Abstract
Microglia of the central nervous system serve a variety of functions that may ultimately lead to the development or detriment of neighboring neuronal and vascular cells. These scavengers of the nervous system have been associated with a variety of neurodegenerative disorders, but the toxic potential of microglia is equally balanced by the protective nature of these cells to exclude foreign microorganisms and promote new tissue proliferation and reorganization. To this extent, our work outlines a series of endogenous microglial cellular pathways that can constitute protection for microglia against during oxygen-glucose deprivation (OGD). We demonstrate in both primary microglia and the microglial cell line EOC 2 that endogenous microglial protection against OGD relies upon the activation and expression of the phosphatidyl-inositol 3-kinase pathways of mammalian target of rapamycin (mTOR) and protein kinase B (Akt1), since pharmacological inhibition of mTOR or Akt1 as well as the gene silencing of Akt1 protein expression leads to significantly increased microglial apoptotic cell injury, DNA fragmentation, and membrane phosphatidylserine exposure. The mTOR pathway may offer endogenous protection through mechanisms that do not entirely rely upon inhibition of glycogen synthase kinase-3β (GSK-3β) activity while Akt1 appears to converge upon the necessary blockade of GSK-3β. Closely aligned to these endogenous protective mechanisms is the subcellular presence and nuclear translocation of nuclear factor-κB p65 (NF-κB p65), since microglial cell injury is significantly increased during the gene silencing of NF-κB p65. Elucidating the underlying pathways that can afford endogenous protection and maintain functional integrity of microglia should offer new prospects for the treatment of a broad range of nervous system disorders.
Keywords: Akt1, apoptosis, gene silencing, GSK-3β, inflammation, macrophage, microglia, NF-κB, oxygen-glucose deprivation, protein kinase B, oxidative stress, phosphatidylserine, siRNA
Introduction
Microglia are monocyte derived cells in the central nervous system that are usually maintained in a quiescent state, but can become activated during a variety of pathological insults (1-5) and lead to the removal of injured as well as non-injured cells (6,7). Through this process, microglia can generate reactive oxygen species and cytokines that have been associated with several neurodegeneration disorders, such as Alzheimer's disease and Huntington's disease (8-11). Furthermore, during periods of acute ischemic cell injury, activation of microglia can parallel the induction of cellular apoptosis and correlate well with the severity of the ischemic insult (12,13).
Yet, the toxic potential of microglia is equally balanced by the protective nature of microglia to exclude foreign microorganisms from the central nervous system and promote cellular repair that serves as a basis for new tissue proliferation and reorganization (4,14,15). In light of beneficial attributes of microglia, recent investigations have emphasized the importance of protecting the integrity and function of glial cells with exogenous agents (16). For example, work involving the trophic factor erythropoietin has demonstrated that excessive microglial cell activation can be modulated through the control of cellular membrane phosphatidylserine (PS) exposure (17,18), protein kinase B (12), and the regulation of caspases (19,20). Interestingly, erythropoietin also has the capacity to independently maintain microglial cell integrity during paradigms that would otherwise lead to microglial cell death (21). In this regard, other studies have focused upon the use of low concentrations of free radical exposure to prevent injury to glial cells during oxidative stress (22,23).
With our present studies, we have forged upon an alternative investigative approach to elucidate intrinsic cellular pathways that are endogenously controlled by microglia and can maintain microglial cell integrity during oxidative stress. In particular, cellular mechanisms that may be closely integrated with endogenous microglial protection are the phosphatidylinositol 3-kinase (PI 3-K) pathways of the mammalian target of rapamycin (mTOR) and protein kinase B, or Akt1. mTOR is a pro-survival factor that leads to enhanced survival in neurons and astrocytes (24,25) and may be necessary for the control of cellular metabolism (26). Akt1, which has been associated with microglial cell proliferation (27-29), plays a central role during nervous system injury to enhance cell survival during oxidative stress (12,13,30-33) and specifically modulate the inflammatory activity of microglia (13,31,34). Although mTOR is controlled by the PI 3-K and Akt1 pathways and possesses the reciprocal ability to enhance Akt1 activity (35,36), these cellular mechanisms do not function in isolation to promote cell survival. In fact, Akt1 is tied to the inhibition of glycogen synthase kinase-3β (GSK-3β) activity to increase cell survival during oxidative stress and, as a result, GSK-3β is considered to be a therapeutic target for some neurodegenerative disorders (37-40). GSK-3β also may control monocyte chemoattractant protein 1 (MCP-1) transcription through nuclear factor-κB (NF-κB) (41) to influence inflammatory cell activation (42). In addition, some injury models suggest that microglia also can upregulate NF-κB (43) and may require NF-κB for tissue repair (44). Although NF-κB has not consistently been found to enhance cell survival in all cell systems (45,46), NF-κB does represent a critical pathway that is responsible for the activation of inhibitors of apoptotic proteins (IAPs), the maintenance of Bcl-xL expression (47,48), and the induction of cytoprotection by trophic factors (49-51).
Here we show that primary cerebral microglia and the microglial cell line EOC 2 have the novel capacity to provide endogenous cellular protection, albeit to a limited extent, to prevent eventual cellular demise during oxygen-glucose deprivation (OGD)-induced oxidative stress. This endogenous microglial protection against OGD relies upon the activation and expression of the PI 3-K pathways mTOR and Akt1, since pharmacological inhibition of mTOR or Akt1 as well as the gene silencing of Akt1 protein expression leads to significantly increased apoptotic cell injury, DNA fragmentation, and membrane PS exposure. mTOR provides endogenous protection through pathways that do not entirely rely upon inhibition of GSK-3β activity while Akt1 appears to converge upon the required blockade of GSK-3β. Microglia also employ an additional level of endogenous protection through the presence of NF-κB p65 with its nuclear translocation to initiate anti-apoptotic programs.
Materials and methods
Primary microglial cultures
Per our prior protocols (12,13,31), microglia were obtained from the cerebral cortex of E-19 Sprague-Dawley rat pup, mechanically dissociated, and seeded in 75 cm2 plastic flasks at a density of 8.5×106 cells per flask. Microglia were purified from mixed cultures with reciprocal shaking at 180 rpm for 15 h and then re-seeded at 105 cells/ml for cell adhesion of 3-h duration to yield an almost pure preparation of microglia (>98%). Microglial cells were identified by α-naphthyl acetate esterase, OX-42, and isolectin B4 from griffonia simplicifolia (Sigma, St. Louis, MO). The cells did not stain for glial fibrillary acidic protein (GFAP).
Microglia cell line cultures
The microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA). Cells were maintained in Dulbecco's modified Eagle's medium (ATTC), supplemented with 10% fetal bovine serum (Sigma), 50 µg/ml penicillin and streptomycin and 20% media from the LADMAC cell line (ATCC) which contains colony stimulating factor-1 (CSF-1) secreted by LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5×106 cells per well or 4×106 cells per dish.
Experimental treatments
Oxygen-glucose deprivation (OGD)
OGD in microglial cultures was performed by replacing media with glucose-free HBSS containing 116 mmol/l NaCl, 5.4 mmol/l KCl, 0.8 mmol/l MgSO4, 1 mmol/l NaH2PO4, 0.9 mmol/l CaCl2, and 10 mg/l phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37°C for 6 h or per the experimental paradigm. For mammalian target of rapamycin (mTOR) inhibition, rapamycin (Sigma) at 1, 10, and 20 nM was applied to cultures 1 h prior to OGD. To inhibit Akt activation (phosphorylation), the Akt selective inhibitor D-2,3-dieoxy-myo inositol l-(R)-2-methoxy-3-(octa-decyloxy) propyl hydrogen phosphate (SH-6) (Alexis, San Diego, CA) was applied to microglial cultures 1 h prior to OGD. The glycogen synthase kinase (GSK)-3β inhibitors SB216763 [3-(2,4-Dichlorophenyl)-4-(l-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione] (SB21) or SB415286 [3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione] (SB41) (Tocris, Ellisville, MO) were applied continuously to the microglial cultures 1 h prior to OGD.
Assessment of primary microglia and EOC 2 cell survival
Cell injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 h following treatment with OGD per our previous protocols (49). The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10-20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures.
Assessment of DNA fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (13,30). Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3′-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Assessment of membrane phosphatidylserine (PS) residue externalization
Phosphatidylserine (PS) exposure was assessed through the established use of Annexin-V. Per our prior protocols (12,13), a 30-µg/ml stock solution of Annexin-V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/m in warmed calcium containing binding buffer (10 mmol/l HEPES, pH 7.5, 150 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 1.8 mmol/l CaCl2). Plates were incubated with 500 µl of diluted Annexin-V for 10 min. Images were acquired with ‘blinded’ assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
Small interfering RNA (siRNA) transfection
Akt1 siRNA
Primary rat microglia were plated into 35 mm dishes or 24-well plates. To silence Akt1 gene expression, commercial reagents using the SMARTpool Akt1 siRNA kit (Upstate, Lake Placid, NY) were used. Transfection of siRNA duplexes were performed with Oligofectamine reagent according to manufacturer's guidelines (Invitrogen, Carlsbad, CA).
NF-κB p65 siRNA
NF-κB p65 siRNA was selected by targeting the sequence 5′-AACATCCCTCAGCACCATCAA-3′ and was designed by using Silencer® siRNA construction kit synthesized by Ambion (Austin, TX). Primary rat microglia were seeded into 35 mm dishes and transfection of siRNA duplexes was performed in cells using the siPORT™ Amine transfection agent (Ambion) according to the guidelines provided by the manufacturer. For both Akt1 siRNA and NF-κB p65 siRNA, experimental assays were performed 72 h post-transfection and for each siRNA assay, negative controls contained multiple siRNAs including the target siRNA and positive controls were absent of the target siRNA.
Expression of phosphorylated Akt1, phosphorylated β-catenin, phosphorylated glycogen synthase kinase, and NF-κB p65
Cells were homogenized and following protein determination, each sample (50 µg/lane) was then subjected to 7.5% SDS-polyacrylamide gel electrophoresis. After transfer, the membranes were incubated with a rabbit polyclonal antibody against a mouse monoclonal antibody against phosphorylated Akt1 (p-Akt1, Ser473, 1:1000, Active Motif, Carlsbad, CA), a rabbit antibody against phosphorylated-β-catenin (1:1000) (p-β-catenin, Ser33/37 Thr41, Cell Signaling, Beverly, MA), a rabbit antibody against phosphorylated GSK-3β (p-GSK-3β, Ser9), or a primary rabbit antibody against NF-κB p65 (1:200) (Santa Cruz Biotechnologies, Santa Cruz, CA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody (goat anti-mouse IgG, 1:2000 (p-Akt1) or goat anti-rabbit IgG, 1:15000 (p-β-catenin, p-GSK-3β, NF-κB p65). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
NF-κB p65 immunocytochemistry
For immunocytochemical staining of NF-κB p65, microglial cells were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were then incubated with rabbit anti-NF-κB p65 antibody (1:150, Santa Cruz Biotechnology) overnight at 4°C and then with biotinylated anti-rabbit IgG (1:50, Vector Laboratories) for 2 h followed by Texas Red streptavidin (1:50, Vector Laboratories) for 1 h followed by incubation with Texas Red streptavidin (1:50, Vector Laboratories) for 1 h. Cells are washed in PBS and then stained with DAPI (Sigma) for nuclear identification. Protein for NF-κB p65 was imaged with fluorescence at the wavelengths of 565 nm (red) and 400 nm (DAPI nuclear staining).
Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Student's t-test. Statistical significance was considered at P<0.05.
Results
OGD leads to progressive cell injury, DNA fragmentation, and membrane PS exposure
In Fig. 1A, microglial survival was significantly reduced over a 12-h course following OGD application to 61±4% (4 h), 36±3% (6 h), 33±2% (8 h), and 28±3% (12 h) when compared with untreated control cultures (91±3%, P<0.01). Since an OGD exposure period of 6 h resulted in survival rate of approximately 40% (60% microglial cell loss), this duration of OGD toxicity was employed for the remainder of the experimental paradigms.
Figure 1.
OGD leads to progressive primary microglial cell apoptotic injury that is reduced by mTOR activation. (A), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and cell survival was determined 24 h after OGD. The cell survival was progressively decreased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (B), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and DNA fragmentation with TUNEL was determined 24 h after OGD. Apoptotic DNA fragmentation was progressively increased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (C), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and membrane PS exposure with Annexin-V was determined 24 h after OGD. Early apoptotic PS externalization was progressively increased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (D), The mTOR inhibitor rapamycin at concentrations of 1.0-20.0 nM was applied to the EOC 2 microglial cell line 1 h prior to a 6-h period of OGD and cell survival was assessed 24 h later. Increased microglial cell injury by rapamycin administration during OGD was evident in microglial when compared with cultures exposed to OGD alone (*P<0.01 vs. control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM. Control indicates untreated cultures.
We also assessed early apoptotic membrane PS exposure and late apoptotic DNA fragmentation in microglia during progressive OGD exposure. Primary cultured microglia were exposed to a series of time periods (2, 4, 6, 8, and 12 h) of OGD. Cellular genomic DNA fragmentation was determined with the TUNEL assay and cell membrane PS exposure was assessed by Annexin-V labeling 24 h after OGD exposure. In Fig. 1B, a significant increase in DNA fragmentation was present in primary microglia following a 2-h (25±4%), 4-h (43±3%), 6-h (66±5%), 8-h (68±3%), and 12-h (70±3%) period of OGD when compared with untreated controls (9±3%). Similarly, as shown in Fig. 1C, a significant increase in membrane PS exposure was observed in microglia 24 following a 2-h (33±3%), 4-h (48±4%), 6-h (68±3%), 8-h (70±3%) and 12-h (72±5%) period of OGD when compared with untreated control (15±3%).
Loss of mTOR activity enhances microglial cell injury during OGD
mTOR is a pro-survival factor that leads to enhanced survival of cells (24,25) and may modulate intracellular energy reserves (26). As a result, we examined the effects of loss of mTOR activity during its inhibition with a series of concentrations of rapamycin that have been shown to block mTOR activity (35,36). Rapamycin falls under the macrolide group of antibiotics and is believed to form an inhibitory complex with the FKBP-rapamycin-binding domain of mTOR which may destabilize mTOR and prevent phosphorylation of downstream pathways involving cytosolic phosphorylated 70 kDa ribosomal protein kinase (p70s6) and 4E-BP1 (52,53). Although rapamycin 1 nM led to a less than significant reduction in microglial survival, further increases in rapamycin concentration with 10 and 20 nM significantly reduced microglial survival during OGD, demonstrating that the mTOR pathway can provide endogenous protection to microglia during oxidative stress (Fig. 1D).
Akt1 offers endogenous protection to microglia during OGD exposure
Western blot assay was performed for phosphorylated Akt1 (p-Akt1) (activated form of Akt1) (Fig. 2A) 12 h following OGD. For Akt1 phosphorylation, Ser473 was examined since phosphorylation of Ser473 is a primary component for the activation of Akt1 (54). During OGD, the expression of p-Akt1 was slightly increased, suggesting that this mild up-regulation of Akt1 activity may offer some endogenous level of protection during OGD. This increased expression of p-Akt1 by OGD was blocked by application of the specific Akt1 inhibitor SH-6 (20 µmol/l) (55).
Figure 2.
Akt1 confers endogenous protection to microglial during OGD. (A), Equal amounts of primary microglial protein extracts (50 µg/lane) were immunoblotted with anti-phospho-Akt1 (p-Akt1, active form) antibody 12 h following a 6-h period of OGD exposure. Application of the specific Akt1 inhibitor SH6 (20 µM) or transient transfection of Akt1 siRNA in primary microglia for 72 h prior to OGD blocked the expression of p-Akt1 (*P<0.01 vs. control; †P<0.01 vs. OGD). (B), Representative images and quantification of data are illustrated for trypan blue staining in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA significantly decreased cell survival during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. (C), Representative images and quantification of data are illustrated for DNA fragmentation with TUNEL in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA for 72 h prior to OGD significantly increased DNA fragmentation during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate DNA fragmentation in microglia while white arrows illustrate absence of genomic DNA injury. (D), Representative images and quantification of data are illustrated for early apoptotic PS exposure with Annexin-V in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA for 72 h prior to OGD significantly increased membrane PS externalization during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM. Control indicates untreated cultures.
In Fig. 2B, application of SH-6 (20 µmol/l) at a concentration that blocks activation of Akt1 through phosphorylation (Fig. 2A) during OGD resulted in enhanced cell injury during OGD, illustrating that microglia require some level of Akt1 activation for endogenous protection. Green arrows indicate. trypan blue uptake into microglia while white arrows illustrate lack of trypan blue uptake. In a similar manner for analysis with DNA fragmentation and membrane PS exposure, SH-6 (20 µmol/l) administration resulted in a significant increased DNA fragmentation (Fig. 2C) and increased membrane PS exposure (Fig. 2D) in microglia, further supporting the premise that primary microglia employ activation of Akt1 to maintain cell integrity and prevent apoptotic demise. Green arrows indicate DNA fragmentation in microglia while white arrows illustrate lack of genomic DNA injury. SH-6 (20 µmol/l) administered in the absence of OGD was not toxic to microglia (data not shown).
Since pharmacological inhibition with SH-6 can have limitations in modulating the specific activity of a protein, we have incorporated into our studies siRNA gene silencing of Akt1 to specifically knockdown Akt1 activity. Microglia were transfected with Akt1 siRNA and the expression of total Akt1 and p-Akt1 was observed through Western blot analysis. Gene silencing of Akt1 during OGD exposure prevents phosphorylation of Akt1 (Fig. 2A). As shown in Fig. 2B, loss of Akt1 expression during Akt1 gene silencing further decreased microglial cell survival from 38±3% during OGD alone to 11±2% with Akt1 siRNA administration. Results with Akt1 gene silencing during DNA fragmentation (Fig. 2C) and PS exposure (Fig. 2D) also demonstrated that the absence of Akt1 expression led to increased DNA fragmentation and membrane PS exposure during OGD. Transfection with Akt1 siRNA alone did not significantly alter cell survival, DNA fragmentation, or membrane PS exposure when compared to controls (data not shown).
GSK-3β inhibition confers protection to microglia downstream from mTOR and Akt1 against apoptotic DNA fragmentation and membrane PS exposure
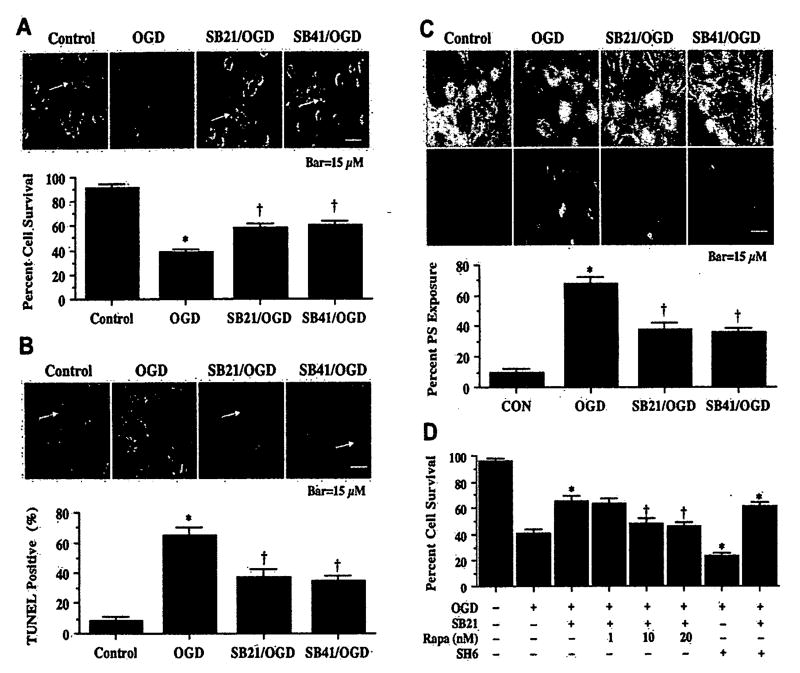
In our studies that examine the effects of GSK-3β inhibition, we used the agents SB216763 and SB415286 that are selective for inhibition of GSK and do not block the activity of Akt, mitogen-activated protein kinases, p70s6, or pyruvate dehydrogenase kinase, isozyme 1 (PDK1) (56). Twenty-four hours following OGD exposure, cellular injury and microglial trypan blue uptake is evident in primary microglia (Fig. 3A). In contrast, administration of the GSK-3β inhibitors [SB216763, (SB21), 5 µM or SB415286, (SB41), 25 µM] 1 h prior to OGD in concentrations consistent with the current literature (57) increase microglial cell survival and prevent trypan blue uptake. Green arrows indicate trypan blue uptake into microglia while white arrows illustrate the absence of trypan blue uptake. Quantification of these results reveals that inhibition of GSK-3β activity with SB21 or SB41 1 h prior to OGD significantly increases microglial survival when compared to OGD alone, suggesting that inhibition of GSK-3β activity also can confer endogenous protection to microglia.
Figure 3.
Inhibition of glycogen synthase kinase-3β (GSK-3β) activity enhances microglia integrity during OGD. The GSK-3β inhibitors SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) were applied to primary microglia 1 h prior to a 6-h period of OGD. Cell survival, DNA fragmentation, and membrane PS exposure were determined 24 h following OGD using the trypan blue dye exclusion method, TUNEL assay, or Annexin-V labeling respectively. (A), Representative images and quantification of data illustrate trypan blue staining in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased cell staining and increased microglial survival during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. (B), Representative images and quantification of data illustrate DNA fragmentation with TUNEL in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased TUNEL staining and decreased microglial DNA fragmentation during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate DNA fragmentation in microglia while white arrows illustrate absence of genomic DNA injury. (C), Representative images and quantification of data illustrate membrane PS exposure with Annexin-V in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased Annexin-V staining and decreased microglial PS externalization during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). (D), The mTOR inhibitor rapamycin (Rapa) at concentrations of 1.0-20.0 nM in conjunction with SB216763 (SB21, 5 µM) or SH6 (20 µM) was applied to the EOC 2 microglial cell line 1 h prior to a 6-h period of OGD and cell survival was assessed 24 h later. Concurrent inhibition of mTOR activity with rapamycin during SB21 administration reduced the beneficial effects of GSK-3β inhibition and worsened microglial survival to levels slightly greater than during OGD alone. In contrast, concurrent inhibition of Akt1 activity did not alter protection during GSK-3β inhibition or lead to a synergistic benefit (*P<0.01 vs. OGD; †P<0.01 vs. OGD/SB21). In all cases, each data point represents the mean and SEM. Control or CON indicates untreated cultures.
Similarly in Fig. 3B, administration of the GSK-3β inhibitors [SB216763, (SB21), 5 µM or SB415286, (SB41), 25 µM] 1 h prior to OGD significantly reduced microglial apoptotic DNA fragmentation. Green arrows indicate DNA fragmentation in microglia while white arrows illustrate the absence of genomic DNA injury. Furthermore, inhibition of GSK-3β also was able to reduce early apoptotic membrane PS exposure (Fig. 3C), suggesting that the GSK-3β is a central mediator of endogenous protection for microglia during OGD injury.
Given the significance of GSK-3β inhibition for endogenous microglial protection, we subsequently examined whether blockade of the mTOR and Akt1 pathways in conjunction with GSK-3β inhibition could alter survival in the microglial EOC 2 cell line (Fig. 3D). Using a series of rapamycin concentrations to inhibit mTOR as in Fig. 1D, concurrent administration of rapamycin 10 and 20 nM with SB21 reduced the beneficial effects of GSK-3β inhibition and worsened microglial EOC 2 survival to levels slightly greater than during OGD alone, suggesting that endogenous microglial protection with mTOR activation does not entirely rely upon the GSK-3β pathway. In contrast, concurrent administration of SH6 (20 µmol/l) with SB21 did not alter protection during GSK-3β inhibition, suggesting that endogenous Akt1 microglial cell protection may be primarily mediated by blockade of GSK-36 activity.
NF-κB p65 is sequestered in the cytoplasm of microglia during OGD
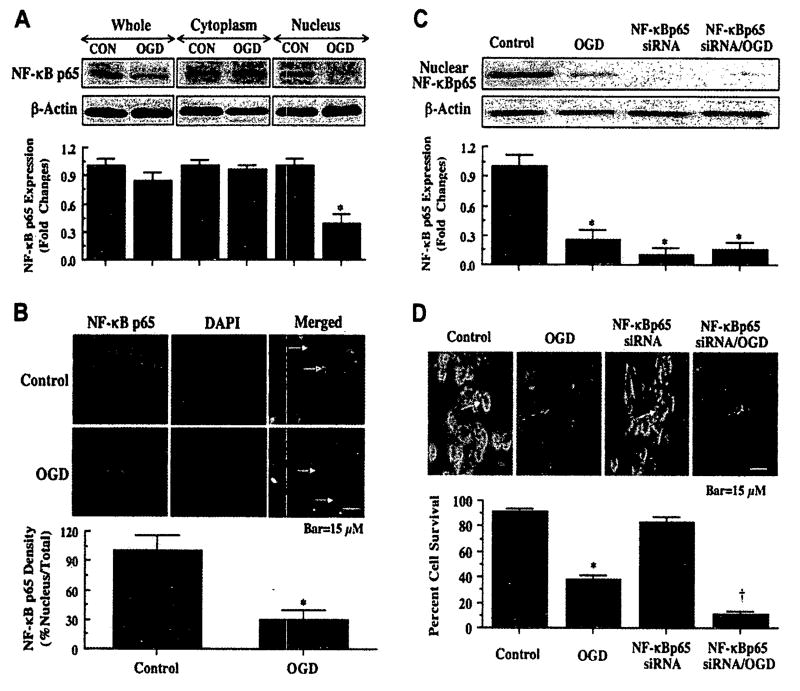
Each of the family members of NF-κB [p65 (RelA), RelB, and c-Rel] contains a C-terminal transactivation domain that can lead to gene transcription. Yet, primary gene activation is controlled by the p65 member that consists of two potent transactivation domains within its C terminus (58). As a result, we chose to examine whether OGD altered subcellular localization of NF-κB p65 in microglia. In Fig. 4A, we determined the expression of NF-κB p65 in the cytoplasmic and nuclear fractions separately by Western blot analysis 6 h following OGD. In untreated control cells, NF-κB p65 was present in both cytoplasmic and nuclear fractions. However, in the presence of OGD, significant expression of NF-κB p65 was present in the cytoplasmic fraction with loss of NF-κB p65 expression in the nucleus (Fig. 4A), suggesting that OGD may lead to microglial cell injury through the prevention of NF-κB p65 translocation to the nucleus blocking the transcription of ‘anti-apoptotic’ programs. In addition, the expression of total NF-κB p65 in whole cell extracts was slightly reduced during OGD exposure which may be indicative of the loss of NF-κB p65 in nuclear fractions during OGD injury.
Figure 4.
OGD prevents the nuclear translocation of NF-κB in microglia and gene silencing of NF-κB enhances microglial cell injury. (A), Equal amounts of protein extracts (50 µg/lane) from whole cells, the cytoplasm, and the nucleus were immunoblotted with anti-NF-κB p65 antibody 6 h following a 6-h period of OGD. Expression of NF-κB p65 was significantly diminished in whole and nuclear fractions during OGD exposure (*P<0.01 vs. CON). CON, untreated cultures. (B), Subcellular location of NF-κB p65 was followed at 6 h following OGD with immunofluorescent staining with anti-NF-κB p65 antibody and visualized with Texas-Red streptavidin. Microglial cell nuclei were stained with DAPI. In merged images, cells with OGD with green arrows show neuronal nuclei with strong NF-κB p65 staining (pink) and white arrows show neuronal cytoplasm with decreased NF-κB p65 staining (purple), illustrating OGD prevent nuclear translocation of NF-κB p65 in primary microglia. Control, untreated microglia. (C), Equal amounts of microglial protein extracts (50 µg/lane) were immunoblotted with anti-NF-κB p65 antibody 6 h following a 6-h period of OGD exposure. Transfection of NF-κB p65 siRNA in microglia for 72 h significantly blocked the expression of NF-κB p65 in control cell and during OGD (*P<0.01 vs. control). (D), Transient transfection of NF-κB p65 siRNA in primary microglia for 72 h prior to OGD was performed and cell survival was determined 24 h following OGD using trypan blue dye exclusion method. Transfection of NF-κB p65 siRNA in microglia prior to a 6-h period of OGD resulted in increased cell injury when compared to OGD alone (*P<0.01 vs. control; †P<0.01 vs. OGD). NF-κB p65 siRNA alone was not toxic to cells. Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. In all cases, each data point represents the mean and SEM. Control, untreated control cultures.
We also examined the subcellular translocation of NF-κB p65 in microglia during OGD with immunofluorescent staining for NF-κB p65 and DAPI at 6 h following OGD (Fig. 4B). With OGD exposure, significant immunofluorescent staining for NF-κB p65 in the cytoplasm of microglia with minimal nuclear staining is present. This is evident by the ability to detect DAPI nuclear staining (purple in color) in cells during merged OGD images since prominent NF-κB p65 staining is not present in the nucleus (Fig. 4B). In contrast, in untreated control cells, NF-κB p65 is present in nuclear and cytosolic subcellular regions as shown by the pink nuclear regions on merged images of NF-κB p65 and DAPI (Fig. 4B). Quantification of nuclear NF-κB p65 staining reveals minimal NF-κB p65 in the nucleus during OGD exposure.
Gene silencing of NF-κB increases microglial cell injury
Microglia were transfected with NF-κB p65 siRNA prior to OGD and Western blot analysis for NF-κB p65 was performed 6 h following OGD. The expression of NF-κB p65 in the nucleus of microglia was significantly reduced 6 h following OGD (Fig. 4C). NF-κB p65 siRNA gene silencing further reduced the nuclear expression of NF-κB p65 in microglia in either untreated cultures or in cells exposed to OGD.
To investigate whether loss of NF-κB contributes to microglial injury during OGD, microglia were transfected with NF-κB p65 siRNA prior to OGD and cell survival was determined by trypan blue exclusion method 24 h following OGD. As shown in Fig. 4D, representative images illustrate that OGD exposure leads to significant trypan blue uptake in microglia 24 h following OGD. Gene silence by NF-κB p65 siRNA transfection prior to OGD further increased the uptake of trypan blue in microglia. NF-κB p65 siRNA transfection alone did not change trypan blue uptake and was not toxic. Quantification of the data illustrates that primary microglial cell survival is significantly decreased by NF-κB p65 gene silencing during OGD exposure (Fig. 4D), illustrating that NF-κB p65 also forms an important component for endogenous microglial cell protection during OGD injury.
Discussion
Microglia of the central nervous system serve a variety of functions that may ultimately lead to the development or detriment of neighboring neuronal and vascular cells. Microglia can lead to cell injury and removal through oxidative stress mechanisms (2,7,18), cytokine release (38,59), and phagocytic engulfment (13,19,34,60,61). However, microglia also serve to protect the central nervous system from foreign organisms and may be essential for new tissue development following injury (4,14,15).
Since microglia may form an essential component for the body to either prevent or at least impede the progression of neurodegenerative disease, several studies have focused upon the ability of extrinsic factors to modulate (16,62) and afford protection to glial cells (21-23). Our present investigations examine an alternative approach to elucidate intrinsic cellular pathways that are endogenously controlled by microglia and that have the potential to maintain microglial cell integrity during oxidative stress. We illustrate that progressive exposure to OGD leads to increased primary microglial cell injury, DNA fragmentation, and membrane PS exposure 24 h following oxidative stress (Fig. 1A-C). Microglial cell injury reaches a plateau within a 6-h exposure of OGD. This susceptibility of microglia to OGD paralleled prior reports of cell injury for microglia and macrophage cell lines during administration of free radicals or exposure to OGD (21-23).
Although PI 3-K plays a critical role for cellular survival during oxygen deprivation and free radical generation, it is the downstream pathways of mTOR and Akt1 that may offer the greatest potential for endogenous microglial cytoprotective mechanisms. mTOR is a pro-survival factor that controls cellular energy balance (26) and leads to enhanced survival in neuronal and non-neuronal cells (24,25). We demonstrate that endogenous mTOR activity is necessary for microglial survival in the EOC 2 cell line during OGD, since inhibition of mTOR activity with the inhibitor rapamycin (35,36) results in enhanced microglial cell injury, illustrating that mTOR activation confers a minimal level of protection during OGD (Fig. 1D).
In a similar vein, Akt1 also appears to serve a significant function to not only assist with microglial cell survival, but also be required for the reduction in apoptotic DNA degradation and membrane PS exposure. The Akt pathway has been linked to the maintenance of microglial cell proliferation (27-29) and in a number of cell populations, Akt1 can protect cells during oxidative stress (12,13,30-33). For our examination of the Akt1 pathway, we either inhibited the activation and phosphorylation of Akt1 with the specific Akt1 inhibitor SH-6 or eliminated the expression of Akt1 with the gene silencing of Akt1 (Fig. 2A). We show that loss of Akt1 activity either during SH-6 administration or during Akt siRNA transfection significantly increased primary microglial cell injury, DNA fragmentation, and membrane PS externalization, demonstrating that microglial endogenous Akt1 activation can enhance cell survival and increase tolerance to apoptotic DNA fragmentation and early PS exposure (Fig. 2B-D).
Interestingly, endogenous cellular protection for microglia does not solely rely upon the PI 3-K pathways of mTOR and Akt1, but involves the regulation of GSK-3β activity. Prior studies have shown that apoptotic cell injury can be prevented during the inhibition of GSK-3β activity while pathology in the nervous system has been associated with increased activity of GSK-3β (37-40). In addition, inhibition of GSK-3β through phosphorylation can occur through a number of proteins that include tyrosine kinases, G protein-coupled receptors, protein kinase C, Akt, and mTOR (63). In Fig. 3A-C, we show that blockade of GSK-3β activity with either SB216763 (SB21) or SB415286 (SB41) is capable of reducing primary microglial cell injury, genomic DNA fragmentation, and microglial membrane PS exposure, illustrating that microglia rely upon the inhibition of GSK-3β activity for cellular protection. Yet, concurrent inhibition of mTOR activity with rapamycin during SB21 administration reduced the beneficial effects of GSK-3β inhibition and worsened microglial survival to levels slightly greater than during OGD alone. These results illustrate that endogenous microglial protection with mTOR may require pathways that are independent from GSK-3β, since inhibition of mTOR with rapamycin in other cell systems has been reported to not alter the phosphorylation of GSK-3β (64). In contrast, concurrent inhibition of Akt1 activity with SB21 administration did not alter protection during GSK-3β inhibition or lead to a synergistic benefit, suggesting that endogenous Akt1 microglial cell protection may be primarily mediated through the blockade of GSK-3β activity.
GSK-3β also may influence inflammatory cell activation through MCP-1 (42) that is mediated by NF-κB transcriptional activity (41). This observation led us to examine the capacity of NF-κB to provide endogenous cellular protection to microglia during oxidative stress. NF-κB proteins are composed of several homo- and heterodimer proteins that can bind to common DNA elements. It is the phosphorylation of IκB proteins by the IκB kinase (IKK) and their subsequent degradation that lead to the release of NF-κB for its translocation to the nucleus to initiate gene transcription (65). Dependent upon Akt1 controlled pathways, the transactivation domain of the p65 subunit of NF-κB is activated by IκB kinase (IKK) and the IKKα catalytic subunit and leads to the induction of several anti-apoptotic mechanisms (66). In addition to the potential for NF-κB to block apoptotic injury, microglia have been shown to increase NF-κB expression (43) and may independently use NF-κB during periods of tissue repair (44). Our present work provides another perspective for the NF-κB pathway and shows that NF-κB p65 nuclear expression is significantly reduced in microglia during OGD with both Western analysis and imunocytochemistry (Fig. 4A and B). Furthermore, NF-κB p65 appears to be a necessary constituent for microglial endogenous protection, since gene silencing of NF-κB p65 results in significantly increased cell injury during OGD (Fig. 4D).
The maintenance of microglial cell integrity and survival are critical factors for effective tissue repair and reorganization that is intimately tied to the prevention of permanent central nervous system injury. Although the deployment of exogenous cytoprotective agents to prevent microglial demise is rapidly gaining increased attention, it is the knowledge of endogenous microglial cellular pathways that may hold an equal or possibly greater value for the preservation of microglial function. To this end, our work is the first to outline a series of endogenous microglial cellular pathways that can constitute protection against oxidative stress (Fig. 5). In particular, we illustrate that endogenous microglial protection against OGD relies upon the PI 3-K pathways of mTOR and Akt1. The mTOR pathway may offer endogenous protection through mechanisms that do not entirely rely upon inhibition of GSK-3β activity while Akt1 appears to converge upon the necessary blockade of GSK-3β. Microglia also employ the expression of NF-κB p65 with its nuclear translocation to initiate anti-apoptotic programs as an additional level of endogenous protection. Future work that targets endogenous microglial cytoprotective pathways may open new prospects for the treatment of both acute onset and chronic progressive disorders of the nervous system.
Figure 5.
Endogenous microglial protection is conveyed through a series of cellular pathways that rely upon the regulation of mTOR, Akt1, GSK-3β, and NF-κB. During oxidative stress, endogenous protection in microglial cells (MG) requires the activation of Akt1 and the mTOR pathways. The activation of these cellular mechanisms, especially those involving Akt1, can lead to the inhibitory phosphorylation (p) of GSK-3β to block early and late apoptotic pathways that involve genomic DNA degradation and membrane PS exposure. In addition, GSK-3β activity may be linked to NF-κB gene transcription, but it is the maintenance of NF-κB expression with its subsequent nuclear translocation that can offer an additional level of endogenous protection in microglial cells during oxidative stress to promote anti-apoptotic pathways.
Acknowledgments
This research was supported by the following grants (K.M.): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, and NIH NIEHS (P30 ES06639).
References
- 1.Di Rosa M, Del'Ombra N, Zambito AM, Malaguarnera M, Nicoletti F, Malaguarnera L. Chitotriosidase and inflammatory mediator levels in Alzheimer's disease and cerebrovascular dementia. Eur J Neurosci. 2006;23:2648–2656. doi: 10.1111/j.1460-9568.2006.04780.x. [DOI] [PubMed] [Google Scholar]
- 2.Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2:409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- 3.Li F, Chong ZZ, Maiese K. Cell life versus cell longevity: the mysteries surrounding the NAD(+) precursor nicotinamide. Curr Med Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabet N. Acetylcholinesterase inhibitors for Alzheimer's disease: anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- 9.Obal I, Jakab JS, Siklos L, Engelhardt JI. Recruitment of activated microglia cells in the spinal cord of mice by ALS IgG. Neuroreport. 2001;12:2449–2452. doi: 10.1097/00001756-200108080-00032. [DOI] [PubMed] [Google Scholar]
- 10.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- 11.Singhrao SK, Neal JW, Morgan BP, Gasque P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington's disease. Exp Neurol. 1999;159:362–376. doi: 10.1006/exnr.1999.7170. [DOI] [PubMed] [Google Scholar]
- 12.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 14.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 15.Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- 16.Maiese K. Inflammatory glial cells of the nervous system: assistants or assassins? Curr Neurovasc Res. 2005;2:187–188. doi: 10.2174/1567202054368380. [DOI] [PubMed] [Google Scholar]
- 17.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Chong Z, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3β, β-catenin, and nuclear factor-κB. Curr Neurovasc Res. doi: 10.2174/156720206778018758. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshioka Y, Kitao T, Kishino T, Yamamuro A, Maeda S. Nitric oxide protects macrophages from hydrogen peroxide-induced apoptosis by inducing the formation of catalase. J Immunol. 2006;176:4675–4681. doi: 10.4049/jimmunol.176.8.4675. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka Y, Yamamuro A, Maeda S. Nitric oxide at a low concentration protects murine macrophage RAW264 cells against nitric oxide-induced death via cGMP signaling pathway. Br J Pharmacol. 2003;139:28–34. doi: 10.1038/sj.bjp.0705206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong R, Park CS, Rezaei Abbassi N, Tang SJ. Roles of glutamate receptors and the MTOR signaling pathway in activity-dependent dendritic protein synthesis in hipocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 27.Ito S, Sawada M, Haneda M, Fujii S, Oh-Hashi K, Kiuchi K, Takahashi M, Isobe K. Amyloid-beta peptides induce cell proliferation and macrophage colony-stimulating factor expression via the PI3-kinase/Akt pathway in cultured Ra2 microglial cells. FEBS Lett. 2005;579:1995–2000. doi: 10.1016/j.febslet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-betal represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–7023. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- 29.Suh HS, Kim MO, Lee SC. Inhibition of granulocyte-macrophage colony-stimulating factor signaling and microglial proliferation by anti-CD45RO: role of Hck tyrosine kinase and phosphatidylinositol 3-kinase/Akt. J Immunol. 2005;174:2712–2719. doi: 10.4049/jimmunol.174.5.2712. [DOI] [PubMed] [Google Scholar]
- 30.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt 1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 31.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 32.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Zhou R, Xiang G. Stepholidine protects against H2O2 neurotoxicity in rat cortical neurons by activation of Akt. Neurosci Lett. 2005;383:328–332. doi: 10.1016/j.neulet.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Chong ZZ, Kang J, Li F, Maiese K. mGluRI targets microglial activation and selectively prevents neuronal cell engulfment through Akt and caspase dependent pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L, Claret FX, Rassidakis GZ. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006;66:6589–6597. doi: 10.1158/0008-5472.CAN-05-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer's disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Qin W, Peng Y, Ksiezak-Reding H, Ho L, Stetka B, Lovati E, Pasinetti GM. Inhibition of cyclooxygenase as potential novel therapeutic strategy in N141I presenilin-2 familial Alzheimer's disease. Mol Psychiatry. 2006;11:172–181. doi: 10.1038/sj.mp.4001773. [DOI] [PubMed] [Google Scholar]
- 41.Steinbrecher KA, Wilson W, III, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 43.Guo G, Bhat NR. Hypoxia/reoxygenation differentially modulates NF-kappaB activation and iNOS expression in astrocytes and microglia. Antioxid Redox Signal. 2006;8:911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- 44.Sanz O, Acarin L, Gonzalez B, Castellano B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J Neurosci Res. 2002;67:772–780. doi: 10.1002/jnr.10140. [DOI] [PubMed] [Google Scholar]
- 45.Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett. 2006;399:91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen EA, Ananieva O, Brown ML, Chang Y. Growth, differentiation, and malignant transformation of pre-B cells mediated by inducible activation of v-Abl oncogene. J Immunol. 2006;176:6831–6838. doi: 10.4049/jimmunol.176.11.6831. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, Era T, Kanakura Y. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J Biol Chem. 2004;279:55578–55586. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- 51.Sae-Ung N, Matsushima T, Choi I, Abe Y, Winichagoon P, Fucharoen S, Nawata H, Muta K. Role of NF-kappa B in regulation of apoptosis of erythroid progenitor cells. Eur J Haematol. 2005;74:315–323. doi: 10.1111/j.1600-0609.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 53.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells. 2004;9:359–366. doi: 10.1111/j.1356-9597.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 54.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 55.Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 56.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fulop T, Larbi A, Douziech N, Levesque I, Varin A, Herbein G. Cytokine receptor signalling and aging. Mech Ageing Dev. 2006;127:526–537. doi: 10.1016/j.mad.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 60.De Simone R, Ajmone-Cat MA, Minghetti L. Atypical anti-inflammatory activation of microglia induced by apoptotic neurons: possible role of phosphatidylserine-phosphatidylserine receptor interaction. Mol Neurobiol. 2004;29:197–212. doi: 10.1385/MN:29:2:197. [DOI] [PubMed] [Google Scholar]
- 61.De Simone R, Ajmone-Cat MA, Nicolini A, Minghetti L. Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J Neuropathol Exp Neurol. 2002;61:237–244. doi: 10.1093/jnen/61.3.237. [DOI] [PubMed] [Google Scholar]
- 62.Manoonkitiwongsa PS, Schultz RL, Whitter EF, Lyden PD. Contraindications of VEGF-based therapeutic angiogenesis: effects on macrophage density and histology of normal and ischemic brains. Vascul Pharmacol. 2006;44:316–325. doi: 10.1016/j.vph.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-IodobenzyI)-adenosine-5′-N-methylcarboxamide confers cardio-protection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3beta. J Pharmacol Exp Ther. 2006;318:124–131. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- 65.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 66.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]