >1,500 mg/day, 9.9 points for doses 801 to 1,500 mg/day, and 2.2 points for doses <800 mg/day.23 The Finnish Behavioral Study noted a 20-point reduction in Verbal IQ for VPA doses >1,500 mg/day, 16.6 points for 800 to 1,500 mg/day, and 4.2 points for <800 mg/ day.25 The Swedish Birth Registry did not conduct a dose analysis.16 The North American13 and UK Pregnancy Registries did not find a significant dose effect for VPA.13,18 The mean VPA dose was 1,033 (434) mg/ day for those with malformations and 983 (431) mg/day for those without malformations in the North American Pregnancy Registry.13 Even though the differences were not significant, the UK Pregnancy Registry reported malformation rates of 9.1% for doses >1,000 mg/day, 6.1% for 600 to 1,000 mg/day, and 4.1% for <600 mg/day.18 In summary, the majority of studies finding greater adverse pregnancy outcomes for VPA have shown a dose effect.
Many clinicians and women at risk are unaware of the increased risk posed by in utero VPA exposure that has been revealed by the recent accumulation of data distributed across multiple studies. None of the above studies meets class I criteria for evidenced-based medicine. Randomized clinical trials are unlikely to be conducted in this population owing to practical and ethical issues. Nevertheless, the probability of finding worse outcomes for VPA in 14 cohorts (10 statistically worse) is extremely unlikely, especially in view of the differences in methodology, geographic locations, and outcome measures across these studies. The evidence is highly compelling that VPA poses a higher risk of anatomic teratogenesis than other commonly used AEDs. The current data suggest an increased risk of behavioral teratogenesis for VPA, but additional studies are needed to confirm this risk separately. Nevertheless, the overall increased risk of poor outcomes for VPA is clear. Clinicians should consider this risk in the choice of AED for women and should specifically advise their female patients of this risk. Although VPA will continue to be an important treatment option in women who fail other AEDs, we advise that VPA not be used as the AED of first choice for women of child-bearing potential, and, when used, its dose should be limited, if possible.
Many questions remain unanswered. The exact risk for many AEDs remains uncertain, and human data for most new AEDs are almost nonexistent. There is a critical need for additional research to understand the underlying mechanisms and identify genetic predispositions. Several pregnancy registries across the world are seeking additional information on AED risk for anatomic teratogenesis. Clinicians should encourage their pregnant patients on AEDs to join these registries.
Registry information
Registries may be contacted as follows: North American Pregnancy Registry has a toll-free number (1-888-AED-AED4); in Europe and other continents, patients should ask their physician about enrolling in the EURAP Registry (http://www.eurapinternational.org).
Acknowledgments
Supported by grants 2 RO1 NS038455 from the NIH/NINDS, 1 R01050659 from the NIH/NINDS, and RB219738 from the UK Epilepsy Research Foundation.
Appendix
NEAD Study Group
Arizona Health Sciences Center, Tucson: David Labiner, MD, Jennifer Moon, PhD, Scott Sherman, MD; Baylor Medical Center, Irving, TX: Deborah T. Combs Cantrell, MD, Cheryl Silver, PhD; Case Western Reserve University, Cleveland, OH: Monisha Goyal, MD, Mike R. Schoenberg, PhD; Columbia University, New York, NY: Alison Pack, MD; Emory University, Atlanta, GA: Page Pennell, MD, Megan Denham, BA, Charles Epstein, MD, Jennifer Gess, MA, Sandra Helmers, MD, Thomas Henry, MD, Jean Montgomery, RN, BSN; Georgetown University, Washington, DC: Gholam Motamedi, MD, Erin Flax, BS; Harvard–Brigham and Women’s, Boston, MA: Edward Bromfield, MD, Katrina Boyer, PhD, Barbara Dworetzky, MD; Harvard–Massachusetts General, Boston, MA: Andrew Cole, MD, Lucila Halperin, BA, Sara Shavel-Jessop, BA; Henry Ford Hospital, Detroit, MI: Gregory Barkley, MD, Barbara Moir, MS; Medical College of Cornell University, New York: Cynthia Harden, MD, Tara Tamny-Young, PhD; Medical College of Georgia, Augusta: Gregory Lee, PhD, Morris Cohen, EdD; Minnesota Epilepsy Group, St. Paul: Patricia Penovich, MD, Donna Minter, EdD; Ohio State University, Columbus: Layne Moore, MD, Kathryn Murdock, MA; Riddle Health Care, Media, PA: Joyce Liporace, MD, Caroline Kohl, PsyD, George Ledakis, PhD; Rush University Medical Center, Chicago, IL: Andres Kanner, MD, Donna Bergen, MD, Michael N. Nelson, PhD; Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO: William Rosenfeld, MD, Michelle Meyer, MEd; St. Mary’s Hospital, Manchester, UK: Jill Clayton-Smith, MD, Maria Briggs, RGN, Mila Kuzmyshcheva, BS, George Mawer, MD, Usha Kini, MD; University of Alabama, Birmingham: Roy Martin, PhD; University of Cincinnati, OH: Michael Privitera, MD, Jennifer Bellman, PsyD, David Ficker, MD; University of Florida, Gainesville: Kimford J. Meador, MD, David Loring, PhD, Eugene Moore, BS; University of Kansas School of Medicine–Wichita: Lyle Baade, PhD, Kore Liow, MD; University of Liverpool, UK: David Chadwick, MD, Gus Baker, PhD, Sara Dutton, BSc, Joanne Eatock, BA, Rebecca Entwistle, BA, James Kelly, BSc, Lauren McEwan, MSc, Laura Purdy, BSC; University of Miami, FL: Eugene Ramsay, MD, Patricia Arena, PhD; University of Southern California, Los Angeles: Laura Kalayjian, MD, Christianne Heck, MD, Sonia Padilla, PsyD; University of Washington, Seattle: Daniel Drane, PhD, John Miller, MD, Gail Rosenbaum, BA, Alan Wilensky, MD; University of Utah, Salt Lake City: Tawnya Constantino, MD, Julien Smith, PhD; Walton Centre for Neurology and Neurosurgery, Liverpool, UK: Naghme Adab, MD, Lynn Owen, Loretta Kerr, Louise Pate, DipN, Gisela Veling-Warnke, MD; Wake Forest University, Winston-Salem, NC: Maria Sam, MD, Cormac O’Donovan, MD, Cecile Naylor, PhD, Shelli Nobles, MS, Cesar Santos, MD.
Executive Committee
Dartmouth Medical School, Hanover, NH: Gregory Holmes, MD; Kaiser Permanente, Oakland, CA: Lynn Ackerson, PhD; Stanford University, CA: Maurice Druzin, MD, Martha Morrell, MD, Lorene Nelson, PhD; Texas A&M University Health Science Center, Houston: Richard Finnell, PhD; University of Oregon, Portland: Mark Yerby, MD; University of Toronto, Ontario, Canada: Khosrow Adeli, PhD, Peter Wells, PharmD.
Data Management Center
Emmes Corporation, Rockville, MD: Mark Wolff, PhD, Evangela Covert, BS, Phyllis Zaia, BS, Jaclyn McKenna, BS, Stacey Whitlock, Steve Russell, BS, Maureen Costas, BA.
Footnotes
Disclosure: Drs. Clayton Smith, Finnell, and Mawer have given expert testimony on fetal anticonvulsant syndrome. Additional disclosure information is available online at www.neurology.org.
References
- 1.Pennell PB. Pregnancy in women who have epilepsy. Neurol Clin. 2004;22:799–820. doi: 10.1016/j.ncl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Tomson T, Gram L, Sillanpaa M, et al. Epilepsy and pregnancy. Petersfield: Wrightson Biomedical Publishing; 1997. [Google Scholar]
- 3.Finnell RH, Nau H, Yerby MS. General principals: teratogenicity of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. Vol. 4. New York: Raven Press; 1995. pp. 209–230. [Google Scholar]
- 4.Meador KJ. Neurodevelopmental effects of antiepileptic drugs. Curr Neurol Neurosci Rep. 2002;2:373–378. doi: 10.1007/s11910-002-0013-6. Review. [DOI] [PubMed] [Google Scholar]
- 5.Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39:251–259. doi: 10.1136/jmg.39.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cost LG, Steardo L, Cuomo V. Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharm Rev. 2004;56:103–147. doi: 10.1124/pr.56.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Practice Parameter: management issues for women with epilepsy (summary statement) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1998;51:944–948. doi: 10.1212/wnl.51.4.944. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists: ACOG educational bulletin. Seizure disorders in pregnancy. Int J Gynaecol Obstet. 1997;56:279–286. [PubMed] [Google Scholar]
- 10.Commission on Genetics, Pregnancy and the Child, International League Against Epilepsy. Guidelines for the care of women of child-bearing age with epilepsy. Epilepsia. 1993;43:588–589. doi: 10.1111/j.1528-1157.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 11.Perucca E. Birth defects after prenatal exposure to antiepileptic drugs. Lancet Neurol. 2005;4:781–786. doi: 10.1016/S1474-4422(05)70224-6. [DOI] [PubMed] [Google Scholar]
- 12.Holmes LB, Wyszynski DF, Lieberman E. The AED (Antiepileptic Drug) Pregnancy Registry: a 6 year experience. Arch Neurol. 2004;61:673–678. doi: 10.1001/archneur.61.5.673. [DOI] [PubMed] [Google Scholar]
- 13.Wyszynski DF, Nambisan M, Surve T, Alsdorf RM, Smith CR, Holmes LB. Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–965. doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 14.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–143. doi: 10.1111/j.1600-0404.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 15.Cunnington M, Tennis P. International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955–960. doi: 10.1212/01.WNL.0000154515.94346.89. [DOI] [PubMed] [Google Scholar]
- 16.Wide K, Winbladh B, Kállén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide population-based register study. Acta Paediatr. 2004;93:174–176. doi: 10.1080/08035250310021118. [DOI] [PubMed] [Google Scholar]
- 17.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–1878. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JI, Russell A, Gutherie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp.2005.074203. e-pub 12 Sept 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canger R, Battino D, Canevini MP, et al. Malformations in offspring of women with epilepsy: a prospective study. Epilepsia. 1999;40:1231–1236. doi: 10.1111/j.1528-1157.1999.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko S, Battino D, Andermann E, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–158. doi: 10.1016/s0920-1211(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 21.Samren EB, Van Duijn CM, Koch S, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology. 2003;60:575–579. doi: 10.1212/01.wnl.0000044157.28073.dc. [DOI] [PubMed] [Google Scholar]
- 23.Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:15–21. doi: 10.1136/jnnp.70.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA. Liverpool and Manchester Neurodevelopment Study Group. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 25.Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 26.Holmes LB, Harvey EA, Coull BA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–1138. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]


