Abstract
Natural orifice endoscopy in small animal models has been limited in the past by instrument size and optical performance. In this report, we investigate the feasibility of using a recently developed microvideo endoscopy system to evaluate the colon microcirculation. Using a murine model of acute colitis, microvideo endoscopy was useful in mapping the topography of inflammation as well as identifying relevant structures in the microcirculation. We conclude that natural orifice endoscopy is a useful method for the minimally invasive longitudinal assessment of the colonic mucosal microcirculation.
Keywords: microcirculation, endoscopy, corrosion casting, scanning electron microscopy, fluorescent dyes
INTRODUCTION
Intravital microscopy (IVM) is a well-established technique for observing tissues in normal and pathologic conditions. Using either episcopic (epi-illumination) or diascopic (transmitted light) illumination, traditional IVM techniques have been well suited for the observation of superficial blood vessels in the skin or exteriorized organs. Recently, in vivo imaging has been extended to solid organs by combining IVM with multi-photon microscopy (MP-IVM) (1). MP-IVM allows effective imaging at tissue depths of up to 500um (2), but image acquisition rates are generally too slow for most rapid biologic processes. A third approach, natural orifice (NO-IVM) microscopy, has been limited until recently by the technical demands of endoscopic imaging in small animal models.
In large animals and humans, natural orifices amenable to endoscopic imaging include the upper respiratory tract, upper gastrointestinal tract, urinary tract and lower gastrointestinal tract. In murine models, the examination of the upper respiratory and gastrointestinal tracts produces ventilatory compromise during oral intubation. Similarly, the endoscopic examination of bladder mucosa is associated with urethral edema and urinary obstruction. In contrast, the lower gastrointestinal tract in mice has been successfully examined by microvideo endoscopy (3).
In this report, we investigated the utility of microvideo endoscopy of the lower gastrointestinal tract to assess the mucosal microcirculation in mice. Microvideo endoscopy was used to examine both normal and acutely inflamed colonic mucosa. Our findings suggest that microvideo endoscopy is a promising method for both evaluating the topography of inflammation as well as assessing changes in the mucosal plexus.
METHODS
Mice
Male Balb/c mice (Jackson Laboratory, Bar Harbor, ME), 25–33g, were used in all experiments. The care of the animals was consistent with guidelines of the American Association for Accreditation of Laboratory Animal Care (Bethesda, MD).
TNBS administration
After the mouse abdomen was sheared and cleansed with water, 36ul of a 2.5% 2,4,6- Trinitrochlorobenzene (TNCB)(ChemArt, Egling, Germany) in a 4:1 acetone:olive oil solution was sprayed onto a 1.5cm diameter circular PhastTransfer Filter Paper (Pharmacia, Upsala, Sweden). The TNCB soaked filter paper was applied to the sheared abdomen and secured with Tegaderm (3M, St.Paul, MN) and Durapore Surgical Tape (3M). The TNCB patch was removed 24 hours after application. On post- sensitization day six, 125ul of a 1.75% 2,4,6- Trinitrobenzenesulfonic acid (TNBS) (Sigma, St.Louis, MO) in a 50% ethanol solution was instilled into the rectum. Control mice had only the 50% ethanol solution instilled intrarectally.
Clinical assessment of colitis
Total body weight was assessed daily. Activity level, fur ruffling and rectal prolapse were scored daily on a 0 (normal) to 2 (severe) scale.
Sedation
The mice were sedated with a combination of ketamine (80–100mg/kg) and Xylazine (5–16mg/kg) injected intraperitoneally. The mice were not restrained during the procedure.
Colon preparation
Because colitis in mice is associated with obstipation, luminal stool limited visual inspection. Our institutional protocol requried free access to food and water; however, limited food intact for 24 hours prior to endoscopy might improve visualization. Prior to endoscopy, the colon was prepared with a gravity-fed saline irrigation administered through a soft-tipped catheter. In addition, continuous slaine irrigation was used during the procedure to expand the lumen, minimize mucosal trauma and dislodge inspisated stool.
Microvideo endoscopy
The microvideo endoscopy was performed using a multipurpose rigid endoscope (KSVEA Rigid; 64018 BSA)(Karl Storz, Germany) with a 2.7mm diameter and 18cm length. The rigid optical system included a 30 degree wide angle forward oblique telescope. The KSVEA rigid endoscope used a 175 Watt Xenon light source. The video images were acquired with a 1/2″ interline transfer CCD with on-chip lens and analog image resolution of >450 lines. The video was recorded on NTSC SVHS and digitized to MPEG-1 video files.
Endoscopic technique
After adequate sedation and colon preparation, the mouse was placed in a ventral position. The tail was lifted to expose the anal orifice. In the colitis model, some mice have rectal prolapse and require extra care in positioning the endoscope. The endoscope was gently inserted under video guidance to minimize distal mucosal trauma. During subsequent manipulations, every effort was made to maintain the endoscope in the center of the lumen.
Mucosal grading system
The rigid endoscope was introduced with care taken to avoid any local trauma to the mucosa. After a careful examination of the inflammatory topography, the endoscopic findings were graded on a 4 point scale (4): 1=normal; 2=mild granularity of the mucosa, with contact bleeding; 3=marked granularity and edema of the mucosa with contact and spontaneous bleeding; and 4=severe ulceration of mucosa with hemorrhage. Mucosal trauma related to the endoscope was rarely observed in grade 1 mucosa and only occasionally observed in grade 2 inflammation.
Intravascular flushing and fixation
After systemic heparinization, intraperitoneal anesthesia and thoracotomy, the murine aorta was cannulated with a 2mm olive tipped cannula (Acufirm 1428LL; Dreieich, Germany) via a left ventriculotomy and the systemic circulation was flushed free of visible blood with PBS warmed to 40°C. After 1 cc of the PBS infusate, the circulation was vented through a right atriotomy. Following the PBS flush, 5cc of 2.5% glutaraldehyde warmed to 40°C was infused through the aortic cannula.
Colonic corrosion casting
Following intravascular fixation, the systemic circulation was perfused with 10–15ml of Mercox (SPI, West Chester, PA) diluted with 20% methyl methacrylate monomers (Aldrich Chemical, Milwaukee, WI). After complete polymerization, the tissues were harvested and macerated in 5% potassium hydroxide followed by drying and mounting for scanning electron microscopy (5). The microvascular corrosion casts were imaged after coating with gold in an Argon atmosphere with a Philips ESEM XL30 scanning electron microscope (Eindhoven, Netherlands). Stereo- pair images were obtained using tilt angles from 6 to 20 degrees. Diameters were interactively measured orthogonal to the vessel axis after storage of calibrated images, using AnalySIS software (version 2.1). The quality of the corrosion casts was controlled by semithin light microscopic sections stained with methylene blue. The corrosion casts demonstrated filling of the whole capillary bed from artery to vein without evidence of extravasation or pressure distension.
FITC-dextran plasma marker
To provide a tracer of plasma flow, a 10% 250,000kD FITC-dextran (Sigma) solution in normal saline was prepared. Mice were injected via the tail vein with 150ul of the prepared solution. After a brief circulation period, the endoscope was inserted and fluorescence was visualized with fitted filters (ex 480; em525).
RESULTS
Gross observations of the colon
To evaluate the effectiveness of microvideo endoscopy in a mouse model of colitis, we challenged presensitized mice with intralumenal TNBS. Systemic manifestations—including fur ruffling, diminished activity and occasional rectal prolapse—developed over 4 days (Figure 1A). Total body weight also gradually declined for 4 days after antigen instillation (Figure 1B). In the context of our previous work (6), these clinical observations suggested that a peak inflammatory time point suitable for microvideo endoscopic evaluation was 4 days after the instillation of TNBS. The gross or extralumenal appearance of the colons at 4 days following TNBS instillation showed segmental dilatation with focal areas of transmural inflammation (Figure 2).
Figure 1.
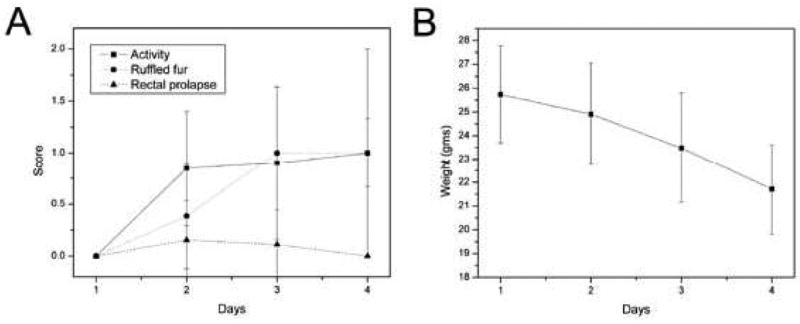
The clinical course of mice after the instillation of TNBS. A) The diminished activity level (increased severity score), ruffled fur and occasional rectal prolapse is characteristic of this model. B) The weight loss declines over 4 days after which the mice generally recover their body weight over the next week.
Figure 2.
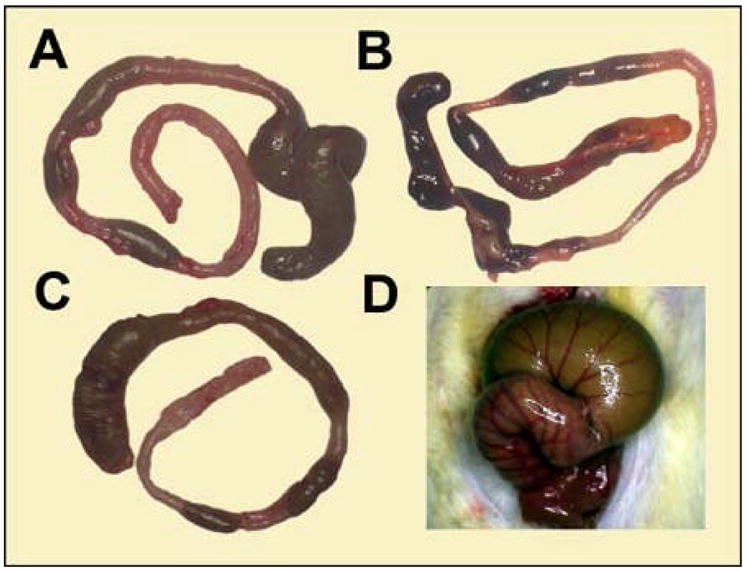
Gross appearance of the hapten-induced colitis. A-C) The dilated appearance of the colon 96 hours after the intralumenal instillation of TNBS. The bowel was variably dilated in the ascending, transverse and descending colons. D) The degree of colonic distension was apparent immediately after laparotomy: a typical dilated transverse colon is shown.
Intralumenal observations of the colon
The mucosal inflammation in TNBS-induced acute colitis was assessed by microvideo endoscopy. Intralumenal examination of the control colons demonstrated minimal stool and pink mucosa (Figure 3A–D). The bowel wall was sufficiently thin to permit faint transmural illumination of the peritoneal cavity (Figure 3C). In the colitis mice, the endoscopic examination demonstrated significant dilatation and lumenal filling with inspisated stool. Microvideo endoscopy demonstrated an edematous-appearing bowel wall with hyperemic mucosa, dilated vessels and superficial ulceration (Figure 4). Although the degree of inflammation varied between colonic segments, the severity ranged from Matts grade 2 to 3. In general, the severity of mucosal inflammation appeared to correlate with lumenal dilatation.
Figure 3.
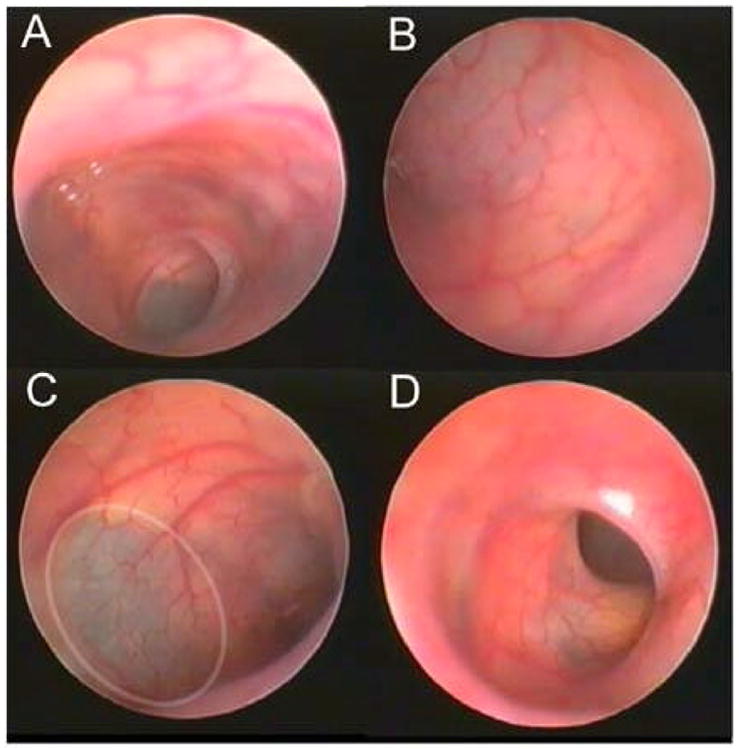
Intralumenal video colonoscopic view of the control colon (N=4). The colonoscopy was notable for the pink mucosa and virtual transparency of the colonic wall. In areas (C, ellipse), the peritoneal contents could be appreciated beyond the colon wall.
Figure 4.
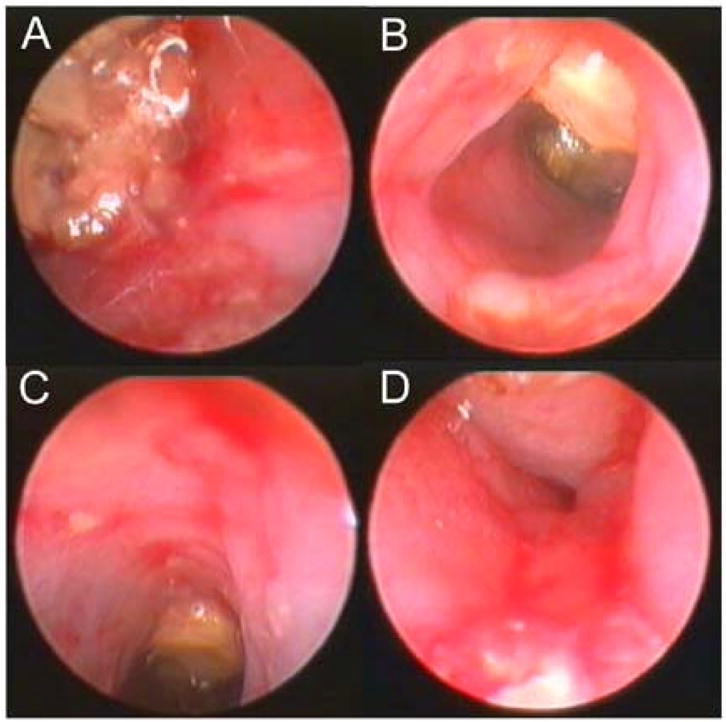
Intralumenal video colonoscopic view of the TNBS-stimulated colon 96 hours after instillation (N=4). The colonoscopy was notable for the thick and edematous wall, superficial mucosal ulceration and inspisated stool.
Mucosal vascular plexus
To provide a structural correlation with the mucosal vessels examined by microvideo endoscopy, the colonic circulation was flushed and corrosion cast injections were performed. The surrounding colonic tissue was corroded away and the resulting casts were examined by 3D scanning electron microscopy (Figure 5). The morphometry of the corrosion casts suggested that the dilated vessels directly observed by microvideo endoscopy were mucosal capillaries and collecting veins. In addition to identifying focal areas of vascular dilatation, the endoscopic examination permitted a more general evaluation of the topography of mucosal inflammation. The spatial variation in mucosal inflammation—presumably related to variability in antigen exposure— could be readily mapped and graded by microvideo endoscopy.
Figure 5.
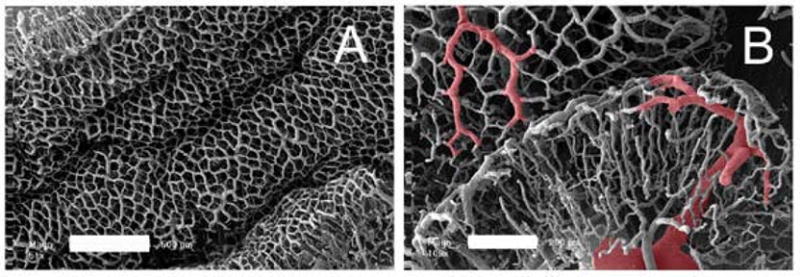
Scanning electron microscopy of the mouse colon. A) The mucosal plexus is characterized by polygonal capillaries surrounding colonic crypts. The variable topography is apparent at this level of resolution (bar=500um). B) The draining veins (red overlay), continuous with the mucosal plexus, are prominent in the setting of acute colitis.
Fluorescence video endoscopy
Because of the importance of microcirculatory changes to the grading of colitis, tracers and image analysis techniques have been recently applied to the evaluation of human colitis (7, 8). To examine the potential for microvideo colonoscopy to detect fluorescent plasma tracers in mice, we adapted fluorescence filters to an existing endoscopy system. Using standard 525nm band pass emission filters, intravenous injection of FITC-dextran was readily visualized in the mucosal vessels (Figure 6). The spatial resolution and signal isolation was sufficient to identify both mucosal capillaries and collecting veins.
Figure 6.
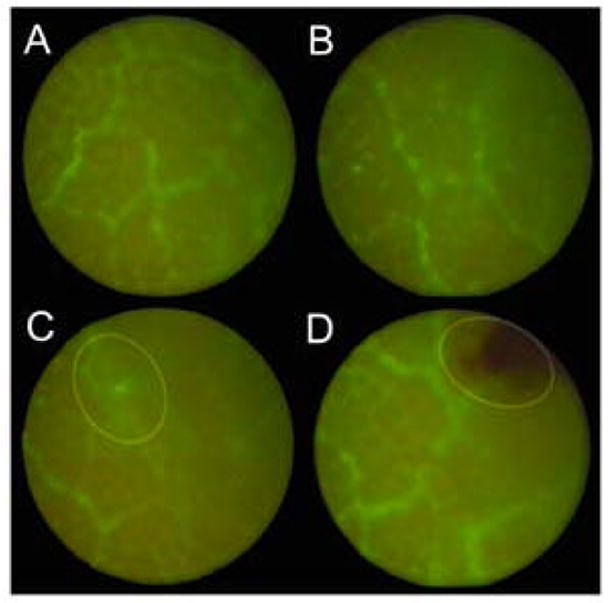
Dilated mucosal vessels in acute colitis enhanced with a FITC-dextran plasma tracer and examined by fluorescence video colonoscopy (N=4). The dilated mucosal vessels are apparent in almost all areas of Matts 2-3 grade colitis. Of interest, local trauma of the mucosa occasionally produced extravasation of the fluorescent tracer into the lumen (C, ellipse), but more frequently produced focal hemorrhage from the friable mucosa (D, ellipse).
DISCUSSION
In this report, we evaluated the utility of microvideo endoscopy in a mouse model of TNBS-induced acute colitis. Microvideo endoscopy 4 days after the instillation of antigen permitted a general assessment of the distribution of colonic inflammation as well as the evaluation of focal microvascular dilatations in the mucosal plexus. Structural studies of the mucosal microcirculation indicated that the microvideo colonoscopy permits an in vivo assessment of both mucosal capillaries and associated collecting veins. These results suggest that microvideo endoscopy is a potentially useful method for the longitudinal evaluation of the dominant microvascular changes in murine acute colitis (6).
Common models of acute colitis include the intralumenal instillation of TNBS (9) and dextran sulfate (10, 11). Both of these approaches produce an inflammatory response in the mouse colon; however, both models are also associated with significant variability in the degree of inflammation produce by the antigen. Typical clinical parameters such as weight loss, ruffled fur and rectal prolapse are remote measures of colon inflammation. Further, the unpredictable mortality in both models suggests the need for more reliable longitudinal assessments of mucosal inflammation. Microvideo endoscopy provides an objective assessment of mucosal inflammation in anesthetized mice. This minimally invasive assessment can be particularly important in longitudinal experiments or experiments involving costly interventions or reagents in limited supply.
Microvideo endoscopy of the colonic mucosal microcirculation in mice also has several limitations. First, the degree of obstipation requires saline irrigation to clear the lumen. Although this manipulation is unlikely to significantly alter the perceived degree of inflammation, mechanical irrigation can result in mucosal injury. Second, a small percentage of mice treated with TNBS developed mucosal inflammation characterized by a diffuse mucosal slough. The white, stringy exudates were ineffectively cleared by saline irrigation resulting in poor visualization of the colonic mucosa. Third, the optical demands of the system required the use of a rigid endoscope. Rigid endoscopy requires experience and skill to avoid mucosal trauma. The rigid endoscope also limits the anatomic extent of the examination to the descending colon. Finally, the 2-dimensional view of the mucosa complicates reliable distance calibration for semi-quantitative morphometry.
In the future, natural orifice imaging will benefit from progressive miniaturization, improved illumination and increased magnification. The 2.7mm endoscope used in this study was relatively large compared to the lumen of the native colon. Smaller diameter rigid endoscopes, and the eventual development of flexible endoscopes, will minimize mucosal trauma and extend colon access. Although the tungsten-halogen light used in this study was sufficiently intense for bright field illumination, future endoscopes will likely benefit from a more flexible optical filter system and brighter fluorescence illumination. Finally, the examination of colon mucosa will be enhanced with increased magnification. The improvements in the endoscopic optical system may soon allow visualization of not only blood vessels in the mucosa, but moving particles within the blood flow stream (12).
Acknowledgments
The authors would like to thank Kerstin Bahr for her technical assistance.
Abbreviations
- 3D
3-dimensional
- FITC
fluorescein isothiocyanate
- PBS
phosphate buffered saline
- SEM
scanning electron microscopy
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TNCB
2,4,6-trinitrochlorobenzene
Footnotes
Supported in part by NIH Grant HL47078 and HL75426
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 2.Stutzmann GE, Parker I. Dynamic multiphoton imaging: a live view from cells to systems. Physiology (Bethesda) 2005;20:15–21. doi: 10.1152/physiol.00028.2004. [DOI] [PubMed] [Google Scholar]
- 3.Becker C, Fantini MC, Wirtz S, Nikolaev A, Kiesslich R, Lehr HA, Galle PR, Neurath MF. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 2005;54:950–954. doi: 10.1136/gut.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med. 1961;30:393–407. [PubMed] [Google Scholar]
- 5.Ravnic DJ, Jiang X, Wolloscheck T, Pratt JP, Huss H, Mentzer SJ, Konerding MA. Vessel painting of the microcirculation using fluorescent lipophilic tracers. Microvasc Res. 2005;70:90–96. doi: 10.1016/j.mvr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ravnic DJ, Konerding MA, Jiang X, Huss HT, Pratt JP, Mentzer SJ. Structural adaptations in the murine colon microcirculation associated with hapten-induced inflammation. Gut. 2006 doi: 10.1136/gut.2006.101824. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi M, Saitoh Y, Fujii T. Adaptive index of hemoglobin color enhancement for the diagnosis of colorectal disease. Endoscopy. 2005;37:386–388. doi: 10.1055/s-2005-861094. [DOI] [PubMed] [Google Scholar]
- 9.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 10.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 11.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ravnic DJ, Zhang Y-Z, Tsuda A, Pratt JP, Huss HT, Mentzer SJ. Multi-image particle tracking velocimetry of the microcirculation using fluorescent nanoparticles. Microvasc Res. 2006 doi: 10.1016/j.mvr.2006.04.006. In press. [DOI] [PubMed] [Google Scholar]


