Abstract
Sialyl Lewis x (sLex) is an important tumor-associated carbohydrate antigen present on the cell surface glycoconjugates involved in leukocyte migration and cancer metastasis. We report the formation of sLex epitope in butyrate-treated human pancreatic adenocarcinoma cells expressing MUC1 and core 2 N-acetylglucosaminyltransferase (C2GnT). Butyrate treatment stimulates not only the transgene but also a group of endogenous glycosyltransferase genes involved in the synthesis of sLex. Current finding raises a concern about the proposed use of butyrate as a cancer therapeutic agent.
Keywords: sLex, glycosyltransferase, butyrate and pancreatic cancer
Introduction
Glycoconjugates located at the outer surface of mammalian cells are characteristics of cell-types and developmental stages [1]. Malignant transformation is often associated with abnormal glycosylation, resulting in the synthesis of altered carbohydrates. Some of these carbohydrate epitopes are involved in selectin-mediated adhesion of leukocytes [2] and cancer cells [3–5] to vascular endothelium. One such carbohydrate is sialyl Lewisx (sLex), NeuAcα2→3Galβ1→4(Fucα1→3)GlcNAc→R, which is a selectin ligand involved in the binding of tumor cells to endothelial cells during metastasis [3–5]. However, regulation of the synthesis of this epitope in metastatic cancer is not completely understood.
sLex is found at the non-reducing terminus of glycoconjugates [6]. The synthesis and functions of mucin glycan-associated sLex are well characterized [6]. Synthesis of sLex on Oglycans begins with attachment of N-acetylgalactosamine (GalNAc) to serine or threonine as catalyzed by polypeptidyl N-acetylgalactosaminyltransferase (ppGalNAcT) [7]. These glycans are then extended by the formation of core 1 (Galβ1–3GalNAc), which is the obligatory precursor of core 2, Galβ1–3(GlcNAcβ1–6)GalNAc [8]. Formation of sLex on core 2 branch proceeds by addition of β1–4galactose to GlcNAc followed by attachment of α2–3 sialic acid or N-acetylneuraminic acid (NeuAc) to galactose and then α1–3fucose to GlcNAc [9]. Elaboration of sLex on core 1 branch occurs by same process after core 1 has been extended with β1–3GlcNAc [10]. Alteration of the activities of the enzymes involved in the synthesis of sLex and its precursors can affect the expression of sLex.
The expression of cell surface carbohydrate antigens are affected by various agents including sodium butyrate [11–13]. Butyrate can alter the activities of endogenous glycosyltransferases in several cell lines, such as CaCo-2 colon carcinoma cells [14] and Chinese Hamster Ovary cells [15]. Butyrate also can modulate the expression of intestinal mucin gene expression [16]. Because butyrate can induce apoptosis in various transformed and nontransformed cell types [17–19] and various cancerous cells [20], it has been considered as a therapeutic agent [21, 22]. In this communication, we report that butyrate treatment of pancreatic adenocarcinoma cells expressing MUC1 and core 2 N-acetylglucosaminyltransferase results in the formation of sLex. This finding raises a concern about the use of butyrate as a cancer therapeutic agent.
Materials and methods
Materials
The MUC1-expressing Pancreatic adenocarcinoma (Panc1/MUC1f) cells stably transfected with pcDNA3.1/Zeo+ containing bovine (b) C2GnT-1 cDNA (Panc1/MUC1f-bC2GnT-1) were previously generated in our laboratory [8]. UDP-[3H]GlcNAc (60 Ci/mmol), UDP-[3H]Gal (60 Ci/mmol), and UDP-[3H]GalNAc (15 Ci/mmol) were obtained from American Radiolabeled Chemicals (St. Louis, MO). Bond Elut C18 cartridges were from Varian (Sunny Vale, CA). Other chemicals were from Sigma (St. Louis, MO) unless indicated otherwise.
Cell culture conditions
Panc 1 / MUC1f-bC2GnT-1 cells were routinely grown in minimal essential medium supplemented with 10% fetal bovine serum and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin). All cell culture reagents were from Hyclone (South Logan, UT).
Sodium butyrate treatment
Panc 1 / MUC1f-bC2GnT-1 cells grown to about 75% confluence in T-25 flasks were treated with varying concentrations of sodium butyrate (0–4 mM). At various time points (0–36 h) after treatment, cells were processed for analysis of glycosyltransferase activities and mRNA expression.
Assay of C2GnT, core 1 β3Galactosyltransferase (core 1β3Gal-T) and ppGalNAcT enzyme activities
C2GnT activity was assayed using total cell lysates as described previously [23]. Control and sodium butyrate treated Panc 1 / MUC1f-bC2GnT-1 cells from a T25 flask were scrapped into 250 μL of 0.25 M sucrose, and then disrupted by forcefully passing the cells successively through 20- and 26-gauge needles. Aliquots of the homogenates were measured for C2GnT activity using UDP-[3H]GlcNAc (~3,500 dpm/nmol) as the sugar donor and 2 mM Galβ1,3GalNAcα-O-Benzyl (Toronto Research Chemicals Inc. Downs view, Ontario, Canada) as the acceptor. The products were isolated with C18 cartridges and quantitated by a Liquid Scintillation Counter (Packard, Meriden, CT). Protein concentration was measured with Coomassive Plus Protein Assay Reagent (Pierce, Rockford, IL) using BSA as the standard. Enzyme activity was calculated by subtracting the endogenous activity measured without exogenous acceptor from total activity and expressed as nmol of sugar donor transferred/h/mg protein. Core 1 β3Gal-T, which catalyzes attachment of galactose in β1–3 linkage to GalNAcα-ser/thr, was assayed as described previously [24], except that 2 mM GalNAc-benzyl was used as the acceptor, and the product was isolated and quantified as described above for assay of C2GnT activity. ppGalNAcT activity was assayed as described [25], except that the reaction mixture contained 0.1 mM UDP-[3H]GalNAc (105 dpm/nmol), 1 mg/ml BSA and 0.1 mg/ml acceptor peptide. The acceptor peptide is a synthetic 29 amino acid MUC2 peptide having the PTTTPITTTTTVTPTPTPTGTQTPTTTPI sequence. Although numerous isoforms of ppGalNAcT possess distinct acceptor substrate preferences [7], the MUC2 tandem repeat sequence can be utilized efficiently by several isoforms of ppGalNAcT [26] and should provide a reasonable measure of overall ppGalNAcT activity.
Western Blot Analysis
Lysates of Panc 1 / MUC1f-bC2GnT-1 cells treated with or without 2 mM sodium butyrate for 36 h were resolved on 6% SDS-polyacrylamide gel electrophoresis. Proteins were electroblotted onto a PVDF membrane (Immobilon-P, 0.2 μm, Millipore, Bedford, MA) overnight at 4° C and 100 mA, then blocked with 5 % BSA in TBS (0.9 % NaCl, 10 mM Tris, pH 7.5) at room temperature (R.T.) for 1 h. The membrane was then incubated for 1 h at R.T. with mouse KM93 anti-sLex monoclonal antibody (Calbiochem, EMD biosciences, LA Jolla, CA) (1:100) in 5 % BSA. The membrane was washed with TBS-T for 5 min and two additional 5 min washes. The membrane was incubated with peroxidase-conjugated goat anti-mouse IgG antibodies (1:2000) in TBS containing 5 % BSA at R.T. for 1 h. The blot was then rinsed with TBS, treated with ECL reagents (Pierce, Rockford, IL) and exposed to ECL-sensitive film (Amersham Pharmacia Biotech, Uppsala, Sweden).
RT-PCR analysis of glycosyltransferase gene expression
Total RNA was extracted from control and sodium butyrate (2 mM) treated pancreatic cancer cells using Tri reagent (MRC, Inc, USA). For cDNA synthesis, 2μg of total RNA were used as the template in a 20μl RT reaction mixture by using Reverse-iTTM 1st Strand synthesis kit (ABgene, UK). The PCR reaction was performed in a Eppendorf Mastercyclerpersonal using 100ng of cDNA template in a total volume of 25μl. PCR analysis of mRNAs of glycosyltransferases and β-actin was carried out by using forward and reverse primers listed below; for core 1 β3Gal-T gene, 5’-GATCCTCATGCAAGGCATTC-3’ and 5’-GCTCCTCCACTCATGTAGCC-3’; for β3GnT-3 (core 1 extending enzyme) gene, 5’-TTCTTCAACCTCACGCTCAAGCAG-3’ and 5’-AGCATCTCATAAGGTAGGAAG CGG-3’; for C2GnT-1 gene, 5’-ACTCGAAACACCTCTCTTTTCTGGC-3’ and 5’-GGT CAGTGTTTTAATGTCTCCAAAG-3’; for C2GnT-2 gene, 5’-TGCATTACTTGTGGG CTCTG-3’ and 5’-TCTGGGGACTTCTCATCCAC-3’; for bC2GnT-1 gene, 5’-CATC GCATCCTGCTTCAGTA-3’ and 5’-ATGTGTCTTTTGCCCACTCC-3’; for β4GalT-1 gene, 5’-AGTGACGTGGACCTCATTCC-3’ and 5’-CCGATGTCCACTGTGATTTG-3’; for β4GalT-4 gene, 5’-GCTGTTGACTTTGTGCCTGA-3’ and 5’-GCCTGGTGGAT GACGTAGAT-3’; for ST3Gal-III gene, 5’-ACTCCAGTGGGAGGAGGACT-3’ and 5’-CGTGACCCCAGAGACTTGTT-3’; for ST3Gal-IV gene, 5’-CTAGCCATCACCAG CTCCTC-3’ and 5’-CCATGAAGAAGGGGTTGAGA-3’; for ST3Gal-VI gene, 5’-TTGCCTCTCTGCTGAGGTTT-3’ and 5’-TCACCCATCAACAATTCCAA-3’; for FucT-IV gene, 5’-GAGAGGCTCAGGCCGTGCTTTT-3’ and 5’-GCAGGAGCCC AATTTCGGGCAC-3’; for FucT-VII gene, 5’-CACCTCCGAGGCATCTTCAACTG -3’ and 5’-CGTTGGTATCGGCTCTCATTCATG-3’; for β-actin gene, 5’-GTGGGGCG CCCCAGGCACCA-3’ and 5’-CTCCTTAATGTCCGGACGATTC-3’. The PCR reaction conditions for all the genes were as follows: 94 °C for 2 min (1 cycle); 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min (30 cycles); and 72 °C for 5 min. The PCR products were subjected to electrophoresis (130V, constant-voltage field) on a 1% agarose gel equilibrated in Tris-borate electrophoresis buffer containing ethidium bromide (1mg/ml). Gels were photographed under UV light.
Results
Effect of sodium butyrate on enzymes involved in the synthesis of mucin glycan core structures
Previously, we showed that sodium butyrate stimulated stably transfected transgenes [27]. In this communication, we confirmed and extended this observation in Panc 1 / MUC1f cells stably transfected with pcDNA3.1 containing bC2GnT-1 cDNA controlled by CMV promoter. As shown in Fig. 1a and 1b, butyrate treatment enhanced C2GnT activity in Panc 1/MUC1f-bC2GnT-1 cells in a time and butyrate concentration dependent fashion. Although 4 mM butyrate treatment yielded highest specific activity, excessive cytotoxicity observed at this concentration led to the decision of using 2 mM butyrate for subsequent study. The time course study showed that 24-h treatment with 2 mM butyrate produced maximal C2GnT activity. In addition, butyrate treatment stimulated not only bC2GnT-1 transgene (Fig. 1c) but also endogenous core 1 β3Gal-T gene (Fig. 1d). This enzyme generates core 1 structure, which serves as the obligatory substrate for C2GnT enzyme. However, butyrate did not have a significant effect on the endogenous ppGalNAcT activity (Fig. 1e), which initiates the synthesis of mucin glycans.
Fig. 1.
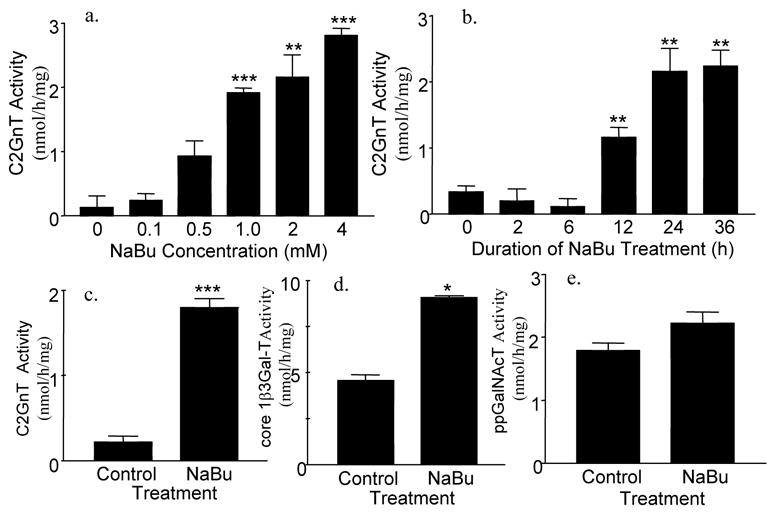
Glycosyltransferase activities in Panc 1 / MUC1f-bC2GnT-1 cells treated with sodium butyrate (NaBu). (a) C2GnT activity in Panc 1 / MUC1f-bC2GnT-1 cells treated with 0-4 mM mM NaBu for 24 h, b) C2GnT activity in Panc 1 / MUC1f-bC2GnT-1 cells treated with 2 mM NaBu for 0-36 h, c) C2GnT activity in Panc 1 / MUC1f-bC2GnT-1 cells treated with and without 2 mM NaBu for 24 h, (d) core 1 β3Gal-T activity in Panc 1 / MUC1f-bC2GnT-1 cells treated with and without 2 mM NaBu for 24 h, and (e) ppGalNAcT activity in Panc 1 / MUC1f-bC2GnT-1 cells treated with and without 2 mM NaBu for 24 h. Results of c-e were obtained from same cell homogenates. *, p<0.05; **, p < 0.01; ***, p<0.001. n = 3 independent T25 flasks.
Sodium butyrate treatment produced sLex epitope
Since expression of sLex glycotope usually accompanies the expression of C2GnT-1 gene [28]. We decided to determine if elevation of C2GnT activity in butyrate-treated Panc 1 / MUC1f-bC2GnT-1 cells would lead to the production of this glycotope. As shown in Fig. 2, treatment of Panc 1 / MUC1f-bC2GnT-1 cells with 2 mM butyrate for 36 h generated sLex carbohydrate epitope.
Fig. 2.
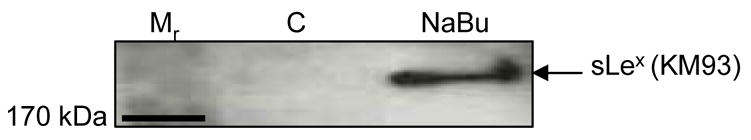
Production of sLex in Panc 1 / MUC1f-bC2GnT-1 cells treated with sodium butyrate. Panc 1 / MUC1f-bC2GnT-1 cells were treated with 2 mM NaBu for 36 h. Proteins were resolved in 6% SDS-PAGE, transferred to PVDF membrane, and probed with sLex-specific monoclonal antibody KM93. The ~250 kDa band, which corresponded to MUC 1 band [7], was detected only in butyrate-treated cells.
Sodium butyrate treatment enhanced the expression of endogenous glycosyltransferase genes involved in the synthesis of sLex
To seek the explanation for the butyrate-induced production of sLex, we employed RT-PCR to measure the expression of bC2GnT-1 and endogenous glycosyltransferase genes involved in the synthesis of sLex with and without butyrate treatment. As shown in Fig. 3, butyrate treatment of Panc 1 / MUC1f-bC2GnT-1 cells stimulated not only bC2GnT-1 transgene (Fig. 3b) but also the following endogenous human glycosyltransferase genes: core 1 β3Gal-T, β3GnT-3 (or core 1 extension enzyme) (Fig. 3a), C2GnT-2, C2GnT-3 (Fig. 3b), α2–3 sialyltransferase (ST3GalT)-IV and -VI (Fig. 3d) and Fucosyltransferase (FucT)-VII (Fig. 3e) as compared to those of untreated Panc 1 / MUC1f-bC2GnT-1 cells. Butyrate treatment did not affect the expression of the following glycosyltransferase genes: β4galactosyl transferases (β4GalT)-1 and -4 (Fig. 3c), ST3Gal-III (Fig. 3d), and FucT-IV (Fig. 3e). Expression of FucT-III gene was not detected in either control or butyrate-treated cells.
Fig. 3.
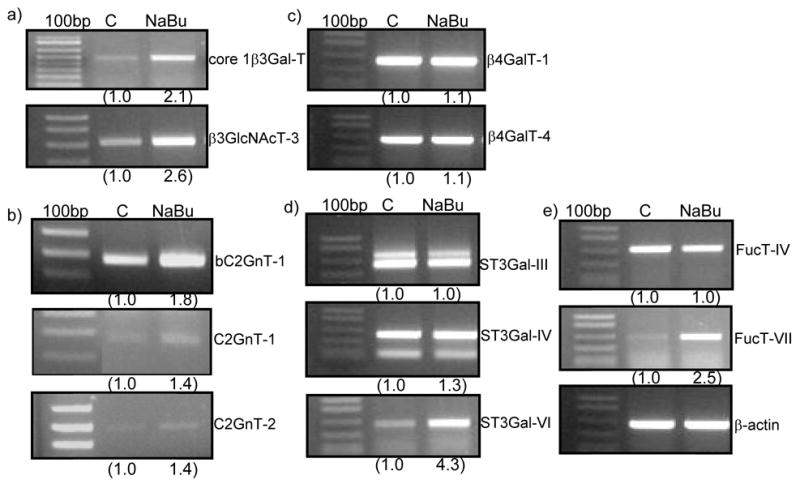
RT-PCR analysis of the expression pattern of glycosyltransferase genes involved in the synthesis of core 1, core 2, and sLex. Panc 1 / MUC1f-bC2GnT-1 cells were treated with and without 2 mM NaBu for 24 h and then analyzed by RT-PCR for the expression of the bovine (b) and human genes involved in the synthesis of core 1, core 2, and sLex. The expression of the genes upregulated by butyrate includes: core 1 β3Gal-T, β3GnT-3, bC2GnT-1, C2GnT-1, C2GnT-3, ST3Gal-VI, and FucT-VII. The expression of the genes not affected by butyrate includes: β4GalT-1 & 4, ST3Gal-III and IV, and FucT-IV and expression of FucT-III was not detected.
Discussion
Carbohydrate ligands containing sLex are involved in leukocyte migration and homing [29] and cancer metastasis [30]. sLex has been found to be elevated in many advanced cancers [30]. In this communication, we report that sLex can be generated by butyrate treatment. Butyrate has been considered as a cancer therapeutic agent because of its ability to induce apoptosis of cancer cells [20]. Given the role of this glycotope plays in cancer metastasis, current observation raise a concern about this therapeutic approach.
Generation of sLex requires a coordination of several enzymatic steps. As depicted in Figure 4, mucin glycan-associated sLex epitope can be synthesized on core 1 and/or core 2 branch elaborated by a group of glycosyltransferases. sLex on core 1 branch is built on the extension of core 1 by β3GlcNAc as catalyzed by β3GnT-3 [14] while sLex on core 2 branch is extended from core 2 β6GlcNAc structure formed by C2GnT [15]. Subsequent steps involved in the synthesis of sLex include sequential actions of GlcNAc: β4galactosyltransferase (β4Gal-T), Galβ1–4GlcNAc: (NeuAc-Gal) α2–3 sialyltransferase (ST3Gal), and NeuAcα2–3Galβ1–4GlcNAc: (Fuc-GlcNAc) α1–3/4 fucosyltransferase (FucT). Core 1β3Gal-T is the only enzyme which can synthesize core 1 structure [31] and β3GnT-3 is the only core 1 extension enzyme [32]. Core 2 can be synthesized by three different isozymes, C2GnT-1, 2, and 3 [33]. Galβ1–4GlcNAc on core 1 and core 2 branchs can be synthesized by β4GalT-1 & 4, respectively. Three different isozymes, ST3Gal-III, IV & VI, can synthesize NeuAcα2–3Galβ1–4GlcNAc from Galβ1–4GlcNAc. sLex is synthesized from NeuAcα2–3Galβ1–4GlcNAc as catalyzed by FucT-III, IV & VII. FucT-VII only uses NeuAcα2–3Galβ1–4GlcNAc as the substrate and is the most efficient enzyme for synthesis of sLex [34]. On the other hand, the primary function of FucT-III is to synthesize sLea although it also can synthesize sLex with low efficiency [35]. The primary products synthesized by FucT-IV are Lex and poly Lex although it is capable of producing sLex with low efficiency [36]. Butyrate treatment enhances not only the enzyme activities for synthesis of core 1 and core 2 structures but also the expression of the genes for extending core 1 structure and for synthesizing sLex. It is noted that expression of several genes, including β4GalT-1 & 4, ST3Gal-III & IV, and FucT-IV, which are also involved in the synthesis of sLex is not stimulated by butyrate. FucT-III gene is not expressed in this cell line. Since sLex is not detected before butyrate treatment, the genes that are activated by butyrate are likely responsible for the production of sLex. Although β4GalT-1 & 4 genes are not affected by butyrate, high basal expression of these two genes would ensure production of sufficient amount of N-acetyllactosamine for generation of subsequent intermediate and final product. With the exception of bC2GnT-1, all other enzymes are generated by endogenous genes. This point is of particular significance when we try to evaluate the potential side effects of using butyrate as a cancer therapeutic agent.
Fig. 4.
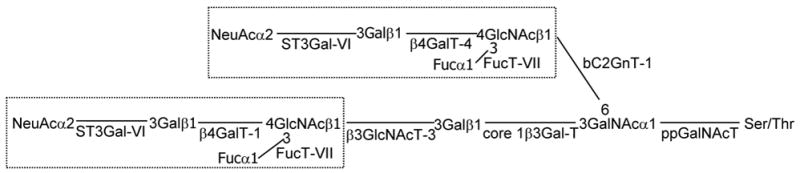
Schematic diagram of the biosynthetic pathway of biantennary sLex located on core 1 and core 2 mucin glycans. Only key glycosyltransferases involved in the synthesis of sLex on MUC1 of Panc 1 / MUC1f-bC2GnT-1 cells are shown.
Butyrate is known to modulate mucin gene expression in the intestinal epithelial goblet cells [16]. It also can enhance sLex production in HT29 cells through stimulation of FucT-III but not FucT-IV gene [37]. FucT-VII gene is not expressed in HT29 cells. Expression of other glycosyltransferases was not examined in this study. It is not clear whether they are affected by butyrate treatment. Our study provides a comprehensive analysis of the expression pattern of all glycosyltransferase genes involved in the synthesis of sLex in Panc 1 / MUC1f-bC2GnT-1 cells. As compared with HT29 cells, Panc 1 / MUC1f-bC2GnT-1 cells express FucT-IV and VII genes but not FucT-III gene. Butyrate treatment stimulates FucT-VII but not FucT-III and FucT-IV genes. Therefore, FucT-VII is likely the FucT gene responsible for the conversion of NeuAcα2–3Galβ1–4GlcNAc to sLex in these cells. Stimulation of FucT-VII and ST3Gal-VI along with bC2GnT-1 and core 1 β3Gal-T genes in response to butyrate treatment could explain the production of sLex in Panc 1 / MUC1f-bC2GnT-1 cells treated with butyrate.
Our results show that it takes up to 12 h for the butyrate effect to reach the peak. The result suggests that butyrate treatment affects gene expression rather than posttranslational modification, such as phosphorylation. Inhibition of histone deacetylation results in altered chromatin structure and gene expression [38], is one mechanism proposed for butyrate effect. However, it is not clear if same mechanism is responsible for the stimulation of endogenous glycosyltransferase genes involved in the synthesis of sLex. Since butyrate effect appears to be selective because not all glycosyltransferase genes are activated, it is predicted that the glycosyltransferase genes that are stimulated by butyrate may share common signaling pathway and/or transcription factors. Further study is needed in order to prove this hypothesis.
Acknowledgments
This work was supported in part by NIHRO1 HL48282, CFF-NIH grant, and NRI-Cancer Glycobiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukuda M, Hiraoka N, Yeh JC. C-Type Lectins and Sialyl Lewis X Oligosaccharides: Versatile Roles in Cell–Cell Interaction. J Cell Biol. 1999;147:467–470. doi: 10.1083/jcb.147.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 3.Kannagi R, Izawa M, Koike T, Miyazaki K, kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl Lex determinant on myeloid and tumor cells. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 5.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 6.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 7.Clausen H, Bennett EP. A family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases control the initiation of mucin-type O-glycosylation. Glycobiology. 1996;6:635–646. doi: 10.1093/glycob/6.6.635. [DOI] [PubMed] [Google Scholar]
- 8.Beum PV, Singh J, Burdick M, Hollingsworth MA, Cheng PW. Expression of core 2 β-1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J Biol Chem. 1999;274:24641–24648. doi: 10.1074/jbc.274.35.24641. [DOI] [PubMed] [Google Scholar]
- 9.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 Oligosaccharide Biosynthesis Distinguishes between Selectin Ligands Essential for Leukocyte Homing and Inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 10.Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Andersen V, Lance P. Expression and regulation of glycosyltransferases for N-glycosyl oligosaccharides in fresh human surgical and murine tissues and cultured cell lines. Clin Sci Colch. 1995;89:397–404. doi: 10.1042/cs0890397. [DOI] [PubMed] [Google Scholar]
- 12.Brooks SE, Timmerman J, Lau CC, Tsao SW, Knapp RC, Sheets EE. Effect of differentiation agents on expression of CA 125, alkaline phosphatase, and cytokeratins in human ovarian adenocarcinoma cells. Gynec Oncol. 1991;42:265–272. doi: 10.1016/0090-8258(91)90356-a. [DOI] [PubMed] [Google Scholar]
- 13.Bloom EJ, Siddiqui B, Hicks JW, Kim YS. Effect of sodium butyrate, a differentiating agent, on cell surface glycoconjugates of a human pancreatic cell line. Pancreas. 1989;4:59–64. doi: 10.1097/00006676-198902000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Amano J, Oshima M. Expression of the H type 1 blood group antigen during enterocytic differentiation of Caco-2 cells. J Biol Chem. 1999;274:21209–21216. doi: 10.1074/jbc.274.30.21209. [DOI] [PubMed] [Google Scholar]
- 15.Datti A, Dennis JW. Regulation of UDP-GlcNAc:Gal beta 1–3GalNAc-R beta 1–6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc) in Chinese hamster ovary cells. J Biol Chem. 1993;268:5409–5416. [PubMed] [Google Scholar]
- 16.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 17.Soldatenkov VA, Prasad S, Voloshin Y, Dritschilo A. Sodium butyrate induces apoptosis and accumulation of ubiquitinated proteins in human breast carcinoma cells. Cell Death Differ. 1998;5:307–312. doi: 10.1038/sj.cdd.4400345. [DOI] [PubMed] [Google Scholar]
- 18.Mandal M, Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell Growth and Differ. 1996;7:311–318. [PubMed] [Google Scholar]
- 19.Wang XM, Wang X, Li J, Evers BM. Effects of 5-azacytidine and butyrate on differentiation and apoptosis of hepatic cancer cell lines. Ann Surg. 1998;227:922–931. doi: 10.1097/00000658-199806000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janson W, Brandner G, Siegel J. Butyrate modulates DNA-damageinduced p53 response by induction of p53-independent differentiation and apoptosis. Oncogene. 1997;15:1395–1406. doi: 10.1038/sj.onc.1201304. [DOI] [PubMed] [Google Scholar]
- 21.Conley BA, Egorin MJ, Tait N, Rosen DM, Sausville EA, Dover G, Fram RJ, Van Echo DA. Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clin Cancer Res. 1998;4:629–634. [PubMed] [Google Scholar]
- 22.Kasukabe T, Rephaeli A, Honma Y. An anti-cancer derivative of butyric acid (pivalyloxmethyl buterate) and daunorubicin cooperatively prolong survival of mice inoculated with monocytic leukaemia cells. Br J Cancer. 1997;75:850–854. doi: 10.1038/bjc.1997.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ropp PA, Little MR, Cheng PW. Mucin biosynthesis. Purification and characterization of a mucin β6 N-acetylglucosaminyltransferase. J Biol Chem. 1991;266:23863–23871. [PubMed] [Google Scholar]
- 24.Cheng PW, Bona SJ. Mucin biosynthesis. Characterization of UDP galactose: alpha-N-acetylgalactosaminide beta 3 galactosyl transferase from human tracheal epithelium. J Biol Chem. 1982;257:6251–6258. [PubMed] [Google Scholar]
- 25.Nishimori I, Perini F, Mountjoy KP, Sanderson SD, Johnson N, Cerny RL, Gross ML, Fontenot JD, Hollingsworth MA. N-acetylgalactosamine glycosylation of MUC1 tandem repeat peptides by pancreatic tumor cell extracts. Cancer Res. 1994;54:3738–3744. [PubMed] [Google Scholar]
- 26.Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J, Clausen H. Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine: Polypeptide N-acetylgalactosaminyl transferase family, GalNAc-T1, -T2, and -T3. J Biol Chem. 1997;272:23503–23514. doi: 10.1074/jbc.272.38.23503. [DOI] [PubMed] [Google Scholar]
- 27.Choi KH, Basma H, Singh J, Cheng PW. Activation of CMV promoter-controlled glycosyltransferase and beta -galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2'-deoxycytidine. Glycoconj J. 2005;22:63–69. doi: 10.1007/s10719-005-0326-1. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi J, Shinohara H, Nonomura C, Ando H, Takaku S, Nojiri H, Nakamura M. Not core 2 beta 1,6-N-acetylglucosaminyltransferase-2 or -3 but -1 regulates sialyl-Lewis x expression in human precursor B cells. Glycobiology. 2005;15:271–280. doi: 10.1093/glycob/cwi005. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda M, Spooncer E, Oates JE, Dell A, Klock JC. Structure of sialylated fucosyllactosaminoglycan isolated from human granulocytes. J Biol Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 30.Ohyama C, Tsuboi S, Fukuda M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. The EMBO J. 1999;18:1516–1525. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zu T, Cummings RD, Canfield WM. Purification, Characterization, and Subunit Structure of Rat Core 1 β1,3-Galactosyltransferase. J Biol Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki K, Kurata-Miura K, Ujita M, Angata K, Nakagawa S, Sekine S, Nishi T, Fukuda M. Proc Natl Acad Sci U S A. 1997;94:14294–14299. doi: 10.1073/pnas.94.26.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beum PV, Cheng PW. Biosynthesis and Function of b1,6 branched mucin type glycans. In: Wu AM, editor. The molecular immunology of complex carbohydrates. Kluwer Academic/ Plenum publishers; New York: 2001. pp. 279–312. [Google Scholar]
- 34.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T. Expression cloning of a novel β1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis x carbohydrate determinants in leukocytes. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 35.Weston BW, Nair RP, Larsen RD, Lowe JB. Isolation of a novel human α (1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group β (1,3/1,4)fucosyltransferase gene. Synthetic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J Biol Chem. 1992;267:4152–4160. [PubMed] [Google Scholar]
- 36.Grabenhorst E, Nimtz M, Costa J, Conradt HS. In vivo specificity of human α1,3/4-fucosyltransferases III-VII in the biosynthesis of Lewisx and sialyl Lewisx motifs on complex-type Nglycans.Coexpression studies from BHK-21 cells together with human trace protein. J Biol Chem. 1998;273:30985–30994. doi: 10.1074/jbc.273.47.30985. [DOI] [PubMed] [Google Scholar]
- 37.Hanski C, Klussmann E, Wang J, Bohm C, Ogorek D, Hanski ML, Kruger-Krasagakes S, Eberle J, Schmitt-Graff A, Riecken EO. Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue. Glycoconj J. 1996;13:727–33. doi: 10.1007/BF00702336. [DOI] [PubMed] [Google Scholar]
- 38.Hebbes TR, Thorne AW, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988;7:1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


