Abstract
We previously reported data from 103 patients with hematological malignancies (median age 54 years) who received peripheral blood stem cell (PBSC) grafts from HLA-matched unrelated donors after nonmyeloablative conditioning and were given postgrafting immunosuppression consisting of mycophenolate mofetil (MMF; administered from day 0 until day +40 with taper through day +96) and cyclosporine (CSP; given from day -3 to day +100, with taper through day 180) (historical patients). The incidences of grade II-IV acute and extensive chronic graft-versus-host disease (GVHD) were 52% and 49%, respectively, and the 1-year probabilities of relapse, nonrelapse mortality, and progression-free survival were 26%, 18%, and 56%, respectively. Here, we treated 71 patients with hematological malignancies (median age 56 years) with unrelated PBSC grafts and investigated whether postgrafting immunosuppression with an extended course of MMF, given at full dosing until day 150 and then tapered through day 180, and a shortened course of CSP, through day 80, would promote tolerance induction and reduce the incidence of GVHD (current patients). We observed 77% grade II-IV acute and 45% extensive chronic GVHD (P=0.03, and P=0.43, respectively, in current compared to historical patients). The 1-year probabilities of relapse, nonrelapse mortality, and progression-free survival were 23%, 29%, and 47%, respectively, (P=0.89, P=0.02, and P=0.08 compared to the historical patients). We conclude that postgrafting immunosuppression with extended MMF and shortened CSP failed to decrease the incidence of GVHD among unrelated PBSC recipients given nonmyeloablative conditioning.
INTRODUCTION
In order to extend allogeneic hematopoietic cell transplantation (HCT) from unrelated donors (URD) to include older patients with hematological malignancies, those with comorbid conditions, and those who had failed high-dose HCT, several groups of investigators have developed reduced-intensity or nonmyeloablative conditioning regimens [1-5], which have relied mainly or exclusively on immune-mediated graft-versus-tumor effects for tumor eradication [6-9]. Based on experimental canine studies [10] and on subsequent clinical trials in HLA-identical sibling recipients [8], we investigated a nonmyeloablative regimen consisting of fludarabine, 3 × 30 mg/m2, and 2 Gy total body irradiation (TBI) for unrelated HCT [5,11-13]. Postgrafting immunosuppression included mycophenolate mofetil (MMF) and cyclosporine (CSP).
In a previous prospective study, MMF was given at 15 mg/kg orally thrice daily, from the evening of day 0 through day +40 with taper through day +96, and CSP was given at 5 mg/kg orally twice daily, from day -3 to day 100, with taper through day 180 [14]. Sustained engraftment was achieved in 95% patients, and cumulative incidences of grade II-IV acute GVHD and extensive chronic GVHD were 52% and 49%, respectively. Two-year probabilities of progression-free survival, relapse, and nonrelapse mortality were 49%, 31%, and 19%, respectively.
Several studies have suggested that CSP prevented activation-induced death of T cells, and thus potentially delayed the eradication of alloreactive donor T-cells, preventing tolerance induction [15-18]. Conversely, antimetabolites such as MMF could delete alloreactive T cells by inducing apoptosis [19,20], thereby favoring tolerance induction. Based on these experimental findings, we investigated whether earlier discontinuation of CSP at day 80 along with prolonged MMF administration (taper initiated at day 150) would decrease the incidence of GVHD after nonmyeloablative unrelated HCT.
PATIENTS AND METHODS
Eligibility criteria
Eligibility criteria were those previously reported [14]. Briefly, patients were included in this multi-institutional international protocol if they had hematological malignancies treatable by unrelated HCT and were older than 50 years. Younger patients were candidates if they were considered at risk for transplant-related mortality with myeloablative conditioning because of medical comorbidities or preceding failed high-dose HCT or if they refused conventional HCT. Seventy-one patients were enrolled from October 2003 to January 2005. Their characteristics are shown in Table 1. Briefly, median age was 56 (range, 15-75) years. Diagnoses were acute myeloid leukemia (n=20), myelodysplastic syndrome (n=14), non-Hodgkin lymphoma (n=11), fludarabine-refractory chronic lymphocytic leukemia (n=8), multiple myeloma (n=6), chronic myeloid leukemia (n=5), Hodgkin lymphoma (n=4), myeloproliferative disorders (n=2), and acute lymphoblastic leukemia (n=1). Forty-seven patients were ≥ 50 years old, 16 had failed high-dose HCT, seven had planned tandem autologous-allogeneic HCT, and one had morbid obesity. Sixty-nine percent of the patients had HCT-comorbidity index (HCT-CI) scores ≥ 1, including 41% who had scores ≥ 3. Patients received HCT at 10 centers including the FHCRC (n=35), Emory University in Atlanta (n=10), University of Leipzig (Germany, n=6), University of Tuebingen (Germany, n=4), Oregon Health & Science University in Portland (n=4), Rocky Mountain Cancer Center in Denver (n=3), University of Utah in Salt Lake City (n=3), VA Puget Sound Health Care System in Seattle (n=2), Medical College Wisconsin in Milwaukee (n=2), and Stanford University in Palo Alto (n=2). The protocol was approved by the Institutional Review Boards (IRB) at the FHCRC and each of the collaborating sites. All patients signed consent forms approved by the local IRB.
Table 1.
Patients
| Characteristic | Current
patients (Protocol #1668) (n=71) |
Historical
patients (Protocol #1641) (n=103) |
P value |
|---|---|---|---|
| Median patient age, y (range) | 56 (15-75) | 54 (17-69.6) | NS |
| Recipient gender, #M (%)/#F (%) | 49/22 | 63/40 | NS |
| Female donor / male recipient, # pts (%) | 11 (15%) | 21 (20%) | NS |
| Diagnosis at the time of nonmyeloablative conditioning, # (%) pts | |||
| AML/ALL | 21 (30) | 24 (23) | |
| CR1-2 | 16 (23) | 21 (20) | |
| CR>2 | 4 (6) | 1 (1) | |
| > CR | 1 (1) | 2 (2) | |
| Chronic myeloid leukemia | 5 (7) | 5 (5) | |
| CP1 | 3 (4) | 3 (3) | |
| CP2 or AP | 2 (3) | 2 (2) | |
| Fludarabine-refractory chronic lymphocytic leukemia | 8 (11) | 13 (13) | |
| Myelodysplastic syndrome | 14 (20) | 9 (9) | |
| RA | 4 (6) | 2 (2) | |
| RAEB/CR1 | 3 (4) | 4 (4) | |
| tAML CR1/CR2 | 7 (10) | 3 (3) | |
| Multiple myeloma | 6 (8) | 11 (11) | |
| CR/PR/Ref | 2(3)/3(4)/1(1) | 2(2)/6(6)/3(3) | |
| Non-Hodgkin lymphoma | 11 (15) | 24 (23) | |
| CR/PR | 5(7)/1(1) | 9(9)/6(6) | |
| Stable | 0 | 1 (1) | |
| Ref/rel | 5 (7) | 6(6)/2(2) | |
| Hodgkin lymphoma | 4 (6) | 8 (8) | |
| CR/PR | 1(1)/1(1) | 1(1)/3(3) | |
| Ref/rel | 2 (3) | 1(1)/3(3) | |
| Myeloproliferative syndrome other than chronic myeloid leukemia | 2 (3) | 3 (3) | |
| Waldenströms macroglobulinemia | 0 | 2 (2) | |
| Renal cell carcinoma | 0 | 4 (4) | |
| Disease, # pts (%) | |||
| Indolent* | 31 (44) | 40 (39) | NS |
| Aggressive† | 40 (56) | 63 (61) | |
| Comorbidity at HCT (HCT-CI score [27]), # pts (%) | |||
| 0 | 22 (31) | 30 (29) | NS |
| 1-2 | 20 (28) | 38 (37) | |
| ≥ 3 | 29 (41) | 35 (34) | |
| Donor, # pts (%) | |||
| HLA-allele Match | 65 (92) | 92 (89) | NS |
| 1 allele HLA- Mismatch | 6 (8) | 11 (11) | |
| PBSC dose, median (range) | |||
| CD34+ cells (× 106/kg recipient) | 7.4 (1.1-30.0) | 7.3 (0.8-26.3) | NS |
| T-cells (× 108/kg recipient) | 2.6 (0.8-6.7) | 2.7 (0.3-9.3) | NS |
Defined as acute myeloid leukemia in first complete remission, acute lymphoblastic leukemia in first complete remission, myelodysplastic syndrome-refractory anemia, chronic myeloid leukemia in first chronic phase, chronic lymphoblastic leukemia, low-grade non Hodgkin lymphoma, multiple myeloma in partial or complete remission, and Waldenstrom macroglobulinemia.
All other diagnoses.
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CR, complete remission; Rel, relapse; Ref, refractory; CP, chronic phase; AP, accelerated phase; RA, refractory anemia; RAEB, refractory anemia with blast excess; tAML, secondary AML; PBSC, G-CSF-mobilized peripheral blood stem cells.
Stem cell source and HLA matching
All patients were given peripheral blood stem cells (PBSC). Compatibility between patients and donors for HLA-A, -B,-C,-DRB1 and -DQB1 alleles was assessed by high-resolution DNA techniques [5]. Sixty-five pairs were matched at the allelic levels for all 10 alleles at HLA-A, -B,-C, -DRB1 and -DQB1, while six pairs were mismatched for a single HLA-A (n=2), HLA-B (n=3), or HLA-C (n=1) allele.
Conditioning regimen and postgrafting immunosuppression
Conditioning included fludarabine, 30 mg/m2/day on days -4, -3, and -2, in all patients. Sixty-eight received 2 Gy TBI (day 0), while three patients with chronic myeloid leukemia (CML) were given 3 Gy TBI in an attempt to reduce the risk of graft rejection. Postgrafting MMF was given at 15 mg/kg orally thrice daily from the evening of day 0 until day +30, at 15 mg/kg orally twice daily from day 31 until day 150, and then tapered from day 150 until day 180. CSP was given at 5 mg/kg orally twice daily from day -3 to day +80 and then discontinued without taper at day 80 in the absence of GVHD.
GVHD Grading and Therapy, and Supportive Care
Diagnosis, clinical grading and treatment of GVHD were performed according to established criteria [21]. Treatment of GVHD was based on the attending physicians’ assessment of the severity of GVHD. Initial treatment usually consisted of prednisolone, 1-2 mg/kg/day with taper initiated within 14 days. If already discontinued, CSP was usually resumed at full doses. Steroid-refractory acute GVHD was treated per available investigational protocols or other salvage regimens. Extensive chronic GVHD was usually treated with prednisone (1 mg/kg) with or without alternate-day CSP [22].
Standard prophylaxis against infections was used [23]. Patients with chronic GVHD requiring systemic immunosuppressive therapy remained on prophylaxis for Pneumocystis carinii and pneumococcal infections.
Disease-dependent restaging after HCT occurred monthly for the first 3 months and then at 6 months, 1 year, and yearly thereafter. Percentages of donor-host chimerism were assessed by fluorescent in situ hybridization for X and Y chromosomes (FISH) in recipients of sex-mismatched HCT, or by polymerase chain reaction (PCR)–based amplification of variable-number tandem repeat (VNTR) or short-tandem repeat (STR) sequences unique to donors and hosts if patients and donors were sex-mismatched [13].
Persistent, progressive, or relapsed malignancies in the absence of severe manifestations of acute and chronic GVHD were treated by rapid taper and discontinuation of systemic immunosuppression in order to initiate graft-versus-tumor effects. In addition, two patients with low (<30%) donor T-cell chimerism were given pentostatin (4mg/m2), followed two days later by donor lymphocyte infusion (DLI) on a separate protocol as preliminarily reported [24], in an attempt to avert graft rejection [25,26]).
Statistical analyses
Data were analyzed as of 4/18/2006. Overall and progression-free survivals were calculated by the Kaplan-Meier method. Cumulative incidence estimates were calculated for graft rejection, graft failure, acute and chronic GVHD, life-threatening GVHD, steroid initiation, progression/relapse, and nonrelapse mortality. Hazard ratios were estimated by Cox regression models. In order to better compare the outcomes between the two protocols, multivariate models adjusting for pre-transplant risk factors were constructed. Pre-transplant risks factors included in the models were aggressive versus indolent disease, HLA-mismatch between donors and recipients versus not, patient CMV status, patient age (over 50 versus not), female to male recipient versus not, and comorbidity at HCT [assessed by the HCT-comorbidity index (HCT-CI) [27]]. By protocol, graft failure was defined as Common Toxicity Criteria (CTC) grade IV thrombocytopenia and neutropenia persisting/occurring after day 21, lasting more than 2 weeks and refractory to hematopoietic growth factors. Graft rejection was defined as the inability to detect or loss of detection of ≥ 5% donor T-cells [26]. All p-values refer to hazard ratio comparisons, and are 2-sided.
RESULTS
Engraftment
Median donor T-cell chimerism levels on days 28, 180, and 365 after HCT were 89%, 99%, and 100%, respectively, in current patients, versus 92%, 99%, and 100% in historical patients. Sustained engraftment was achieved in 68 current patients (96%) versus in 98 historical patients (95%). Two of 3 current CML patients conditioned with 3 Gy TBI had either protocol defined primary graft failure (without fulfilling the protocol’s criteria for graft rejection (see below), or graft rejection (defined as the inability to detect or loss of detection of ≥ 5% donor T-cells).
GVHD
The cumulative incidences of grade II-IV and grade III-IV acute GVHD were 77% and 26% among current patients versus 52% (P=0.03; P=0.02 after adjusting for pre-transplant risk factors [Table 2]) and 15% (P=0.11; P=0.16 after adjusting for pre-transplant risk factors [Table 2]) among historical patients, respectively (Figure 1A). Specifically, grades II, III and IV acute GVHD were seen in 51%, 16% and 10% of current patients, respectively, versus 38%, 11% and 4% of historical patients, respectively. One of the 7 current cases of grade IV acute GVHD occurred after abrupt discontinuation of postgrafting immunosuppression for progressive disease, and another after pentostatin (4 mg/m2) and DLI for prevention of graft rejection. Three additional cases of grade IV acute GVHD occurred after discontinuation of CSP on day 80. Seven of 11 historical patients mismatched with their donor for a single HLA-class I allele experienced grade II-IV acute GVHD (including one patient with grade IV acute GVHD), while 5 of 6 current patients who mismatched with their donor for a single HLA-class 1 allele had grade II-IV acute GVHD (including one patient with grade IV acute GVHD). The 1-year cumulative incidence of extensive chronic GVHD was 45% among current patients versus 49% among historical patients (P=0.43; P=0.48 after adjusting for pre-transplant risk factors [Table 2]) (Figure 1B).
Table 2.
Outcomes for current (n=71) relative to historical (n=103) patients
| Endpoint | Cumulative Incidence at
1 year |
Adjusted* HR† (95% CI) |
Adjusted* P-value |
|
|---|---|---|---|---|
| Historical
patients |
Current
patients |
|||
| Overall survival | 68% | 55% | 1.58 (1.0-2.5) | 0.05 |
| Progresion-free survival | 56% | 47% | 1.51 (1.0-2.3) | 0.05 |
| Relapse/progression | 26% | 23% | 1.22 (0.7-2.2) | 0.50 |
| Nonrelapse mortality | 18% | 29% | 1.93 (1.1-3.5) | 0.03 |
| Grade II-IV acute GVHD | 52% | 77% | 1.67 (1.1-2.5) | 0.02 |
| Grade III-IV acute GVHD | 15% | 26% | 1.65 (0.8-3.3) | 0.16 |
| Extensive chronic GVHD | 49% | 45% | 0.85 (0.5-1.3) | 0.48 |
Adjusted for disease risk (aggressive versus indolent disease), one HLA-allele mismatch between donor and recipient or not, recipient CMV serostatus, recipient age (over 50 years old versus not), female donor to male recipient, and comorbidity at HCT (HCT-CI score 0,1-2, ≥ 3).
HR for outcomes in current relative to historical patients. HR > 1 refers to worse outcomes.
Figure 1.
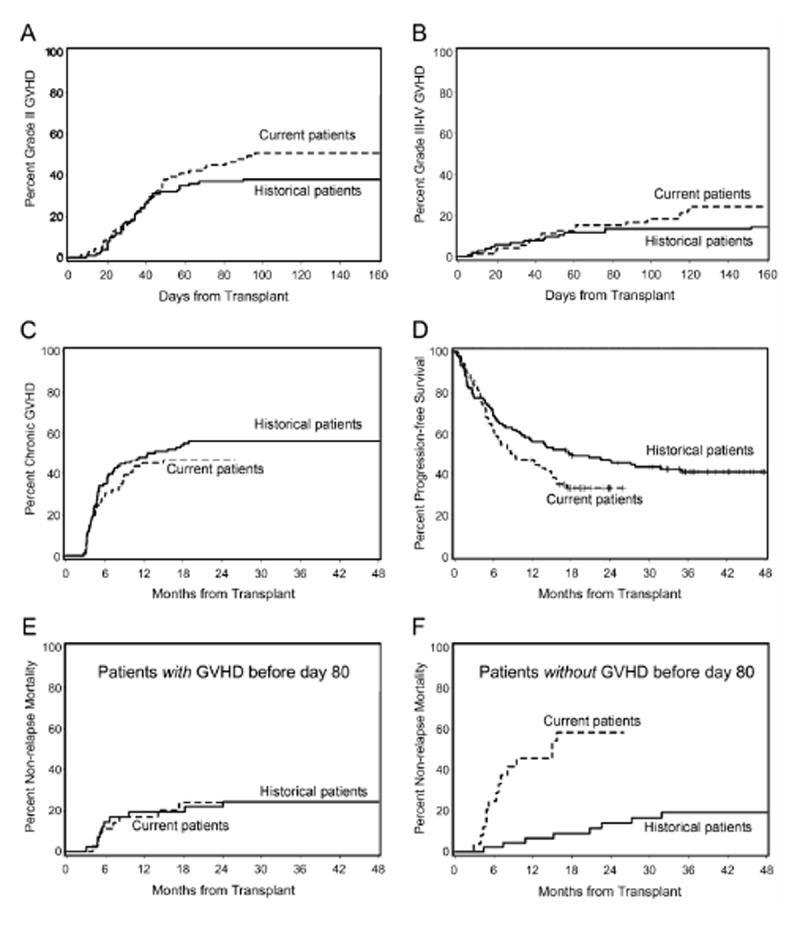
Cumulative incidences of grades II acute GVHD (A), III-IV acute GVHD (B) and chronic extensive GVHD (C) in patients included in the current protocol (protocol #1668; extended MMF and truncated CSP, n=71), or in the historical protocol (protocol #1641; n=103). Progression-free survival in patients included in the current protocol or in the historical protocol (D). Cumulative incidences of nonrelapse mortality in the current and in the historical protocols among patients who experienced (E) or did not experience (F) grade II-IV acute GVHD before day 80.
Relapse, nonrelapse mortality, survival
The current and historical 1-year cumulative incidences of relapse/progression were similar: 23% versus 26%, respectively (P=0.89; P=0.50 after adjusting for pre-transplant risk factors [Table 2]). However, the current 1-year cumulative incidence of nonrelapse mortality was higher, 29%, as compared to the historical one of 18% (P=0.02; P=0.03 after adjusting pre-transplant risk factors [Table 2]). In order to determine whether abrupt CSP discontinuation on day 80 was in part responsible for the increased current nonrelapse mortality, we compared nonrelapse mortality in patients with grade II-IV acute GVHD before day 80, who therefore were continued on CSP, and in patients without grade II-IV acute GVHD on day 80 whose CSP was discontinued. Current and historical nonrelapse mortalities were comparable in patients with acute GVHD before day 80 [HR 1.12 (0.4-3.2), P=0.83], while nonrelapse mortality was higher in current patients without grade II-IV acute GVHD on day 80 [HR 10.1 (3.4-30), P<0.0001] (Figure 1C). Current causes of death in these patients included grade III (n=1) or grade IV (n=4) acute GVHD, chronic GVHD (n=3), graft failure (n=2), infection (n=1), pulmonary embolism (n=1), chronic heart failure (n=1), and unknown (n=1). Taken together, these data suggested that early CSP discontinuation on day 80 contributed to higher nonrelapse mortality.
The current 1-year overall and progression-free survivals were 55% and 47% versus 68% (P=0.06; P=0.05 after adjusting for pre-transplant risk factors) and 56% (P=0.08; P=0.05 after adjusting for pre-transplant risk factors [Table 2]), respectively, among historical patients (Figure 1D).
DISCUSSION
GVHD with or without infection has been the leading cause of nonrelapse mortality after transplantation following nonmyeloablative conditioning [9,28]. Since CSP [15-18] but not MMF inhibited activation-induced death of T-cells [19,20], we reasoned that early CSP discontinuation combined with extended MMF administration might help promoting tolerance induction, and reduce the incidences of late acute and chronic GVHD.
However, we observed that prolonging MMF and truncating CSP administration failed to reduce GVHD. On the contrary, while chronic GVHD was comparable in the two protocols, grade II-IV acute GVHD was significantly more frequent among current than historical patients. There are two possible explanations for this finding. First, from day 28 to day 40, MMF was given b.i.d. in current versus t.i.d. in historical patients. That explanation is unlikely, however, given that t.i.d. MMF administration, while apparently effective in reducing the risk of graft rejection, did not alter the GVHD incidence compared to original b.i.d. MMF administration [5]. Second, and most likely, our hypothesis of tolerance induction through early discontinuation of the calcineurin inhibitor and continuation of the antimetabolite was incorrect. As a result, more current than historical patients developed acute GVHD after day 80 including four with grade IV GVHD. Apparently, administration of a calcineurin inhibitor for at least 6 months after HCT is needed for successful establishment of graft-host-tolerance. This concept is in agreement with the recent observation by Burroughs et al. in patients given grafts from related donors that extending CSP administration through day 180 compared to CSP discontinuation at earlier time points reduced the incidence of grade III-IV acute GVHD from 15–20% to <5% [29].
Likely, as a result of the increase in acute GVHD, nonrelapse mortality in current patients was significantly higher than among historical patients. The increase in nonrelapse mortality occurred among patients without acute GVHD on day 80, while it was similar among patients who experienced acute GVHD before day 80 and, therefore, continued receiving CSP. Strong associations between acute GVHD and nonrelapse mortality have been reported by us and others after nonmyeloablative or reduced-intensity conditioning [9,30-34]. Not surprisingly, the increased nonrelapse mortality among current patients translated into both decreased overall and progression-free survivals compared to historical patients.
The increased incidence of grade II-IV acute GVHD in current patients did not translate into lower risk of relapse. We acknowledge that caution is in order when comparing relapse incidences between the two groups of patients given the heterogeneity of underlying diseases. However, this observation is in agreement with previous studies by our group [9] and by others [30,35,36], showing that occurrence of grade II-IV acute GVHD was not significantly associated with lower risk of relapse after nonmyeloablative conditioning. In contrast, occurrence of chronic GVHD has been significantly associated with lower risk of relapse after nonmyeloablative conditioning, particularly in patients with acute myeloid leukemia or myelodysplastic syndrome [9,35,36].
While 96% of current patients achieved sustained engraftment, similar to what was observed in the previous protocol (95%), two patients with CML had graft rejection/failure, even though the TBI dose had been increased from 2 Gy to 3 Gy. A high rate of graft rejection among CML patients given unrelated grafts after relatively more intense “reduced-intensity” conditioning has been reported by other investigators [1,37]. A graft failure rate of 44% (3 of 8 patients) was described after a reduced intensity conditioning regimen that combined fludarabine (150 mg/m2), i.v. busulfan (6.6 mg/kg), and ATG [37], while 5 of 22 evaluable patients giving unrelated marrows after conditioning with 5.5 Gy TBI and cyclophosphamide also experienced graft failure [1].
In conclusion, postgrafting immunosuppression with extended MMF and shortened CSP increased the incidence of acute GVHD in unrelated HCT recipients given nonmyeloablative conditioning. It would appear that extending calcineurin inhibitors for six months is required for better control of GVHD. Extending that use beyond that time is of questionable value, given that a prospective randomized study comparing 6 vs. 24 months CSP administration failed to show differences in outcomes [38].
Acknowledgments
The authors wish to thank the data coordinators Heather Hildebrandt and Debbie Bassuk, and the study nurses, Mary Hinds, John Sedgwick, and Michelle Bouvier, for their invaluable help in making the study possible. The authors also are grateful Helen Crawford, Bonnie Larson, and Sue Carbonneau for manuscript preparation, all physicians, nurses and support personnel for their care of patients on this study, and the patients for their willingness to participate in this clinical trial.
Grants: Supported in part by grants CA78902, CA92058, CA18029, CA49605, HL36444, CA15704 and HL088021 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. R.S. also received support from the Laura Landro Salomon Endowment Fund. M.S. received support from the Paros Family Fund. F.B. is a research associate of the National Fund for Scientific Research (FNRS) Belgium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hallemeier C, Girgis M, Blum W, et al. Outcomes of adults with acute myelogenous leukemia in remission given 550 cGy of single-exposure total body irradiation, cyclophosphamide, and unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2004;10:310–319. doi: 10.1016/j.bbmt.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–3059. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 3.Nagler A, Aker M, Or R, et al. Low-intensity conditioning is sufficient to ensure engraftment in matched unrelated bone marrow transplantation. Exp Hematol. 2001;29:362–370. doi: 10.1016/s0301-472x(00)00655-x. [DOI] [PubMed] [Google Scholar]
- 4.Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantion by using a nonmyeloablative conditioning regimen. Blood. 2002;99:1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 5.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 6.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 7.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 8.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 9.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 10.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 11.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 12.Baron F, Maris MB, Storer BE, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11:272–279. doi: 10.1016/j.bbmt.2004.12.326. [DOI] [PubMed] [Google Scholar]
- 13.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 14.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339:625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- 16.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 18.Blaha P, Bigenzahn S, Koporc Z, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. erratum appears in Blood. 2003 Sep 15;102(6):1950. [DOI] [PubMed] [Google Scholar]
- 19.Chapuis AG, Paolo RG, D’Agostino C, et al. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med. 2000;6:762–768. doi: 10.1038/77489. [DOI] [PubMed] [Google Scholar]
- 20.Izeradjene K, Revillard JP. Apoptosis of superantigen-activated T cells induced by mycophenolate mofetil treatment. Transplantation. 2001;71:118–125. doi: 10.1097/00007890-200101150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan KM. Graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. Oxford, UK: Blackwell Publishing Ltd; 2004. pp. 635–664. [Google Scholar]
- 22.Koc S, Leisenring W, Flowers MED, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 23.Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–520. doi: 10.1053/bbmt.2002.v8.pm12374456. [DOI] [PubMed] [Google Scholar]
- 24.Sandmaier BM, Maloney DG, Maris MB, et al. Conversion of low donor chimerism following nonmyeloablative conditioning for hematopoietic cell transplantation (HCT) using pentostatin and donor lymphocyte infusion (DLI) Blood. 2004;104(Part 1):57a. #186 abstr. [Google Scholar]
- 25.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 26.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20:1690–1700. doi: 10.1038/sj.leu.2404335. Review. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron F, Sandmaier BM. Current status of hematopoietic stem cell transplantation after nonmyeloablative conditioning. Curr Opin Hematol. 2005;12:435–443. doi: 10.1097/01.moh.0000177830.63033.9d. [DOI] [PubMed] [Google Scholar]
- 29.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 30.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 31.Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;105:4532–4539. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 32.Baron F, Storb R, Storer BE, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopietic cell transplantation. J Clin Oncol. 2006;24:4150–4157. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 33.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 34.Mielcarek M, Burroughs L, Leisenring W, et al. Prognostic relevance of “early-onset” graft-versus-host disease following nonmyeloablative hematopoietic cell transplantation. Br J Haematol. 2005;129:381–391. doi: 10.1111/j.1365-2141.2005.05458.x. [DOI] [PubMed] [Google Scholar]
- 35.Cao TM, Shizuru JA, Wong RM, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105:2300–2306. doi: 10.1182/blood-2004-04-1473. [DOI] [PubMed] [Google Scholar]
- 36.Blaise DP, Boiron JM, Faucher C, et al. Reduced intensity conditioning prior to allogeneic stem cell transplantation for patients with acute myeloblastic leukemia as a first-line treatment. Cancer. 2005;104:1931–1938. doi: 10.1002/cncr.21418. [DOI] [PubMed] [Google Scholar]
- 37.Bornhauser M, Thiede C, Platzbecker U, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res. 2001;7:2254–2262. [PubMed] [Google Scholar]
- 38.Kansu E, Gooley T, Flowers MED, et al. Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood. 2001;98:3868–3870. doi: 10.1182/blood.v98.13.3868. Brief Report. [DOI] [PubMed] [Google Scholar]


