Abstract
Infection and inflammation lead to changes in mood and cognition. Although the “classic” sickness behavior syndrome, involving fatigue, social withdrawal, and loss of appetites are most familiar, other emotional responses accompany immune activation, including anxiety. Recent studies have shown that gastrointestinal bacterial infections lead to enhanced anxiety-like behavior in mice. The bacteria-induced signal is most likely carried by vagal sensory neurons, and occurs early on (within six hours) during the infection. These signals induce evidence of activation in brain regions that integrate viscerosensory information with mood, and potentiate activation in brain regions established as key players in fear and anxiety. The findings underline the importance of viscerosensory signals arising from the gastrointestinal tract in modulation of behaviors appropriate for coping with threats, and suggest that these signals may contribute to affective symptoms associated with gastrointestinal disorders.
Keywords: gut-brain interaction, Campylobacter jejuni, Citrobacter rodentium, vagus, interoception
1. Introduction
In the late 1800s William James, a psychologist at Harvard University, published a provocative theory of emotion: that the perception of emotions follows from perception of our physical responses to cognitive apprehension of external threats (or more pleasant stimuli). That is: the experience of emotion is integrated with somato- and viscerosensory signals that result from cognitively driven motor, neuroendocrine, or autonomic responses, in a kind of brain-body-brain “loop” communication sequence. He further proposed that, when emotions were finally mapped in the brain, rather than existing as separate centers (e.g. anxiety center, happiness center) the neural substrates of emotion would be integrated with somato- and viscerosensory representation (James, 1890). Although James is usually remembered for the assertion that bodily sequelae of emotion-inducing stimuli “are” the emotions, this corollary prediction regarding brain substrates of emotions is proving particularly prescient. Findings from neuroanatomical, neuropsychological and functional neuroimaging studies support James' contention that the brain representations of emotions would be co-represented with information derived from internal tissues (Craig, 2003; Dalgleish, 2004; Nauta, 1971; Price, 1999; Zagon, 2001). Such regions include the medial and orbital prefrontal and anterior cingulate cortex, insula, hypothalamus, amygdala, and bed nucleus of the stria terminalis. These findings underline the important contribution of viscerosensory signals related, for instance, to hunger, satiety, or pain, to emotional states.
The relation of emotions and internal sensory information carries important implications for psychoneuroimmunology. It is now well established that the immune system influences mood and cognition (Dantzer, 2004; Maier, 2003). Furthermore, via autonomic and neuroendocrine outflow, psychological states, including depression and stress, modulate immune system functioning in turn. These interactions exert significant influence on the course of chronic illness, and thus make compelling targets for therapeutic intervention. Understanding the biological and neurological substrates (e.g. brain regions and neurotransmitter systems involved) mediating interactions of the immune and nervous systems, however, is key to such an aim.
2. Infection-induced anxiety
We first became involved with these issues when one of us (ML) observed that mice treated per-orally with live bacteria (Campylobacter jejuni) seemed more anxious than saline-treated control mice (Lyte et al. 1998). This observation was confirmed by comparing the behavior of C. jejuni-treated mice and control mice on the elevated plus maze. However, there was no evidence that the bacteria had reached the systemic circulation (and thus the brain), or that the infection induced circulating pro-inflammatory cytokines. For that matter, the mice showed no evidence of “classic” sickness behavior, which includes suppressed ingestive behavior, social withdrawal, hunched posture, and psychomotor retardation. These observations raised questions regarding what mechanism, if not via the circulation, is used to signal the brain, and what, then, is going on in the brain during this infection?
If bacteria-related signals are not circulating in the blood, the most likely explanation is that the signals are carried instead by viscerosensory nerves that innervate the gut. Viscerosensory nerves in the gut fall into two categories: intrinsic and extrinsic. Intrinsic (or enteric) resident nerve cells serve to mediate secretory and motor functions associated digestion. Some of these intrinsic nerves innervate the lymphoid tissues below the epithelium, and may be sensitive to bacteria or immune generated signals, including cytokines. These intrinsic nerves do not innervate the central nervous system, however, and cannot directly signal the brain. Communication between the intrinsic neurons of the gut with the brain and spinal cord is carried out by two populations of extrinsic nerves. Vagal (parasympathetic sensory) nerves project to the caudal brainstem, and spinal visceral (sympathetic sensory) project to the thoraco-lumbar spinal cord. These nerves innervate the intrinsic neurons, but also are in contact with immune cells and lymphoid tissue, and the subepithelium, a site of host/pathogen interface. The sensitivity of spinal visceral sensory nerves to noxious distension of the colon is inhibited by a diet including bacteria or bacterial products (Kamiya et al., 2006) suggesting that gut bacteria influence these nerves by either a direct or perhaps indirect (via immune constituents) action within the gut. Vagal sensory neurons, in particular, evince a close relationship with immune cells in the submucosa (Williams et al. 1997) and project nerve endings into the mucosa (although not into the lumen). Thus, in our experiments, bacteria related signals that influence brain functions most likely were carried by either the vagus or the spinal visceral sensory nerve fibers.
Based on previous studies implicating the vagus nerve in immune-gut-brain communication (Castex et al., 1995; Goehler et al., 2000, for review), we questioned whether vagal sensory neurons might be the link between infection in the gut and brain responses that support anxiety. To address the issue we used immunohistochemical localization of the immediate-early gene product c Fos. This protein is induced in cells, including neurons, shortly (∼ 45-60 minutes) after cells become activated. Thus, induction of this protein can be used as a marker for activation. Although not all neurons express c-Fos when activated (but many do) and expression of c-Fos protein in a neuron is not necessarily related to the experimental manipulations, c-Fos expression patterns have been widely used to provide information regarding the functions of neuronal ensembles. For these studies, mice were inoculated with Camplyobacter jejuni (Goehler et al., 2005) or Citrobacter rodentium (Lyte et al. 2006), and c-Fos expression in the vagal sensory neurons assessed beginning at four hours after inoculation. C-Fos protein began to appear in the vagal sensory neurons at around five hours post-inoculation, and peaked around seven to eight hours. Given that intestinal transit time to the cecum (the primary site of colonization in Camplyobacter jejuni and Citrobacter rodentium) in a mouse is estimated to be around three hours, and c-Fos protein induction takes about an hour, these findings imply that within one hour of arriving at the cecum, the bacteria were able to generate signals in vagal sensory neurons, and thus signal the brain. Thus, vagal sensory neurons seem to be highly sensitive to the presence of potentially dangerous bacteria in the GI tract, and likely form the link between bacteria and behavior.
A critical piece of puzzle of how infection in the gut influences the brain and behavior regards the signals generated as a consequence of bacterial infection that serve to signal sensory neurons. That is- how do vagal sensory neurons know? This is still an open question, but viscerosensory neurons innervating the gut express receptors for a variety of immune/inflammatory mediators, including histamine, prostaglandins, ATP, adenosine, serotonin, cytokines including interleukin 1 (IL-1) and tumor necrosis factor (Kirkup et al., 2001). Additionally, or alternatively, vagal sensory neurons may respond directly to pathogens or pathogen products. For instance, vagal sensory ganglia have been reported to express TOLL-like receptors (proteins that bind to pathogen products or cellular components), including TOLL-like receptor 4 (TLR4), which signals bacterial lipopolysaccharides (Hosoi et al., 2005). Whether vagal sensory neurons express TLR-4 on their projections to the gut, however, is unknown. Further, Spiller (2002) has pointed out that cholera toxin (CT), a product of the A-B family of heat-labile enterotoxins, binds to GM1 gangliosides, which are expressed on both nerve fibers and enterocytes. CT subunits appear to enter vasoactive intestinal peptide-expressing neurons, specifically, to interact with adenyl cyclase as a basis for its diarrhea-inducing effects. However, other members of this toxin family are expressed by bacteria known to induce signaling to the brain, notably Esherichia coli and Campylobacter jejuni. These toxins could well interact with GM1 gangliosides on vagal sensory neurons. There are clearly multiple possible mechanisms by which pathogen-related signals, or local signals generated in response to pathogens or tissue damage could interact with vagal sensory neurons, and thus signal the brain.
3. Brain substrates for infection-induced anxiety-like behavior
As James predicted and as recent studies now support, brain regions involved in emotions are associated with viscerosensory and autonomic (visceral motor) processing (Craig, 2003; Nauta, 1971; Price, 1999; Zagon, 2001). Previous studies using immune challenges with bacterial products (LPS) or cytokines, such as IL-1, that usually induce symptoms of behavioral depression have indicated that, indeed, these immune stimuli also activate brain regions involved in viscerosensory processing (Goehler et al., 2000, for review). What about pathogen-related stimuli, such as per-orally administered bacteria, that do influence behavior, but do not necessarily induce behavioral depression? To address this issue we evaluated brain response patterns to Campylobacter infection, initially while mice rested in their home cages (Gaykema et al., 2004). These studies have indicated that treatment with these bacteria is strongly associated with the same viscerosensory processing stimuli previously reported for other immune-related stimuli (cytokines and bacterial products) presented exogenously. In particular, activation was seen in the nucleus of the solitary tract (nTS), the site of termination of vagal sensory nerve fibers, as well as in the ventrolateral medulla, which (with the nTS) propagates viscerosensory information to the forebrain, as well as in the lateral parabrachial nucleus, the paraventricular hypothalamus, the central nucleus of the amygdala, and the bed nucleus of the stria terminalis. The latter three regions are well-established for integrating affective states (stress, fear, anxiety) with autonomic and neuroendocrine responses. In addition, cortical areas proposed by both Damasio (1994) and Craig (2003), i.e. the medial prefrontal cortex and insula, also showed activated neurons in the early phases of live bacterial infection of the gut. Although the models of both Damasio and Craig are specific for primate/human brain, the findings that gut infection induces anxiety-like behavior and may involve brain regions that may be homologous in the mouse suggest that the integration of viscerosensory drive with affective experience/behavior is a fundamental organizing feature of mammalian brain.
Whereas mapping activation of brain regions to Campylobacter in resting animals provided us with confirmation that the response to live infections generally matched that of exogenous cytokines etc., we still do not know how infection-induced viscerosensory drive enhances (see below) anxiety-like behavior. To approach this issue we have challenged mice with Camplyobacter and Citrobacter, and assessed their behavior using the open-field holeboard, another apparatus useful for assessing a rodent's innate apprehension of open spaces (Goehler, et al., in preparation; Lyte et al. 2006;). This apparatus also provides a set of nine holes the mice can investigate, setting up a potential conflict between exploration/foraging in the open against the safety of remaining near the walls. We assessed brain activation patterns in infected mice following exposure to the open-field/hole board, and compared the activation patterns with saline-treated mice and infected animals left to rest in their home cages. These experiments were carried out between seven and nine hours after inoculation, during the time range when vagal sensory neurons show evidence of activation (c-Fos protein induction). Whereas all animals exposed to the open-field/hole board showed activation in brain regions previously implicated in behavior and defensive responses, including the septal area, several hypothalamic regions, amygdala, bed nucleus of the stria terminalis, and periaquiductal grey, the responses in the paraventricular hypothalamus, central and basolateral amygdala, and bed nucleus of the stria terminalis were potentiated in the infected animals, who also exhibited enhanced anxiety-like behavior. Notably, the paraventricular hypothalamus is a key player in the induction of neuroendocrine and autonomic response to stress, whereas the basolateral and central nuclei of the amgydala, with the related bed nucleus of the stria terminalis, are strongly implicated in fear and anxiety (Walker et al., 2003). In this way, infection-related viscerosensory signals enhance anxiety in novel environments by exerting additional drive on key brain regions regulating responses to environmental challenges.
4. What may be the adaptive value of enhanced anxiety during gut infection?
An important issue to keep in mind regarding experiments investigating emotional states in animals, especially anxiety, is that the characteristics of the testing procedure influence the findings. In the case of the open field/hole board, this apparatus represents a possibly dangerous novel environment. It can be thought of as analogous to that which an animal might encounter when foraging in an unknown area for food, or in any other exploratory behavior in the wild. The open character of the environment allows potentially for the presence of predators (e.g. hawks). Thus, some innate anxiety is normal when an animal is placed in this apparatus. The critical difference we observed when comparing infected animals with controls was that the infected animals showed enhanced anxiety-like behavior. In particular, the infected animals avoided almost completely the center part of the field, and preferred to remain in the corners (Lyte et al., 2006). They also spent more of their time engaged in “risk assessment” behavior. Overall, the behavior of the infected animals can be described as markedly more cautious.
Why should infected animals be more cautious in an open environment? An important aspect of the findings from these studies is that both vagal sensory neuron signaling and anxiety-like behavior occur rather rapidly following per-oral inoculation. This suggests that gut-brain signaling via the vagus is operating as something like an early warning signal. The consequences of food-poisoning can be severe, and a prompt response from the brain, to initiate supportive neuroendocrine, physiological, and behavioral responses is essential for full-fledged host defense. However, behavioral responses to infection often include symptoms such as somnolence and psychomotor retardation that, while supportive of recuperation, can render an animal vulnerable to predators, particularly if an animal is in an exposed situation, such as an open field. Thus cautiousness and avoidance of open, exposed spaces likely confers an advantage to recently infected animals.
Anxiety is usually conceptualized as a response to a possible threat. Experimentally these typically take the form of exteroceptive threats- those arising from the environment, notably potential predators in the form of predator scents, or of open spaces where predators may lurk. The findings from our studies, and those of others (Basso et al. 2003; Castex, et al. 1995; Lacosta et al. 1999; Rossi-George et al. 2005) indicate, however, that interoceptive threats, such as infection or allergy or systemic immune challenge, also can engage behaviors typical of threat avoidance, and increase drive on brain regions that process threat-related information. These areas also contribute to neurocircuitry that support stress responses. Because stress may potentiate peripheral inflammatory conditions, this increased drive might be predicted to exacerbate ongoing inflammation in the gastrointestinal tract, or other peripheral tissues.
5. Bidirectional interactions of bacteria/immune-brain-gut axis: “bottom-up” and “top-down” influences on gastrointestinal health and mood
One of the core principles of psychoneuroimmunology is the bidirectional influence of the immune and nervous systems on mood and health. That is, affective symptoms associated with illness or inflammation follow not only from stress of dealing with a medical condition, or personality factors (“top-down” brain-mediated) but also from the effects of cytokines, or other products of inflammation, induced by infection or inflammation (“bottom-up” immune and viscerosensory mediated). In the case of infection and inflammation of the gastrointestinal tract, this principle is particularly relevant for the anxiety and depressive symptoms so often concomitant with chronic inflammation in Chrohn's Disease and Inflammatory Bowel Disease, as well as in functional gastrointestinal disorders such as Irritable Bowel Syndrome (IBS).
IBS is a particularly good example of a psychoneuroimmunologic disorder. The condition is diagnosed in people with abdominal pain associated with changes in bowel frequency, in the absence of abnormalities indicating a physical cause such as obstruction (Lydiard, 2001). Thus it is a functional, as opposed to structural, gastrointestinal disorder. It is more common among women, is exacerbated by stress, and is associated with activation of mucosal immune cells (Chadwick et al., 2002). In as many as 30% of patients, symptoms follow a bout of food poisoning, often with Campylobacter jejuni (Spiller, 2002). This condition is termed “post-infective IBS”, and symptoms can persist for a year or more. IBS is associated with a high prevalence of psychiatric symptoms, including depression, panic disorder, generalized anxiety, and post-traumatic stress disorder (Lydiard, 2001). Interestingly, it has been noted that anxiety-related symptoms may be more prominent early in the course of IBS, with depression more common in patients with chronic symptoms (Lydiard, 2001), and that agoraphobia (fear of open spaces, or “fear of the marketplace”) is more common in patients with gastrointestinal symptoms (Mayer et al., 2001). These findings highlight the association of affective symptoms, particularly anxiety, with gastrointestinal disorders. Our findings, and those of others (Basso et al. 2003; Castex, et al. 1995; Lacosta et al. 1999; Rossi-George et al. 2005) suggest that the consequences of inflammation or infection in the bowel may contribute to these symptoms.
From recent findings a picture emerges in which viscerosensory nerves make multifunctional contributions to stress-related and affective responses to gastrointestinal dysfunction. Visceral hypersensitivity and enhanced pain states accompany infection and immune activation, and are most likely mediated by spinal viscerosensory nerves (Kamiya et al., 2006; Liu et al. 2007) that drive ascending nociception pathways to the forebrain, via the pontine lateral parabrachial nucleus (Traub & Murphy, 2002). In this way, activation of spinal viscerosensory fibers likely influence affective states by potentiating pain and discomfort. In contrast, the vagus nerve provides immediate signals relevant to host defense (Liu et al, 2007) and mood modulation (Lyte et al., 2006; Schachter, 2004). Stimulation of the vagus nerve in humans can ameliorate depressive symptoms in some treatment-refractory patients, but can increase or induce symptoms of anxiety and depression in others. Interestingly, disruption of the vagus nerve enhances visceral hypersensitivity following immune challenge (Coelho et al., 2000) implying that the vagus plays an as yet uncharacterized protective role during inflammation, as well. One mechanism for protective effects may be via a vagal efferent suppression of pro-inflammatory cytokine production (Tracey, 2002). Taken together, these findings suggest that the outcome and severity of gastrointestional dysfunction and mood symptoms may be modulated by the interaction of vagal and spinal viscerosensory nerve functions.
The observation that infection in the gut can potentiate anxiety-like behavior suggests implications for the phenomenology of affective symptoms associated with gastrointestinal disorders. Factors previously associated with the development or risk of such symptoms, (e.g. pre-existing psychiatric disorder, somatosizing personality, stress and pain associated the illness) have generally been conceptualized from the top-down perspective, as have many new therapeutic approaches (Mayer et al. 2006). However, if viscerosensory drive via the vagus contributes to affective symptoms in these disorders, interventions addressing underlying infection or inflammation, or other source of stimuli activating the vagus, could also ameliorate symptoms. In addition, understanding that some of the negative affective experiences associated with gastrointestinal disorders may not be under voluntary control of the patients may relieve some of the stress of these disorders.
6. Conclusion
It seems reasonable to assert that linking emotions to homeostatic mechanisms conveys powerful adaptive advantages, as this is a salient way for conditions within the body to influence behavior. In the case of infection-induced anxiety, increasing cautiousness in an animal that may soon become ill could save its life. In a similar way, the development of a conditioned taste aversion (a learned negative association of recently eaten food with illness) following the survival of food poisoning encourages an animal to avoid potentially tainted food in the future. Our recent findings implicate the vagus as a critical link between internal threats and brain regions that cope with such threats, supporting Andrews (1992) description of it as the “great wandering protector”.
Figure 1.
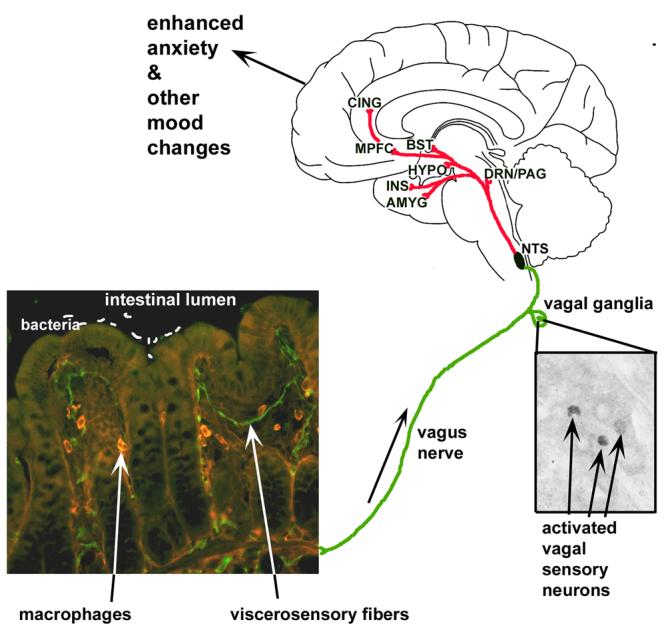
Model diagram of gut-brain interactions underlying bacterial infection-induced enhanced anxiety and other mood and affective changes. Bacteria interact, in some unknown way, with viscerosensory nerves, likely associated with the vagus nerve, that innervate the gastrointestinal (GI) tract, shown here innervating villi of the mouse ileum (unpublished image from authors's archive). Activated vagal sensory neurons (Goehler et al. 2005; Lyte et al., 2006; unpublished image from author's archives) project to the nucleus of the solitary tract (NTS) of the brainstem, which propagates viscerosensory information to brain regions involved in emotion, including the dorsal raphe nuclei and periaquiductal grey (DRN/PAG), several nuclei of the hypothalamus (HYPO), the amygala (AMYG) and bed nucleus of the stria terminalis (BST), as well as cortical area including the insula (INS), medial prefrontal cortex (MPFC) and anterior cingulate (CING). In this way, information about stimuli within the GI tract, including infections, can be integrated with ongoing emotional states and behavior.
Acknowledgements
This work was supported by NIH grants MH64648, MH50431, & MH68834.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews PLR. A Protective Role For Vagal Afferents: A Hypothesis. In: Andrews PLR, Lawes INC, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. CRC Press; London: 1992. pp. 279–302. [Google Scholar]
- Basso AS, Costa Pinto FA, Russo M, Giorgetti Britto LR, de Sa-Rocha LC, Palermo Neto J. Neural correlates of IgE-mediated food allergy. J. Neuroimmunol. 2003;140:69–77. doi: 10.1016/s0165-5728(03)00166-8. [DOI] [PubMed] [Google Scholar]
- Castex N, Fioramonti J, Fargeas MJ, Bueno L. c-fos expression in specific rat brain nuclei after intestinal anaphylaxis:involvement of 5-HT3 receptors and vagal afferent fibers. Brain Res. 1995;688:149–160. doi: 10.1016/0006-8993(95)00526-v. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterol. 2002;120:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first-and second-order representations of bodily states. Nat. Neurosci. 2001;4:207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Coelho A-M, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and the vagus nerve. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G781–G790. doi: 10.1152/ajpgi.2000.279.4.G781. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error. Avon Books Inc.; New York, NY: 1994. [Google Scholar]
- Danzter R. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Camplyobacter jejuni: analysis with Fos immunohistochemistry. Brain, Behav., Immun. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Anderson K, Hansen MK, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemoreceptive pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr NA, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton. Neurosci. 2005;120:104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. II. Henry Holt & Co.; New York, NY: 1890. Chapter XXV. The Emotions. [Google Scholar]
- Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Tougas G, Bienestock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: Potential for novel therapies I. Receptors on visceral afferents. Am J. Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: angiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Liu CY, Mueller MH, Grundy D, Kreis ME. Vagal modulation of intestinal afferent sensitivity to systemic lipopolysaccharide in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2007 doi: 10.1152/ajpgi.00267.2006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 1998;65:63–68. doi: 10.1016/s0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- Lyte M, Wang L, Opitz N, Gaykema RPA, Goehler LE. Anxiety-like behavior during initial stage of infection with agent of colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J. Clin. Psychiatry. 2001;62:38–45. [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune-brain communication: implications for understanding stress, pain, and cognition. Brain Behav. Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J. Clin. Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment. Pharmacol. Ther. 2006;24:919–933. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. The problem of the frontal lobe: A reinterpretation. J. Psychiatry Res. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Ann. NY. Acad. Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: Dependence on tumor-necrosis factor-α. J. Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S. Vagus nerve stimulation: mood and cognitive effects. Epilepsy Behav. 2004;5:S56–S59. doi: 10.1016/j.yebeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Role of nerves in enteric infection. Gut. 2002;51:759–762. doi: 10.1136/gut.51.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nat. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93–102. doi: 10.1016/s0304-3959(01)00381-5. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation. 1997;5:266–270. doi: 10.1159/000097346. [DOI] [PubMed] [Google Scholar]
- Zagon A. Does the vagus nerve mediate the sixth sense? TINS. 2001;24:671–673. doi: 10.1016/s0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]


