Abstract
The fluidity of Synechocystis membranes was adjusted in vivo by temperature acclimation, addition of fluidizer agent benzyl alcohol, or catalytic lipid hydrogenation specific to plasma membranes. The reduced membrane physical order in thylakoids obtained by either downshifting growth temperature or administration of benzyl alcohol was paralleled with enhanced thermosensitivity of the photosynthetic membrane. Simultaneously, the stress-sensing system leading to the cellular heat shock (HS) response also has been altered. There was a close correlation between thylakoid fluidity levels, monitored by steady-state 1,6-diphenyl-1,3,5-hexatriene anisotropy, and threshold temperatures required for maximal activation of all of the HS-inducible genes investigated, including dnaK, groESL, cpn60, and hsp17. The causal relationship between the pre-existing thylakoid physical order and temperature set point of both the transcriptional activation and the de novo protein synthesis was the most striking for the 17-kDa HS protein (HSP17) associated mostly with the thylakoid membranes. These findings together with the fact that the in vivo modulation of lipid saturation within cytoplasmic membrane had no effect on HS response suggest that thylakoid acts as a cellular thermometer where thermal stress is sensed and transduced into a cellular signal leading to the activation of HS genes.
Despite the numerous efforts aimed to understand how cells are capable of sensing changes in ambient temperature, the exact nature of sensors and signals participating in the heat shock (HS) response is presently unknown. According to a classical model, the major trigger of HS protein (HSP) formation is the accumulation of denatured proteins in the cytoplasm (1, 2). A different model raises the possibility that DnaK may act as a direct thermometer (3), whereas the ribosome sensor hypothesis implies that the signal that transduces sensing of the temperature stress to the increased expression of stress genes is regulated at the level of the translation process (4). It also was speculated that the accumulation of reactive oxygen species could activate HSP synthesis (5).
The physical state of cell membranes is known to be a very sensitive monitor of the most diverse environmental changes. This feature was suggested to render cell membranes an ideal location for the primary temperature stress sensor (6–8). Strongly supporting this hypothesis, catalytic hydrogenation of unsaturated lipids in the surface membrane of cyanobacterial cells at constant temperature resulted in the activation of transcription of the desA gene (6), which normally is induced by cold stress (9). The rapid increase in the membrane fluidity induced by the direct physical effect of the temperature upshift during heat shock is well documented (10, 11). On the other hand, all organisms examined to date produce the evolutionary conserved HSPs when they are exposed to a sudden, sublethal increase in the ambient temperature. If the primary HS sensor is membrane associated, one can suppose that any modification of membrane physical properties analogous to heat-induced perturbations also could lead to changes in the level of expression of HS genes. In fact, compounds like alkanols, with the ability to alter membrane fluidity, alter the threshold temperature of the HS response (7, 8, 12). The HS and ethanol stress responses exhibited an extensive similarity and functional overlap (8). Genetic manipulation of the level of membrane lipid unsaturation in yeast modified the temperature set point of HS response (7). Further supporting the view that thermal stress is transduced into a cellular signal at the level of membrane, in parallel with the adaptive readjustment of membrane fluidity, the threshold induction temperature of HSPs was shown to be subject of temperature acclimation in various organisms (8, 13, 14). Age-related diminished HSP induction supposedly is resulted from the altered transbilayer fluidity and lipid asymmetry of membranes (15).
The unicellular blue-green alga Synechocystis PCC 6803 has been used extensively as a powerful model for studying the molecular mechanism of stress response in photosynthetic organisms. Recently, the complete nucleotide sequence of its genome and the potential protein coding regions have been determined (16). Proteins of four size classes (70, 60, 17, and 14 kDa) were induced when Synechocystis cells had been subjected to HS (17). The 70-kDa HSP corresponded to the bacterial DnaK (17, 18). Recently, two additional dnaK homologues have been revealed in the Synechocystis genome (16). The HSP60 proteins are encoded by the groESL operon and the cpn60 gene possessing no groES in the neighboring region (18–21). HSP14 was identified as groES, the cochaperonin of GroEL, the role of HSP17 has not been determined yet. Evidence already has been provided on the growth temperature-dependent regulation of the formation of HSPs in Synechocystis (14).
The objective of the present study was to test the validity of the hypothesis predicting that membrane acts as a temperature sensor in cyanobacterial cells exposed to heat stress. The physical state of cell membranes were adjusted experimentally by changing growth temperature, treating the cells with benzyl alcohol (BA), or applying the technique of surface membrane selective in vivo microheterogeneous lipid hydrogenation. The temperature threshold of HS response for the individual HSPs was followed simultaneously by measuring mRNA formation and de novo protein synthesis. Our results indicate that in parallel with the decrease of the molecular order of thylakoid membranes both the threshold temperature for damage of photosystems and the maximal activation of all the HSPs present in Synechocystis cells lower significantly. We also demonstrate that the thermally induced expression of DnaK and GroESL families are affected differently on alterations in membrane physical state. The causal relationship between the initial level of thylakoid fluidity and the temperature set point of the transcriptional activation was the most spectacular for HSP17, described in the present study. We suggest that it is the thylakoid rather than the plasma membrane where the putative primary heat stress signal is generated.
MATERIALS AND METHODS
Growth of Cells.
Synechocystis PCC 6803 cells were grown photoautotrophically at different (22°C and 36°C) temperatures as in ref. 14. Cultures in exponential growth phase were used for experiments.
Assay of Thylakoid Thermostability.
To examine the thylakoid thermal tolerance in vivo the heat-induced changes in steady-state chlorophyll a (Chl a) fluorescence were measured as in ref. 22 by a Quanta Master QM-1 luminescence spectrophotometer (Photon Technology International, Princeton). The concentration of samples was adjusted to 1 μg of Chl a/ml. The temperature of water-jacketed and stirred cuvette was increased at 0.5°C/min by using a temperature-controlled circulator. The wavelength of excitation was 435 nm and of emission 680 nm at 5 nm slits, respectively. Heat inactivation of photosynthesis was measured with Clark-type oxygen electrode as in ref. 14.
Fluidity Manipulations.
Changes in the membrane fluidity were attained by temperature acclimation of cells at 22°C and 36°C as in ref. 14. Alternatively, microviscosity of cell membranes of cells acclimated to 36°C was reduced by the administration of 30 mM BA, a generally used membrane fluidizer (23), 10 min before heat stress. The double bonds of unsaturated fatty acids were reduced by the nontoxic, nonpermeable, microheterogeneous PdRh-PVPP catalyst, a palladium–rhodium alloy in the form of poly(N-vinylpyrrolidone)-protected colloid, prepared as in ref. 24. For the hydrogenation of total membranes pellets were taken up in growth medium at a chlorophyll concentration of 10 μg/ml and hydrogenated with 0.1 mg/ml of catalyst. For selective hydrogenation of plasma membranes permeaplasts prepared as in ref. 6 were diluted to OD800 = 0.4 and hydrogenated with 0.2 mg/ml of catalyst. Reactions were conducted under atmospheric pressure of H2 for 5 min. To test the effect of the inactive catalyst, the reaction was simulated under N2 pressure. GLC analyses of the fatty acids were carried out according to ref. 6.
Fluidity Measurements of Isolated Thylakoid Membranes.
Three microliters of a 0.2 mM solution of 1,6-diphenyl-1,3,5-hexatriene (in tetrahydrofuran) was added to 3 ml suspension of thylakoids (isolated as in ref. 25) containing 3 μg of Chl a, and the suspension was incubated for 40 min at 4°C. Steady-state fluorescence anisotropy was determined as in ref. 26.
Quantitation of the Fraction of the Motionally Restricted Lipid Pool by Electron Spin Resonance Spectroscopy.
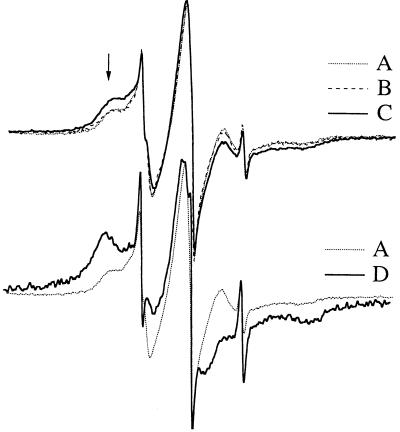
To assess changes in the membrane physical state after thermal adaptation (22°C vs. 36°C) and cytoplasmic membrane-directed hydrogenation, permeaplasts were labeled by the stearic acid spin label (14-SASL) kindly provided by D. Marsh (Max-Planck-Institute für Biophysikalishe Chemie, Goettingen, Germany). Five micrograms of 14-SASL was dried under nitrogen flow from ethanol solution, to which 100 μl of permeaplast suspension (corresponding to 20-ml cells) was added and vortexed. After 10-min incubation at room temperature, samples were loaded into glass capillaries (i.d. 1 mm) and pelleted, and the excess supernatant was removed. ESR spectra were recorded on a Bruker (Rheinstetten, Germany) ECS106 X-band spectrometer. A modulation frequency of 100 kHz, modulation amplitude of 1.0 Gauss, and microwave power of 10 mW was used. Total scan range was 120 Gauss. The amplitude of the low field local extrema (as indicated with the arrow on Fig. 3), a quantity proportional to the fraction of the motionally restricted lipid pool (27), was measured relative to that of the central peak.
Figure 3.
Estimation of the changes of plasma membrane physical state from the relative intensity of the motionally restricted spectral component of 14-SASL. The low field immobile peak arising from the protein immobilized spin label molecules is indicated by an arrow. (Upper) Spectra were recorded from permeaplasts of 22°C grown Synechocystis (A), treated with inactive catalyst under hydrogenation conditions (B), hydrogenated with PdRh-PVPP catalyst (C). (Lower) Spectra taken from permeaplast of cells grown at 22°C (A) or 36°C (D).
HS Treatment, Northern Analysis, Radioactive Labeling, and Thylakoid Association of HSPs.
Cells with altered membrane fluidities were exposed to HS at various temperatures either for 30 min to test mRNA formation from dnaK, groESL, cpn60 and hsp17 genes, or for 60 min to follow the rate of de novo HSP synthesis. For this latter purpose, 14C-amino acid mixture was added to the cells (0.5 μCi/ml) at the 30th min of heat shock at indicated temperatures, and the incubation was continued up to the 60th min. Isolation of thylakoid membranes, SDS/PAGE, and fluorography were carried out according to ref. 25. Total RNA isolation and Northern analysis were performed as in refs. 19–21. The N-terminal amino acid sequence of the putative, heat-inducible HSP17 was determined. The sequence agreed perfectly with that of predicted from a nucleotide sequence of an ORF (sll1514) found in Cyanobase (16). The hsp17 probe was generated by PCR with primers 5′-GCAGATGAACCAACTGTTTG-3′ and 5′-AGGATACCGGCATCGTAATT-3′ using genomic DNA as template.
RESULTS
Influence of Growth Temperature and Fluidization with BA on the Thermostability of Thylakoid Membranes.
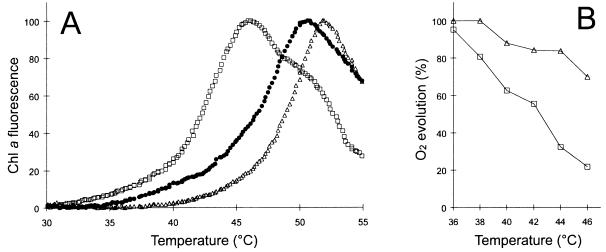
It is suggested, that in photosynthetic cells exposed to high temperature, before impairment of other cellular functions the photosynthetic apparatus and especially photosystem II (PSII) is damaged first (28). Similar to higher plants, Chl a fluorescence in cyanobacteria is considered as an intrinsic probe of the actual thermal stability of PSII-protein complexes (22). The temperature at which a sharp rise in Chl a fluorescence occurs on heating provides an index of the thylakoid thermal stability. When this assay was performed with Synechocystis cells growing at distinct temperatures, the low temperature (22°C)- acclimated cells displayed a clearly decreased level of thermostability compared with their 36°C-grown counterparts (Fig. 1A). Fully supporting this finding, it was shown in a previous study that the downward shift in the growth temperature caused a decrease (ca. 4°C) in the heat inactivation of PSII (25). Ascribed to its general membrane fluidizing effect, a remarkable reduction of thylakoid thermostability could be attained at the addition of 30 mM BA to cells acclimated to 36°C (Fig. 1A). Thus, by the criterion of temperature-induced fluorescence enhancement the thermosensitivity of thylakoid function displayed the following order: 36 + BA > 22 > 36.
Figure 1.
Thylakoid thermostability of Synechocystis cells with different membrane physical states. (A) Temperature-induced fluorescence enhancement yield of Chl a in cells grown at 36°C (▵), 22°C (•), or grown at 36°C and treated with 30 mM BA (□). (B) The profiles of heat inactivation of photosynthesis in 36°C-grown cells in the absence (▵) or in the presence of 30 mM BA (□).
When we measured oxygen evolution in 36°C-grown cells no discernible difference was detected in the basal photosynthetic activity after the administration of 30 mM BA (Fig. 1B). Cells treated with BA and washed thereafter resumed normal rate of growth (data not shown). By contrast, exposure of BA-treated cells to heat stress at various temperatures provoked a strong reduction of thermotolerance as tested by measuring the photosynthetic oxygen evolution (Fig. 1B). Accordingly, the growth of the cells exposed to 46°C for 30 min in the presence of BA was completely blocked (data not shown). These results are consistent with the assumption that BA and temperature have synergistic effect on the cell’s thermotolerance and are in agreement with the Chl a fluorescence data (Fig. 1A).
Fluidity Changes of Thylakoid Membranes Derived from Cells Grown at Contrasting Temperatures or Treated by BA.
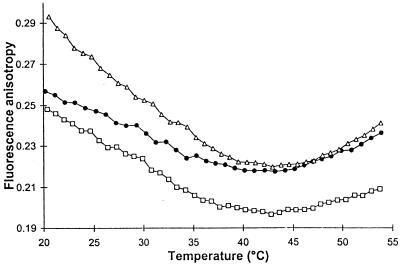
The aim of these experiments was to determine how thylakoid membrane fluidity, measured by steady-state fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene is affected by growth temperature and/or BA treatment. Below ca. 40°C, thylakoid membranes of cells grown at the lower temperature were more fluid at any particular measuring temperature. The higher fluidity detected at identical temperature for thylakoid of 22°C-grown cells compared with that from cultures grown at 36°C was in accordance with the reported changes in fatty acid pattern and ratio of protein to lipid of the membranes (29). Administration of BA to thylakoid samples of 36°C-grown cells resulted in an even greater degree of membrane disorder (Fig. 2). Besides the substantial difference observed in membrane physical states, the profile of anisotropy-temperature curves of 36 and 36+ BA samples were, however, strikingly similar. It is noted that the anisotropy values, decreasing gradually and reaching a minimum by the progress of test temperatures, reflected the order established before on the heat sensitivity for these membranes.
Figure 2.
Effect of temperature on 1,6-diphenyl-1,3,5-hexatriene steady-state fluorescence anisotropy in thylakoid membranes isolated from cells acclimated to 36°C (▵), 22°C (•), or grown at 36°C and treated with 30 mM BA (□).
Modulation of Surface Membrane Physical State by Selective Hydrogenation of the Cytoplasmic Membrane.
Both thermal acclimation and treatment of cells with BA resulted in an overall modulation of the physical order of cytoplasmic as well as thylakoid membranes. To test which particular membrane could represent the “cellular thermometer” during heat stress efforts were made therefore to selectively manipulate the fluidity of the surface membrane of cells without changing their thylakoid membranes. Catalytic hydrogenation of cell’s membrane lipids proved to be a powerful technique to modulate plasma membrane physical order of living cyanobacterial, plant, and mammalian cells (6, 30, 31). The nontoxic PdRh-PVPP-catalyst (see above), which consists of largely uniform, nanosize metal particles, allowed hydrogenation of phospholipid model membranes under gentle conditions with short reaction times (24). Before conducting experiments with living Synechocystis cells, the hydrogenation rate as a function of the catalyst concentration was tested on “total membrane” preparations made from 22°C-adapted cells at room temperature. As highlighted by Table 1 for 0.1 mg/ml of catalyst concentration, a 5-min reaction resulted in a loss in all of the polyunsaturated fatty acids. Only minor differences in rate of hydrogenation were seen for the major lipid classes (i.e., monogalactosyl diacyl glycerol, digalactosyl diacyl glycerol, sulfoquinovosyl diacyl glycerol, and phoshatidyl glycerol) (data not shown). Catalyst concentration of 0.2 mg/ml was selected as “optimal” for cell (permeaplast) hydrogenation, which afforded the best combination of high cell survival and significant lipid saturation. Fraction of the protein-restricted lipid spin-label pool was shown to be a sensitive indicator of the changing level of lipid unsaturation of native membranes (29, 32, 33). Both the lack of time-dependent ESR signal and loss of overall ESR intensity suggested that, within the time scale of our experiments, the fatty acid spin label did not reach internal membrane structures, where 14-SASL normally is completely reduced under the same conditions, within minutes of the labeling (30). The level of in vivo plasma membrane saturation was assessed from the calculated modulation of relative 14-SASL immobilization (see Materials and Methods). As shown in Fig. 3 the lipid immobilization (see arrow) achieved by PdRh-PVPP hydrogenation displayed an intermediate level when compared with the spectra gained from cells grown at contrasting temperatures (22°C vs. 36°C). The calculated ratio of immobilized lipid pool was the following: 0.16 vs. 0.46 for 22°C- and 36°C-grown cells and 0.26 for hydrogenated samples. Further corroborating the surface selective mode of the reaction, lipids of thylakoids isolated from hydrogenated cells remained unaffected (data not shown).
Table 1.
Effect of PdRh-PVPP hydrogenation on the fatty acid composition of total membrane preparations isolated from Synechocystis cells acclimated to 22°C
| Samples | Total fatty acids, %
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | γ18:3 | α18:3 | 18:4 | |
| Control | 48.1 | 3.8 | 1.6 | 4.5 | 10.9 | 21.9 | 5.8 | 3.4 |
| Hydrogenated | 54.0 | 3.3 | 13.4 | 9.7 | 6.7 | 9.8 | 2.1 | 1.0 |
Membrane suspensions were hydrogenated for 5 min at 22°C (for details, see Materials and Methods). Data are averages of three experiments. The range of experimental deviations was within 0.5%.
Temperature Profiles of the Expression of dnaK, groESL, cpn60, and hsp17 Genes in Synechocystis Cells with Modulated Membrane Fluidity.
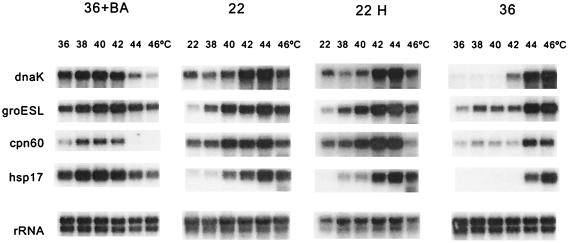
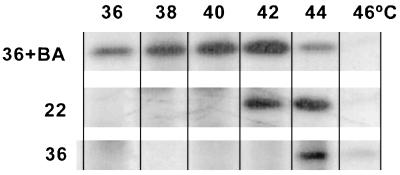
If HS is truly transduced into an intracellular signal at the level of membranes, then any manipulation that modulates membrane physical state should alter the profile and temperature threshold of HS response. To confirm this working hypothesis, we performed Northern blot analysis of RNA from cells acclimated to contrasting temperatures (22°C vs. 36°C), treated by BA (36+BA) or catalytically saturated selectively in their cytoplasmic membrane before various heat exposures. Our results showed that in cells acclimated to 36°C all four HS genes tested (dnaK, groESL, cpn60, and hsp17) were activated at significantly elevated temperatures in comparison with the low temperature-grown counterparts (Fig. 4). The threshold temperature for the maximal activation was 44°C in 36°C samples whereas this value downshifted to 38–40°C in 22°C-grown cells. Moreover, not like dnaK, groEL, and cpn60, the hsp17 gene is transcribed exclusively at 44°C and above in samples of 36°C-grown cells. These results demonstrate that the temperature set points for the activation of the HS genes do not have fixed values and are affected by the growth temperature. The main factors governing the long-term acclimation in cyanobacteria to ambient temperatures are changes in the level of lipid unsaturation and ratio of protein to lipid in the membrane (25, 26, 29, 32), which, in turn, cause shift in the membrane physical state (see Fig. 2). To test whether correlation between growth temperature, thylakoid thermosensitivity, and activation of HS genes are mediated via changes in the membrane physical state, membrane fluidity of isothermally grown cells was shifted before the Northern analysis. Administration of BA to 36°C-grown cells (36+BA), causing reduced molecular order in both the cytoplasmic and thylakoid membranes, dramatically lowered the threshold temperature for HS gene activation (Fig. 4). Further supporting its unique feature, at the administration of the fluidizer hsp17 message was present in a high abundance at the whole temperature range tested. By contrast, the selective modulation of plasma membrane physical state by hydrogenation of 22°C-grown cells resulted in no significant effect on the temperature profile of the induction of HS genes.
Figure 4.
Effect of membrane modulations on the temperature profile of HS gene (dnaK, groESL, cpn60, and hsp17) expression in Synechocystis. Cells, acclimated to 36°C (designated 36), 22°C (designated 22), grown at 22°C and hydrogenated (22 H), grown at 36°C and treated with 30 mM BA (36+BA), were incubated at indicated temperatures, and the extracted total RNA were subjected to Northern analysis. Hybridizations with rRNA were served as loading controls.
Effect of Fluidity Modulation on the Formation and Thylakoid Association of HSP17 Protein.
The level of the incorporation of radiolabeled amino acids into various HSPs in fluidity manipulated cells transferred directly to challenging high temperature (38°C to 46°C) was determined by the fluorography of SDS/PAGE-separated proteins. The results obtained with HSP70 and HSP60s were qualitatively similar to our observations revealed by Northern analysis, following strictly the order of the actual level of membrane fluidity and thermostability values established before (data not shown). The sharp “fluidity-dependence” established at the transcriptional level for hsp17 appeared to be even more striking by following the de novo protein synthesis. Surprisingly, the vast majority of newly synthesized HSP17 was found to be associated with thylakoid membranes in all of the samples investigated, which also was confirmed by Western blotting by using antiserum prepared against HSP17 (unpublished observations). The temperature profile of newly synthesized, thylakoid-associated HSP17 in fluidity-manipulated cells is shown in Fig. 5. In 36°C-grown cells the thylakoid-bound HSP17 is formed only at 44°C, whereas on downshifting the growth temperature the temperature threshold of protein synthesis lowers to 42°C. In response to a more pronounced change caused in the membrane’s physical order by administration of BA, a massive HSP17 synthesis occurred even at the growth temperature of the cells.
Figure 5.
Temperature profile of the de novo synthesis of thylakoid-associated HSP17. Cells acclimated to 36°C (designated 36), 22°C (designated 22), or grown at 36°C and treated with 30 mM BA (36+BA) were labeled with 14C-amino acid mixture and incubated at temperatures indicated. Thylakoids were isolated and proteins were analyzed by SDS/PAGE followed by fluorography.
DISCUSSION
Temperature regulation of HS gene expression is critical for the survival of photosynthetic organisms living in variable thermal environments, and the implication of HSPs in the acquisition of thermotolerance is well established (14, 20, 21, 29, 32). The term heat hardening often is used to describe the transitory thermotolerant state, which has been shown to depend on HSP synthesis (34). The function of HSPs in folding of nascent proteins and refolding of denatured proteins led to their designation as “molecular chaperones.” For optimal function both GroEL and DnaK chaperones require the assistance of co-chaperones, GroES and DnaJ/GrpE, respectively (19–21).
The nature of the signals leading to regulation of HSPs at the level of transcription initiation, i.e., the “cellular thermometer(s)” is still a matter of debate. A relationship between membrane physical state and the HS response was suggested initially on the basis of the observations that poikilotherms, which are able to adjust their membranes to altered growth temperatures, modify the set point of the temperature at which expression of HS genes occurs (7, 8, 12–14). These findings indicated that some disturbances of the pre-existing membrane physical state, which is subject of thermal acclimation, could be involved in the perception of rapid temperature changes and might cause transduction of a signal that induces transcription of HS genes.
The hypothesis that a cellular membrane acts as a “primary sensor” was first evidenced when using the technique of catalytic hydrogenation (6). Hydrogenation of Synechocystis stimulated the transcription of the desA gene in the same way as chilling (9). Because the reaction was confined to the most cold-sensitive constituent of cyanobacterial cells the plasma membrane (35), it could act as the sensor to altered lipid saturation or “fluidity.” Hydrogenated cells displayed an elevated sensitivity to cooling in the activation of desA transcription, indicating that hydrogenation and cooling have additive effects and that cold perception is mediated through changes in membrane fluidity. In the present study we presumed that the membrane sensor hypothesis described for cold-induced desaturases could be extended toward the HS response. Because the highly unsaturated photosynthetic membranes were shown to be the most heat-sensitive parts of the cyanobacterial cells (14, 25, 28, 32, 36) we predicted that they would be the most likely candidates to act as “thermometers.” Perturbation of thylakoid lipid phase was achieved by subjecting cells to temperature acclimation or by addition of BA. Similar to low-temperature acclimation, addition of BA induced a pronounced reduction of the membrane physical order. The heat stability of thylakoids (Fig. 1) tested in vivo was in line with the fluidity-temperature profiles measured on isolated membranes. It should be noted that factors responsible for thermal stability of thylakoids, which are subject of thermal acclimation, were shown to be retained in the isolated membranes (36). The most intriguing finding of the present investigation is that the cells having thylakoid membranes with different physical state revealed strikingly different threshold temperatures for the induction of transcription of all the HSPs studied (Fig. 4). Just like acclimation of Synechocystis cells to lower growth temperature, the fluidizing BA mediated a reduction in both the minimum and maximum temperatures required for the heat activation of the hsp genes, i.e., dnaK, groESL, cpn60, and hsp17. BA and temperature had synergistic effects on the cell’s HS response, closely resembling the synergism described for temperature down-shift and membrane rigidifying hydrogenation during cold exposure (6). Lending support to a model in which thermal stress is transduced into a cellular signal at the level of the thylakoid membranes in the photosynthetic organisms, plasma membrane saturation did not affect temperature threshold of hsp gene activation (Fig. 4). Our study did not seek to elucidate the precise molecular target involved in the capability of thylakoids to activate the pathways leading to HSP synthesis. It should be emphasized that besides the hypothetic thylakoid microdomain(s) acting as “thermometer(s)” the existence of other cellular stress sensors should not be neglected. Recently it was shown that mutations of Escherichia coli causing unbalanced membrane phospholipid compositions induce lipid-specific signals, resulting in the activation of certain regulatory genes (37). We suggest that both alterations of the size of the protein boundary lipid layer (29, 32, 33) and the nonbilayer propensity of membranes as well (38) could play a critical role in the membrane-sensing and -signaling mechanism of HS response. In addition, the actual membrane physical state affected by various factors also may control the diffusion rate of plastoquinone molecules, the mobile electron carriers within membrane bilayer, which were suggested to be the major determinants of the recently described redox signaling pathway (39). This idea is in a full accordance with our recent findings that the transcription rate of Synechocystis chaperonins, groESL and cpn60, is controlled by the redox state of thylakoid membranes (21).
It was an intriguing finding that most of the HSP17, the product of the presently revealed “fluidity gene,” proved to be thylakoid-associated during HS (Fig. 5). Evidence was provided that a pool of GroEL proteins in Synechocystis also became membrane bound under thermal stress (25). Recently, we also have shown in a model using lipid membranes and active protein folding GroESL oligomers that the chaperonin binding to lipids is governed by the composition and physical state of the host membranes. Chaperonins, which associated with unilamellar vesicles and stabilized the membrane at high temperature by increasing its microviscosity, retained their capability to assist protein folding (26). Though the exact function of HSP17 protein in Synechocystis is hitherto unknown, a chaperone-like activity recently was revealed for a 16-kDa alpha-crystallin-like small HSP from Mycobacterium tuberculosis (40). Expression of a gene encoding the rice 16.9-kDa HSP enhanced thermotolerance in E. coli (41). Another small HSP, homologous to HSP17 of Synechocystis, inducible by heat and ethanol, also was found to be peripherally associated with the membrane in Leuconostoc oenos (42). Combining all of these observations one can speculate that although membrane association of chaperons may rigidify and thereby stabilize membranes under heat stress, this modulation simultaneously may lead to a down-regulation of the transcriptional activity of HS genes. Thus, the “cross-talk” between the “primary sensor” membranes and HSPs may suggest the existence of a hitherto unrevealed feedback mechanism of HS gene regulation.
Acknowledgments
This research was supported by the Hungarian National Scientific Research Foundation (OTKA; T 016392 to I.H. and F023159 and FKFP 0318/1997 to F.J.) and by the Prime Minister’s Office, Hungary, Project for Environmental Protection of Lake Balaton (11/97 to L.V. and MEH 96-30 430-36 to V.V.)
ABBREVIATIONS
- Chl a
chlorophyll a
- HS
heat shock
- HSP
heat shock protein
- BA
benzyl alcohol
- PdRh-PVPP
a palladium–rhodium alloy in the form of poly(N-vinylpyrrolidone)-protected colloid
- 14-SASL
stearic acid spin label
References
- 1.Craig E A, Gross C A. Trends Biochem Sci. 1991;16:135–139. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- 2.Bukau B. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 3.McCarty J S, Walker G C. Proc Natl Acad Sci USA. 1991;88:9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Bogelen R A, Neidhart F G. Proc Natl Acad Sci USA. 1990;87:5589–5593. [Google Scholar]
- 5.Jacquuier-Sarlin M R, Polla B S. Biochem J. 1996;318:187–193. doi: 10.1042/bj3180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vígh L, Los D A, Horváth I, Murata N. Proc Natl Acad Sci USA. 1993;90:9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carratù L, Franceschelli S, Pardini C L, Kobayashi G S, Horváth I, Vígh L, Maresca B. Proc Natl Acad Sci USA. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper P W. FEMS Microbiol Lett. 1995;134:121–127. doi: 10.1111/j.1574-6968.1995.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 9.Los D A, Horváth I, Vígh L, Murata N. FEBS Lett. 1993;318:57–60. doi: 10.1016/0014-5793(93)81327-v. [DOI] [PubMed] [Google Scholar]
- 10.Mejia R, Gomez-Eichelmann M C, Fernandez M S. Biochim Biophys Acta. 1995;1239:195–200. doi: 10.1016/0005-2736(95)00152-s. [DOI] [PubMed] [Google Scholar]
- 11.Dynlacht J R, Fox M H. Radiat Res. 1992;130:55–60. [PubMed] [Google Scholar]
- 12.Curran B P G, Khalawan S A. Microbiology. 1994;140:2225–2228. doi: 10.1099/13500872-140-9-2225. [DOI] [PubMed] [Google Scholar]
- 13.Dietz T J, Somero G N. Proc Natl Acad Sci USA. 1992;89:3389–3393. doi: 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehel C, Gombos Z, Török Z, Vígh L. Plant Phys Biochem. 1993;31:81–88. [Google Scholar]
- 15.Igbavboa U, Avdulov N A, Schroeder F, Wood W G J. J Neurochem. 1996;66:1717–1725. doi: 10.1046/j.1471-4159.1996.66041717.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 17.Lehel C, Wada H, Kovács E, Török Z, Gombos Z, Horváth I, Murata N, Vígh L. Plant Mol Biol. 1992;18:327–336. doi: 10.1007/BF00034959. [DOI] [PubMed] [Google Scholar]
- 18.Chitnis P R, Nelson N. J Biol Chem. 1991;266:58–65. [PubMed] [Google Scholar]
- 19.Lehel C, Los D A, Wada H, Györgyei J, Horváth I, Kovács E, Murata N, Vígh L. J Biol Chem. 1993;268:1799–1804. [PubMed] [Google Scholar]
- 20.Glatz A, Horváth I, Varvasovszki V, Kovács E, Török Z, Vígh L. In: Physical Stresses in Plants: Genes and Their Products for Tolerance. Grillo S, Leone A, editors. Berlin: Springer; 1996. pp. 21–29. [Google Scholar]
- 21.Glatz A, Horváth I, Varvasovszki V, Kovács E, Török Z, Vígh L. Biochem Biophys Res Commun. 1997;239:291–297. doi: 10.1006/bbrc.1997.7463. [DOI] [PubMed] [Google Scholar]
- 22.Fork D C, Sen A, Williams W P. Photosyn Res. 1987;11:71–87. doi: 10.1007/BF00117675. [DOI] [PubMed] [Google Scholar]
- 23.Cooper P, Meddings J B. Biochim Biophys Acta. 1991;1069:151–156. doi: 10.1016/0005-2736(91)90117-q. [DOI] [PubMed] [Google Scholar]
- 24.Nádasdi L, Joó F, Horváth I, Vígh L. Appl Catal A. 1997;162:57–69. [Google Scholar]
- 25.Kovács E, Török Z, Horváth I, Vígh L. Plant Phys Biochem. 1994;32:285–293. [Google Scholar]
- 26.Török Z, Horváth I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vígh L. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh D. In: Supramolecular Structure and Function. Pifat-Mrzljak G, editor. Berlin: Springer; 1986. pp. 48–62. [Google Scholar]
- 28.Berry J A, Bjorkman O. Annu Rev Plant Physiol. 1980;31:491–553. [Google Scholar]
- 29.Vígh L, Török Z, Kovács E, Glatz A, Balogh N, Horváth I. In: Biochemical and Cellular Mechanisms of Stress Tolerance in Plants. Cherry J H, editor. Berlin: Springer; 1994. , NATO ASI Series, Vol. H86, pp. 77–95. [Google Scholar]
- 30.Vígh L, Joó F, Cséplö A. Eur J Biochem. 1985;146:241–244. doi: 10.1111/j.1432-1033.1985.tb08645.x. [DOI] [PubMed] [Google Scholar]
- 31.Benkö S, Hilkman H, Vígh L, van Blitterswijk W J. Biochim Biophys Acta. 1987;896:129–135. doi: 10.1016/0005-2736(87)90173-8. [DOI] [PubMed] [Google Scholar]
- 32.Vígh L, Lehel C, Török Z, Gombos Z, Balogh N, Horváth I. In: Plant Lipid Biochemistry, Structure, and Utilization. Quinn P J, Harwood J L, editors. London: Portland; 1990. pp. 373–381. [Google Scholar]
- 33.Schlame M, Horváth I, Vígh L. Biochem J. 1990;265:79–85. doi: 10.1042/bj2650079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders B M. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- 35.Vígh L, Joó F. FEBS Lett. 1983;162:423–427. [Google Scholar]
- 36.Nishiyama Y, Los D A, Hayashi H, Murata N. Plant Physiol. 1997;115:1–8. doi: 10.1104/pp.115.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue K, Matsuzaki H, Matsumoto K, Shibuya I. J Bacteriol. 1997;179:2872–2878. doi: 10.1128/jb.179.9.2872-2878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Kruijff B. Nature (London) 1997;386:129–130. doi: 10.1038/386129a0. [DOI] [PubMed] [Google Scholar]
- 39.Huner N P A, Maxwell D P, Gray G R, Savitch L V, Krol M, Ivanov A G, Falk S. Physiol Plant. 1996;98:358–364. [Google Scholar]
- 40.Yuan Y D, Crane D, Barry C E. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh C H, Chang P F L, Yeh K W, Lin W C, Chen Y M, Lin C Y. Proc Natl Acad Sci USA. 1997;94:10967–10972. doi: 10.1073/pnas.94.20.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jobin M P, Delmas F, Garmyn D, Diviès D, Guzzo J. Appl Environ Microbiol. 1997;63:609–614. doi: 10.1128/aem.63.2.609-614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]