Abstract
The factors that regulate trophoblast invasion of the uterine vasculature are incompletely understood. In this paper we show that macaque trophoblasts express the mucin, MUC1, and that it is involved in trophoblast-endothelial interaction. Immunocytochemistry, Western blotting and RT-PCR analyses confirmed that MUC1 was expressed by isolated early gestation macaque trophoblasts. MUC1 was also detected in endovascular trophoblasts in sections of placental-decidual tissue during early gestation. A blocking antibody against MUC1 reduced trophoblast adhesion to uterine endothelial cells and also blocked trophoblast transendothelial migration. MUC1 is known to bind to Intercellular Adhesion Molecule-1 (ICAM-1) in other systems. Incubation in the presence of a blocking antibody against Intercellular Adhesion Molecule-1 (ICAM-1) or recombinant ICAM-1 modestly, but significantly, reduced transendothelial trophoblast migration. These results are consistent with the idea that MUC1 is involved in trophoblast adhesion to uterine endothelial cells and in trophoblast transendothelial migration.
Keywords: adhesion, placenta, uterus, mucins
INTRODUCTION
The mucin, MUC1, is a Type 1 transmembrane protein expressed on the apical surface of a wide variety of normal glandular epithelial cells and is overexpressed in a non-polarized fashion in many tumor cells [1]. MUC1 is also expressed by activated T-cells [2] and dendritic cells [3, 4]. Because of its large size and unusual physical properties resulting from extensive O-glycosylation of the variable tandem repeat region, MUC1 is generally thought of as having lubricating or anti-adhesive effects on cell-cell and cell-substrate interactions [5-8]. In tumor cells, the anti-adhesive effects of MUC1 are thought to facilitate metastasis [7, 9]. In the female reproductive tract, MUC1 is expressed by the uterine epithelium where it plays roles in protecting against infection and regulating blastocyst implantation. The presence of MUC1 is refractory to implantation but generalized (in the mouse) or localized (in the human) loss of MUC1 during the window of implantation is associated with blastocyst attachment [10-13].
Ambiguously, other data suggest that MUC1 may facilitate cell-cell adhesion. MUC1 has been shown to bind intercellular adhesion molecule-1 (ICAM-1) and E-selectin [14]. In the latter case, binding is likely due to the presentation of sialyl Lewis(x) or sialyl Lewis(a) antigens by MUC1 [15]. Uterine MUC1 has been shown to present selectin ligands that may function in blastocyst attachment [16]. The adhesion of tumor cells to endothelium was shown to be blocked by anti-MUC1 and anti-ICAM-1 antibodies [17, 18]. MUC1 and ICAM-1 also appear to be involved in regulating transendothelial migration of breast carcinoma cells [19].
In human and non-human primates, blastocyst attachment to the endometrial epithelium is followed by trophoblast invasion of the endometrium and endometrial vasculature. The attachment of endovascular trophoblasts to endothelium and their migration within the uterine vasculature is a physiologically important, yet poorly understood, phenomenon. This interaction between trophoblasts and endothelial cells is important for normal placental development and for establishing a blood supply to the developing fetus. Several candidate adhesion molecule systems have been proposed to play a role in trophoblast-endothelial cell adhesion [20-26]. In vitro adhesion assays using human and macaque trophoblasts suggest that trophoblast-endothelial adhesion involves β1 integrin, αVβ3 integrin, VE-cadherin, and VCAM-1 [27-30].
Human villous and extravillous trophoblasts and mouse trophoblasts express MUC1 [31-33]. These observations have attracted little attention and, to date, no studies have investigated the role of MUC1 in trophoblast adhesion and migration. We have therefore tested the idea that MUC1 is expressed by early gestation macaque trophoblasts and that it plays a role in the attachment of trophoblasts to endothelium. The results show that macaque trophoblasts express MUC1 and that trophoblast-endothelial cell adhesion and trophoblast transendothelial migration are mediated by MUC1.
MATERIALS AND METHODS
Trophoblast Isolation and Culture
All procedures involving animals were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under the approval of the University of California Davis, Animal Care and Use Committee. Trophoblast cells were isolated from 40-65 day Rhesus monkey (Macaca mulatta) placental/decidual tissue using procedures we have previously detailed [34]. FACS analysis of the purified trophoblast population revealed that 75% of the cells were β1 integrin-positive [35], consistent with a predominantly extravillous trophoblast phenotype.
Endothelial Cell Culture
Human uterine microvascular endothelial cells (UtMVECs, passage 3) were purchased from Clonetics Corporation (San Diego, CA) and maintained in endothelial basal medium-2 (EBM-2, Clonetics) supplemented with human recombinant epidermal growth factor, human fibroblast growth factor, vascular endothelial growth factor, ascorbic acid (Vitamin C), hydrocortisone, human recombinant insulin-like growth factor, heparin, gentamicin, amphotercin, and 5% fetal calf serum (FCS). Cells between passages 5-10 were used for experiments.
Immunocytochemistry
A hamster polyclonal antibody (CT2) against the cytoplasmic domain of MUC1 was generously provided by Dr Sandra Gendler, Mayo Clinic, Arizona. The mouse monoclonal antibody B27.29 was provided by Fujirebio Diagnostics, Inc., Malvern, PA, and was raised against a partially purified mucin fraction from the ascites fluid of a cancer patient. This antibody recognizes a sequence within the MUC1 tandem repeat domain [36]. Monoclonal antibodies against Leukocyte Function Antigen-1 (LFA-1) were obtained from Abcam (Cambridge MA).
For immunofluorescence staining, adherent cells on 8-chamber LabTek culture slides were first fixed with ice-cold methanol for 10 min (for CT2 and all other antibodies except B27.29) or with 4% paraformaldehyde for 15 min (for B27.29). The slides were then washed in PBS and incubated overnight with primary antibodies (each at 10 μg/ml) followed by incubation with secondary antibodies for 1 h. Primary antibody controls consisting of isotype-matched mouse or Armenian hamster immunoglobulins (Abcam, Cambridge, MA) were always included. Primary antibodies were detected using FITC-labeled goat anti-hamster IgG (Abcam, Cambridge MA) or AlexaFluor-488-labeled goat anti-mouse IgG antibodies (Invitrogen Corporation, Carlsbad CA). For some experiments, cells were double-stained using anti-MUC1 antibody and rabbit anti-cytokeratin (Zymed, San Francisco CA). The rabbit anti-cytokeratin was detected using an AlexaFluor-594-labeled goat anti-rabbit antibody.
Immunohistochemistry
Immunohistochemical staining was performed on sections of paraffin-embedded tissues. Placentas and attached decidua basalis were obtained from four animals (Macaca mulatta) on day 30-40 of pregnancy. Tissues were fixed in phosphate-buffered 4 per cent paraformaldehyde for 4 h, rinsed in phosphate buffer, and embedded in paraffin. After sectioning (6 μm) of paraffin-embedded tissues, slides were de-paraffinized and rehydrated. Some of these specimens were made available to us by Dr Allen Enders in the form of archived paraffin-embedded blocks and sections.
Prior to immunostaining for MUC1, sections were immersed in 10 mM sodium citrate, pH 6.0, and heated to 100°C for 10 min using a microwave oven. The sections were allowed to cool for 15 min then washed with PBS. For detection of cytokeratin, sections were incubated with pepsin (1 mg/ml) in 10 mM Tris-HCl, pH 2.0 for 10 min and then washed with PBS. Sections were incubated overnight with the anti-MUC1 antibody (CT2, see above), a rabbit anti-cytokeratin antibody (Zymed), or a monoclonal anti-Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) antibody (DAKO, Carpinteria CA). Control sections were incubated with non-immune Armenian hamster Ig or rabbit Ig. Antibodies were used at 10 μg/ml. After washing, the sections were incubated with a FITC-conjugated rabbit anti-Armenian hamster Ig antibody, an AlexaFluor-488-conjugated goat anti-rabbit Ig, or an AlexaFluor-488-conjugated goat anti-mouse Ig for 1 h. After further washing, the sections were mounted in glycerol-based medium and viewed with a fluorescence microscope. Detection of MUC1 in archived paraffin-embedded sections that had been stored for greater than two years was unsatisfactory due to high levels of autofluorescence that could not be reduced. However, autofluorescence was significantly reduced if archived blocks from the same specimens were freshly re-sectioned.
RT-PCR Analysis
Trophoblasts were cultured for 2 days. Messenger RNA was extracted from the cells (50 × 106) using a Poly(A)Pure kit (Ambion Inc., Austin TX). Three hundred ng mRNA was denatured by heating to 70°C for 10 minutes. The denatured mRNA was then reverse transcribed in a total volume of 20 μl containing DTT (10mM), dGTP, dCTP, dTTP, and dATP (1 mM each), random hexamers (5 μM; GE Healthcare BioSciences Corp. Piscataway NJ), RNasin (1 U/μl; Promega Biosciences, San Luis Obispo CA) and 1 X RT buffer with the addition of 200 U SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad CA) for 2 h at 42°C. To control for genomic contamination, H2O was substituted for reverse transcriptase. The reaction was terminated by incubation at 70°C for 15 min.
For PCR, 2 μl of the resultant cDNA was amplified in a 50 μl reaction volume containing AccuPower PCR PreMix (Bionexus, Inc., Oakland CA) and 50 pmoles each of anti-sense and sense primers for MUC1. Two sequential (nested) PCR reactions were carried out. Primers designated MUC1-3-5’ and MUC1-4-3’ were used for the first reaction and primers MUC1-1-5’ and MUC1-2-3’ were used for the second reaction (see below). For the first PCR reaction, conditions were 95°C for 5 min and then 35 cycles of: 95°C, 20 sec, 50°C, 30 sec, 72°C, 1min, 72°C, 7min, 4°C, hold. For the second PCR reaction, conditions were: 95°C for 5 min followed by 35 cycles of 95°C, 20 sec, 46°C, 30 sec, 72°C, 1 min, 72°C, 7 min, and 4°C, hold. PCR reaction products were analyzed on 2% agarose gels and ethidium bromide-stained bands were photographed.
MUC1 Primer Design
Two sets of primers directed against the highly conserved cytoplasmic tail region of human MUC1 were prepared. Primers MUC1-1-5’ and MUC1-2-3’ were modified (restriction sites were removed) from published primer sequences [37] and yield a 207 bp amplicon. Primers MUC1-3-5’ and MUC1-4-3’ were based on published sequences [38]. Primer Sequences were: MUC1-1-5’: 5’- CCGCCGAAAGAACTACGG, MUC1-2-3’: 5’- CAAAGTTGGCAGAAGTGGC; MUC1-3-5’: 5’-AACCTCCAGTTTAATTCCTCTCTGGA, MUC1-4-3’: 5’- CTACAAGTTGGCAGAAGT
Western blotting
Cultures were washed with Dulbecco’s Modified PBS containing Ca2+ and Mg2+. The cells were then lysed on ice by the addition of M-PER Mammalian Protein Extraction Reagent (Pierce Biochemicals, Rockford IL) supplemented with 1% Protease Inhibitor Cocktail (Sigma-Aldrich Co, St. Louis MO). The lysate was homogenized by repeated passage through a 27 gauge needle, then mixed with an equal volume of Laemmli sample buffer (BioRad Laboratories, Hercules CA) containing 5% β-mercaptoethanol and heated in a boiling water bath for 5 minutes. The samples were immediately chilled on ice and loaded on to SDS-polyacrylamide gels (Gradiopore; 8%; Promega, San Luis Obispo, CA) at 20 μg protein per lane. After electrophoresis the proteins were transferred to nitrocellulose membrane (BioRad, Hercules CA). The membrane was blocked for 1 hour in 1% non-fat dried milk solution. The blocked membrane was incubated overnight with the anti-MUC1 antibody (CT2, see above), then washed and incubated with rabbit anti-Armenian hamster IgG labeled with horseradish peroxidase (Abcam, Cambridge MA). After further washing, the membrane was incubated with chemiluminescent substrate (SuperSignal West Dura; Pierce) for 5 min at room temperature. The membrane was then exposed to X-ray film (Pierce). Band intensities were quantified using Kodak 1D software (Kodak Imaging Systems, New Haven CT).
Cell-cell adhesion Assay
Trophoblast adhesion to confluent endothelial cell monolayers was carried using the fluorescence-based assay described previously [27] but with some modifications. Endothelial cells (UtMVECs) were plated (20,000 cells per well) and cultured for 24-48 h until confluent. Prior to use in experiments, UtMVECs were incubated with Tumor Necrosis Factor-α (TNF-α) (10 ng/ml) for 12 h. Trophoblasts were incubated with 10 μM CFDA-SE Cell Tracer Kit (Invitrogen, Carlsbad CA) for 15 minutes in PBS at 37°C, then washed in EBM containing 1% bovine serum albumin and added (250,000 cells per well) to the confluent UtMVEC cultures in a 96-well dish. The cells were incubated at 37°C for 2 h and non-adherent cells were removed by gentle washing. The dish was then placed in a Millipore 2300 fluorescence plate reader and fluorescence values were obtained. Trophoblast adhesion was expressed as a percentage of the total fluorescence of the input cells.
For antibody blocking experiments, trophoblasts were labeled with CFDA-SE as above and then pre-incubated with azide-free blocking antibodies (50 μg/ml) for 20 min. After washing, the trophoblasts were added to the endothelial monolayers as just described. In some experiments, the endothelial cells were pre-incubated with blocking antibodies for 20 min prior to the additions of the labeled trophoblasts. The blocking antibodies (azide-free) used were sheep anti-Intercellular Adhesion Molecule-1 (ICAM-1; R&D Systems, Minneapolis, MN), monoclonal anti-E-selectin (Chemicon Inc., Temecula CA), and anti-MUC1 (B27.29 and DF3; Fuziribio Inc).
Transmigration Assay
Endothelial cells (20,000 cells per insert) were cultured to confluency on 3 μm pore size FluoroBlok inserts coated with Matrigel (BD Biosciences, San Jose CA). Trophoblasts were labeled in suspension with Vybrant CFDA-SE (Invitrogen) then incubated with blocking antibodies or recombinant ICAM-1 as described above. In some cases the endothelial cells were pre-incubated with anti-ICAM-1 antibody. The labeled trophoblasts were then added (250,000 cells per insert) to the adherent endothelial monolayers (pre-incubated with TNF-α as described above). The cells were co-cultured for different times at 37°C. Antibodies were present throughout the incubation. At each time point, the culture plate was placed in a Cytofluor 2300 plate reader and the level of fluorescence in the lower chamber was measured. The level of fluorescence in the lower chamber provides a direct measure of trophoblast invasion which was expressed as a percentage of the total fluorescence of the input trophoblasts.
Statistical Analyses
All experiments were repeated at least 3 times using cells from different placentas in each case. Cells from different placentas were not pooled. Statistical analysis was performed by one-way ANOVA followed by Tukey-Kramer multiple comparison and linear trend post-tests using the Prism software program (GraphPad Inc., San Diego, CA). Two-way repeated measures ANOVA with Bonferroni post-testing, or non-linear regression analysis was used for some data. Data are expressed as means ± SEMs and differences were considered significant if p < 0.05.
RESULTS
Expression of MUC1 by macaque trophoblasts
Figure 1A shows the MUC1 immunofluorescence pattern when cultures of adherent early gestation trophoblasts were permeabilized and stained with an antibody raised against the cytoplasmic domain of MUC1. Punctate staining was prominent around and over nuclei whereas a more diffuse pattern was seen in the cytoplasm. A matching bright field image is shown in Fig. 1B. Figure 1C and D show an immunoglobulin control and corresponding bright field, respectively. Fig. 1E shows more discreet punctate fluorescence when non-permeabilized cells were stained with the B27.29 antibody raised against the extracellular domain of MUC1. Staining appeared to be largely over the nucleus and little or no staining was evident over the cytoplasm. Figure 1F shows the corresponding bright field image. An antibody control and corresponding bright field image are shown in Figs. 1G and 1H, respectively.
Fig. 1. Immunofluorescence detection of MUC1 in isolated macaque trophoblasts.
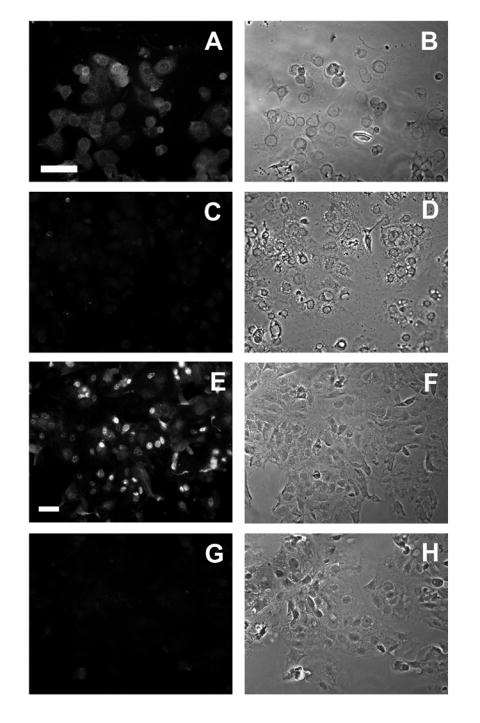
Trophoblast cells were cultured for 24 h then stained with CT2 (A) or B27.29 (E) anti-MUC1 antibodies and viewed by immunofluorescence microscopy (see Methods). The immunofluorescence images C and G show cells incubated with the corresponding control immunoglobulins. The images in the right-hand column are the respective matching phase contrast views. The horizontal bar represents 50 μm. The images are typical representatives from four separate experiments.
Sections of early gestation macaque placental/decidual tissue were also stained with anti-MUC1 antibody (CT2) and examined by immunofluorescence microscopy. Adjacent sections were incubated with control immunoglobulin for the MUC1 antibody and with antibodies against PECAM-1 and cytokeratin. As expected, and as reported by others [39], strong MUC1 staining was found associated with epithelial cells on the decidual surface and epithelial cells lining uterine glands. An example of the latter is shown in Fig. 2A. Uterine epithelial cells did not stain using the control immunoglobulin (Fig. 2B) and were negative for PECAM-1 (Fig. 2C). As expected, uterine epithelium was also positive for cytokeratin (Fig. 2D). Examination of freshly sectioned villous tissue incubated with the anti-MUC1 antibody (Fig. 2E) revealed weak fluorescence staining of the trophoblast layer that was often difficult to distinguish from the control (Fig. 2F). Villous trophoblasts were positive for PECAM-1 as previously reported [24] and were also positive for cytokeratin (Fig. 2G and 2H, respectively). Figure 2I-L shows a series of sections through a uterine blood vessel heavily invaded by trophoblasts. Large numbers of PECAM-1-positive and cytokeratin-positive trophoblasts can be seen. Positive MUC1 staining (Fig. 2I) was associated with trophoblasts closest to the vessel lumen. Staining intensity was less than that seen for uterine epithelial cells but could readily be distinguished from the antibody control. Figure 2 M-P shows another invaded vessel at higher magnification. Again, positive MUC1 staining was found for trophoblasts lining the vessel lumen. For comparison, Fig. 2Q-T shows the immunofluorescence staining pattern of a non-invaded artery. The endothelium is clearly identified by positive PECAM-1 staining (Fig. 2S, arrow) but there is no cytokeratin staining (Fig. 2T) and no MUC1 staining (Fig. 2Q) associated with this vessel profile. We did not observe MUC1 staining within the trophoblastic shell (not shown).
Fig. 2. Immunofluorescence detection of MUC1 in macaque placental/decidual tissue.
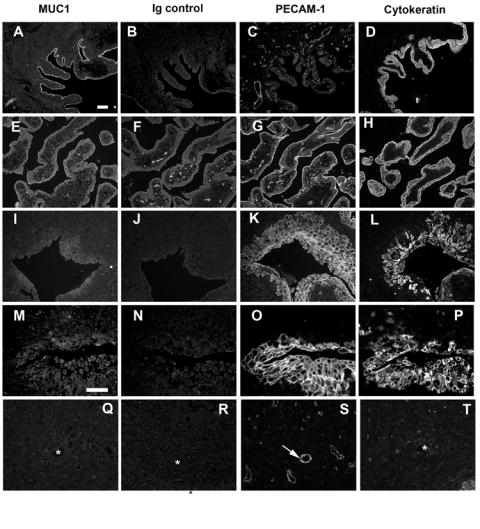
Serial sections of paraffin-embedded placental/decidual tissues were stained with antibodies against MUC1 (CT2), PECAM-1, or cytokeratin as described in Methods. The results of incubation with control immunoglobulin for the anti-MUC1 antibody are also illustrated. Panel A-D shows a typical large uterine gland. Panel E-H shows villous tissue. Panel I-L shows an invaded artery. Panel M-O shows a different invaded vessel at higher magnification. Panel P-S shows a non-invaded vessel. The arrow indicates endothelium stained with PECAM-1. The asterisks indicate the location of the corresponding vessel lumen in adjacent sections. The bars represent 50 μm.
Expression of MUC1 by isolated macaque trophoblasts was also assessed by Western blot analysis using the CT2 antibody (Fig. 3A). Two bands with molecular masses between 30 kDa and 15 kDa were detected. Two or more bands within this size range are typically detected using this antibody in other MUC1-expressing cells [40]. For comparison, MUC1 expression by human trophoblast-derived Jar choriocarcinoma cells was also examined (Fig. 3A). A smeared band was found possibly due to greater expression of MUC1 by these cells.
Fig. 3. Analysis of MUC1 expression in trophoblasts by Western blot and RT-PCR.
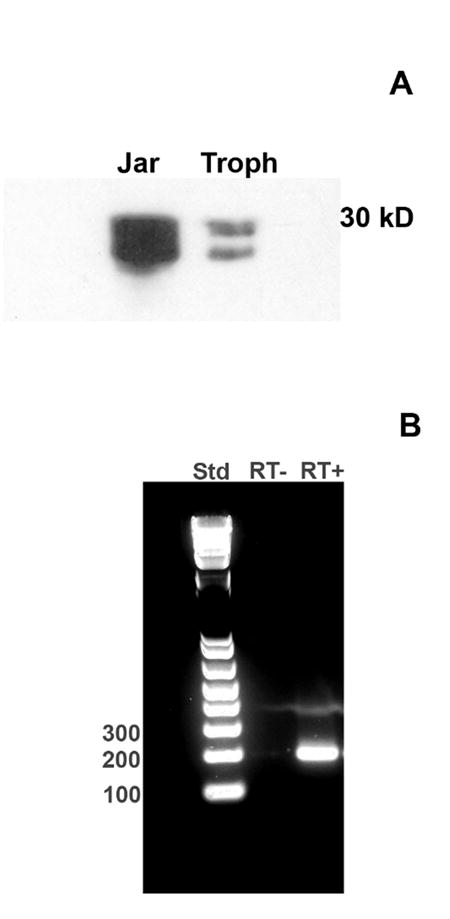
(A) Trophoblast lysates and human Jar choriocarcinoma cell lysates were examined by Western blot using the CT2 anti-MUC1 antibody (see Methods). A representative blot is shown. (B) Total RNA was extracted from macaque trophoblast cultures and reverse transcribed. The resulting cDNA was used as a template for PCR using primers directed against MUC1 as described in Methods. PCR products were analyzed on 2% agarose gels. DNA standards (Std; left lane) were included. The middle lane shows that no band was produced in the absence of reverse transcription (RT-). The right lane (RT+) shows a prominent band of the predicted size (207 bp). The band was excised from the gel and sequenced. The sequence data confirmed identity with MUC1.
Further confirmation of MUC1 expression by macaque trophoblasts was carried out using RT-PCR analysis. Figure 3B shows that an amplicon of the predicted size (207 bp) was produced (RT+). No band was detected in the absence of reverse transcription (RT-) confirming the absence of genomic material. The band was excised from the gel and sequence data (not shown) confirmed the identity with MUC1.
MUC1 is involved in the adhesion of macaque trophoblasts to endothelial cells
Trophoblast adhesion to uterine endothelial cells was measured using a fluorescence-based assay that we have described previously [27]. When trophoblasts were pre-incubated with an antibody (B27.9) known to block MUC1-mediated adhesion and then added to confluent endothelial cell cultures, adhesion was significantly reduced (p<0.01) compared to non-antibody treated cells (Fig. 4). Since B27.29 reacts with the tandem repeat domain and with carbohydrate domains [41] we repeated the experiment using another anti-MUC1 antibody (DF3) which only reacts with the DTRPAPGS core peptide sequence. Adhesion was also significantly reduced using this antibody (results not shown).
Fig. 4. Effect of anti-MUC1 antibody on trophoblast adhesion to endothelial cells.
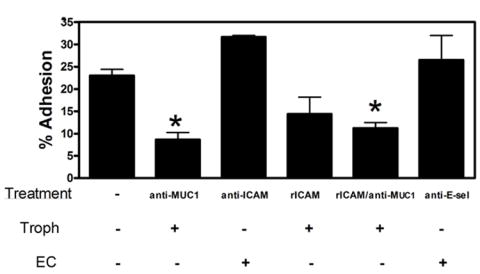
Trophoblasts were labeled with the fluorescent cell tracer CFDA-SE and added to confluent endothelial (UtMVEC) monolayers as described in Methods. Adhesion was measured after 2 h. For some experiments trophoblasts were pre-incubated with anti-MUC1 antibody or with recombinant human ICAM-1 (rICAM-1). In other experiments the endothelial cells were pre-incubated with anti-ICAM-1 or anti-E-selectin (E-sel). Adhesion is expressed as a percentage of the initial input trophoblasts and values are means ± SEMs from 3 experiments. Data were analyzed by ANOVA with Dunnett’s Multiple comparison post-test. Values marked with an asterisk were significantly different from the control (p<0.01).
Since MUC1 has been reported to bind ICAM-1, we carried out experiments to determine whether ICAM-1 was involved in trophoblast-endothelial adhesion. When trophoblasts were added to endothelial cells that had been pre-incubated with an adhesion-blocking anti-ICAM antibody, adhesion was not significantly different from the control. Two other commercial anti-ICAM-1 antibodies were also without effect on trophoblast adhesion (not shown). Pre-incubation of trophoblasts with recombinant human ICAM-1 reduced subsequent binding to endothelial cells but the effect was not quite statistically significant (q=2.82, p>0.05). Increasing the concentration of antibody or recombinant ICAM-1 did not change these results (not shown). Pre-incubation of trophoblasts with the anti-MUC1 antibody prior to incubation with recombinant ICAM-1 significantly reduced trophoblast adhesion compared to the control but the extent of the effect was similar to that of the anti-MUC1 antibody used alone. Pre-incubation of trophoblasts with an antibody against LFA-1, a major ICAM-1 counter receptor, failed to inhibit adhesion to endothelial cells (results not shown). MUC-1 is also reported to bind to E-selectin [14], but trophoblast adhesion was unaffected by pre-incubation of endothelial cells with an adhesion-blocking antibody against E-selectin (Fig. 4).
Treatment of trophoblasts with anti-MUC1 antibody reduces transmigration across endothelial cell monolayers
We next examined the effects of the anti-MUC1 antibody on the time course of trophoblast transmigration across endothelium using commercial invasion chambers (Fig. 5). Mean trophoblast migration values for each of the antibody-treated co-cultures were consistently less than control values at all time points. Regression analysis of these time course data indicated that the anti-MUC1 antibody significantly (F=5.597; p<0.001) reduced trophoblast migration and when individual time points were analyzed by 2-way ANOVA, a significant effect of the anti-MUC1 antibody was found at the 4, 5, and 6 h time points. Regression analysis indicated that trophoblast migration was significantly reduced by recombinant ICAM-1 (F=3.109, p<0.05) and by the anti-ICAM-1 antibody (F=2.967, p<0.05). Analysis by 2-way ANOVA showed that a significant effect of the rICAM-1 and the anti-ICAM-1 antibody on trophoblast migration was achieved at the 5 and 6 h, and the 6 h time points, respectively.
Fig. 5. Effect of anti-MUC1 antibody on trophoblast transendothelial migration.
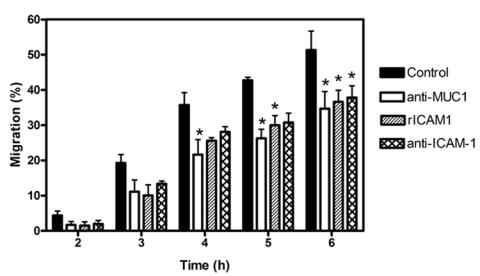
Trophoblasts were labeled with the fluorescent cell tracer CFDA-SE and added to confluent endothelial monolayers cultured on TransWell FluorBlok inserts. Transendothelial migration of trophoblasts to the lower chamber was measured at different time points as described in Methods. For some experiments trophoblasts were pre-incubated with anti-MUC1 antibody or with recombinant human ICAM-1 (rICAM-1) and then added to the endothelial cells without washing. In other experiments the endothelial cells were pre-incubated with anti-ICAM-1 antibody which was maintained in the culture medium throughout the experiment. Trophoblast migration is expressed as a percentage of the initial input trophoblasts and values are means ± SEMs from 3-4 experiments. Data were analyzed by non-linear regression with an F test. The results are described in the text. Data were also evaluated by 2-way ANOVA with a Bonferroni post-test. The asterisks indicate values that are significantly different (p<0.05) from the corresponding control value.
DISCUSSION
The results presented here demonstrate that early gestation macaque trophoblasts express MUC1 and that MUC1 is involved in trophoblast-endothelial interactions. In sections of early gestation macaque placental/decidual tissue MUC1 expression was detected in endovascular trophoblasts but was not found in the trophoblastic shell. MUC1 expression by villous trophoblasts was often equivocal and weak at best. Expression of MUC1 by human endovascular trophoblasts was not reported although MUC1 was detected in first trimester villous and extravillous trophoblasts [32]. The expression of MUC1 by invasive trophoblasts in intact macaque tissue was substantiated by showing robust MUC1 expression at the protein and mRNA levels in isolated early gestation macaque trophoblast cells. We have previously shown that these cells have an invasive phenotype in vitro [35, 42]. In addition to the human placental study cited above, muc1 has also been shown to be expressed by mouse labyrinthine trophoblast [33] and by human trophectoderm [8]. The expression of MUC1 by trophoblasts is rarely acknowledged but the expression of MUC1 by invasive trophoblasts in the macaque is consistent with the idea that it may be involved in mediating the interaction between endovascular trophoblasts and uterine endothelium.
Our finding that an antibody known to block MUC1-mediated adhesion in other systems also blocked trophoblast adhesion to endothelial cells supports the idea that MUC1 on trophoblasts is involved in trophoblast adhesion to endothelium. Other molecules implicated in human and non-human primate trophoblast attachment to endothelium include β1 integrin, αVβ3 integrin, VE-cadherin, and VCAM-1 [27-30, 43]. How these different adhesion systems are orchestrated to allow trophoblast attachment and migration along endothelium is not known. Our data also suggest that MUC1 could be involved in trophoblast transendothelial migration in vivo since the MUC1 antibody also reduced trophoblast migration across endothelial monolayers. Trophoblast transendothelial migration is an important step in the trophoblast-mediated remodeling of the uterine vasculature. MUC1 has been reported to be involved in tumor cell transmigration [19] and may play a role in T-cell motility [2].
The identity of the endothelial counter-receptor for MUC1 is clearly of significance. Since MUC1 has been reported to bind to ICAM-1 [17, 44] and ICAM-1 is expressed by endothelial cells and involved in leukocyte transmigration, ICAM-1was an obvious counter-receptor candidate for trophoblast MUC1. However, when endothelial cells were pre-incubated with an anti-ICAM-1 antibody no significant inhibition of trophoblast adhesion was observed. The finding that pre-incubation of trophoblasts with recombinant ICAM-1 reduced adhesion (albeit to a not quite statistically significant level) is suggestive of some involvement of ICAM-1. The observation that the combined effect of the anti-MUC1 antibody and recombinant ICAM-1 on adhesion was not additive, is consistent with the idea that trophoblasts bind ICAM-1 via MUC1. We could find no expression of the major ICAM-1 counter receptors, LFA-1 and MAC-1 by macaque trophoblasts and corresponding blocking antibodies had no effect on trophoblast-endothelial adhesion (results not shown). Stronger support for the conclusion that ICAM-1 is involved in trophoblast-endothelial interaction was provided by the modest, but statistically significant, effect of recombinant ICAM-1 and anti-ICAM-1 on trophoblast transendothelial migration. Human MUC1 is reported to bind to domain 1 of ICAM-1 [45] and this is the site targeted by the antibody. Studies of T-cell migration across ICAM-1-/- endothelioma cells [46] in which different ICAM-1 mutants had been re-expressed demonstrated that the extracellular domain of ICAM-1 supports T cell adhesion while the cytoplasmic tail was required for transendothelial migration. In the present case it is possible that while trophoblast attachment to endothelial cells shows minimal dependency on ICAM-1, the interaction between trophoblasts and endothelial ICAM-1 is sufficient to induce signaling activity in the ICAM-1 cytoplasmic domain which affects subsequent transmigration. In a paper submitted for publication elsewhere, we show that endothelial ICAM-1 expression and distribution are altered by co-culturing UtMVECs with trophoblasts.
Other potential MUC1 ligands can be considered. Because of extensive glycosylation, MUC1 can mediate binding to lectins. Sialyl Lewis(x) [14, 47] and the Thomsen-Friedenreich antigen [48-50] are presented by MUC1 on the surface of certain tumor cells and may promote adhesion to endothelial cells expressing selectins and galectin-3, respectively [51-54]. We found no evidence that E-selectin was involved in trophoblast-endothelial attachment or that UtMVECs express either galectin-3 or galectin-1 (immunofluorescence results not shown).
In summary, the results show that invasive trophoblasts in the macaque express MUC1 and that MUC1 is involved in trophoblast attachment to, and migration across, endothelium. While our data support a modest role for endothelial ICAM-1 in MUC1-mediated trophoblast adhesion and transmigration, the data do not rule out the possibility of other as yet undiscovered MUC-1 ligands on uterine endothelium.
Acknowledgments
Early gestation macaque placental tissue was made available to us through the cooperation of the staff at the California Regional Primate Research Center, University of California, Davis. We are particularly indebted to Sara Davis, Dr. Andy Hendryckx, and Katy Lanz. We also thank Dr Allen Enders for providing some of the tissue blocks used in this study. The work was supported by Philip Morris USA Inc. (GCD) and NIHR01HL068035-01A1 (GCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Twanda L. Thirkill, Department of Cell Biology and Human Anatomy, School of Medicine, University of California, Davis
Tim Cao, Department of Biomedical Engineering, University of California, Davis.
Michael Stout, Department of Cell Biology and Human Anatomy, School of Medicine, University of California, Davis.
Thomas N. Blankenship, Department of Cell Biology and Human Anatomy, School of Medicine, University of California, Davis
Abdul Barakat, Department of Mechanical and Aeronautical Engineering, University of California, Davis.
Gordon C. Douglas, Department of Cell Biology and Human Anatomy, School of Medicine, University of California, Davis
Literature Cited
- 1.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–53. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 2.Correa I, Plunkett T, Vlad A, Mungul A, Candelora-Kettel J, Burchell JM, Taylor-Papadimitriou J, Finn OJ. Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology. 2003;108:32–41. doi: 10.1046/j.1365-2567.2003.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloosen S, Thio M, Vanclee A, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, Germeraad WT, Bos GM. Mucin-1 is expressed on dendritic cells, both in vitro and in vivo. Int Immunol. 2004;16:1561–71. doi: 10.1093/intimm/dxh157. [DOI] [PubMed] [Google Scholar]
- 4.Wykes M, MacDonald KP, Tran M, Quin RJ, Xing PX, Gendler SJ, Hart DN, McGuckin MA. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J Leukoc Biol. 2002;72:692–701. [PubMed] [Google Scholar]
- 5.Hilkens J, Wesseling J, Vos HL, Storm J, Boer B, van der Valk SW, Maas MC. Involvement of the cell surface-bound mucin, episialin/MUC1, in progression of human carcinomas. Biochem Soc Trans. 1995;23:822–6. doi: 10.1042/bst0230822. [DOI] [PubMed] [Google Scholar]
- 6.Ligtenberg MJ, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–24. [PubMed] [Google Scholar]
- 7.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meseguer M, Aplin JD, Caballero-Campo P, O’Connor JE, Martin JC, Remohi J, Pellicer A, Simon C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64:590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 9.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervenak JL, Illsley NP. Episialin acts as an antiadhesive factor in an in vitro model of human endometrial-blastocyst attachment. Biol Reprod. 2000;63:294–300. doi: 10.1095/biolreprod63.1.294. [DOI] [PubMed] [Google Scholar]
- 11.DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, Gendler S, Hilkens J, Carson DD. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45:127–58. doi: 10.1016/s0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 12.Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–47. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 13.Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Baeckstrom D, Brevinge H, Hansson GC. Comparison of sialyl-Lewis a-carrying CD43 and MUC1 mucins secreted from a colon carcinoma cell line for E-selectin binding and inhibition of leukocyte adhesion. Tumour Biol. 1997;18:175–87. doi: 10.1159/000218028. [DOI] [PubMed] [Google Scholar]
- 15.Hey NA, Aplin JD. Sialyl-Lewis x and Sialyl-Lewis a are associated with MUC1 in human endometrium. Glycoconj J. 1996;13:769–79. doi: 10.1007/BF00702341. [DOI] [PubMed] [Google Scholar]
- 16.Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ. MUC1 is a scaffold for selectin ligands in the human uterus. Front Biosci. 2006;11:2903–8. doi: 10.2741/2018. [DOI] [PubMed] [Google Scholar]
- 17.Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244–9. [PubMed] [Google Scholar]
- 18.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973–82. doi: 10.1002/1097-0142(20010601)91:11<1973::aid-cncr1222>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Rahn JJ, Chow JW, Horne GJ, Mah BK, Emerman JT, Hoffman P, Hugh JC. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22:475–83. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 20.Blankenship TN, King BF. Macaque intra-arterial trophoblast and extravillous trophoblast of the cell columns and cytotrophoblastic shell express neural cell adhesion molecule (NCAM) Anat Rec. 1996;245:525–531. doi: 10.1002/(SICI)1097-0185(199607)245:3<525::AID-AR9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Burrows TD, King A, Loke YW. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta. 1994;15:21–33. doi: 10.1016/s0143-4004(05)80233-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Fisher SJ, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blankenship TN, Enders AC. Expression of platelet-endothelial cell adhesion molecule-1 (PECAM) by macaque trophoblast cells during invasion of the spiral arteries. Anat Rec. 1997;247:413–419. doi: 10.1002/(SICI)1097-0185(199703)247:3<413::AID-AR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Genbacev O, Fisher SJ. The human placenta remodels the uterus by using a combination of molecules that govern vasculogenesis or leukocyte extravasation. Annals New York Academy of Sciences. 2003;995:73–83. doi: 10.1111/j.1749-6632.2003.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 27.Douglas GC, Thirkill TL, Blankenship TN. Vitronectin receptors are expressed by macaque trophoblast cells and play a role in migration and adhesion to endothelium. Biochimica et Biophysica ACTA/General Subjects. 1999;1452:36–45. doi: 10.1016/s0167-4889(99)00109-3. [DOI] [PubMed] [Google Scholar]
- 28.Thirkill TL, Douglas GC. The vitronectin receptor plays a role in the adhesion of human cytotrophoblast cells to endothelial cells. Endothelium. 1999;6:277–90. doi: 10.3109/10623329909078495. [DOI] [PubMed] [Google Scholar]
- 29.Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S, Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303:101–13. doi: 10.1016/j.yexcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Cartwright JE, Balarajah G. Trophoblast interactions with endothelial cells are increased by interleukin-1beta and tumour necrosis factor alpha and involve vascular cell adhesion molecule-1 and alpha4beta1. Exp Cell Res. 2005;304:328–36. doi: 10.1016/j.yexcr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Richter DU, Jeschke U, Bergemann C, Makovitzky J, Luthen F, Karsten U, Briese V. Expression of the Thomsen-Friedenreich (TF) tumor antigen in human abort placentas. Anticancer Res. 2005;25:1675–8. [PubMed] [Google Scholar]
- 32.Jeschke U, Richter DU, Hammer A, Briese V, Friese K, Karsten U. Expression of the Thomsen-Friedenreich antigen and of its putative carrier protein mucin 1 in the human placenta and in trophoblast cells in vitro. Histochem Cell Biol. 2002;117:219–26. doi: 10.1007/s00418-002-0383-5. [DOI] [PubMed] [Google Scholar]
- 33.Shalom-Barak T, Nicholas JM, Wang Y, Zhang X, Ong ES, Young TH, Gendler SJ, Evans RM, Barak Y. Peroxisome proliferator-activated receptor gamma controls Muc1 transcription in trophoblasts. Mol Cell Biol. 2004;24:10661–9. doi: 10.1128/MCB.24.24.10661-10669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douglas GC, King BF. Isolation and morphologic differentiation in vitro of villous cytotrophoblast cells from Rhesus monkey placenta. In Vitro Cell Dev Biol. 1990;26:754–758. doi: 10.1007/BF02623616. [DOI] [PubMed] [Google Scholar]
- 35.Soghomonians A, Barakat AI, Thirkill TL, Blankenship TN, Douglas GC. Effect of shear stress on migration and integrin expression in macaque trophoblast cells. Biochimica et Biophysica ACTA/General Subjects. 2002;1589:233–46. doi: 10.1016/s0167-4889(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Sejbal J, Kotovych G, Koganty RR, Reddish MA, Jackson L, Gandhi SS, Mendonca AJ, Longenecker BM. Structurally defined synthetic cancer vaccines: analysis of structure, glycosylation and recognition of cancer associated mucin, MUC-1 derived peptides. Glycoconj J. 1995;12:607–17. doi: 10.1007/BF00731254. [DOI] [PubMed] [Google Scholar]
- 37.Meerzaman D, Xing PX, Kim KC. Construction and characterization of a chimeric receptor containing the cytoplasmic domain of MUC1 mucin. Am J Physiol Lung Cell Mol Physiol. 2000;278:L625–9. doi: 10.1152/ajplung.2000.278.3.L625. [DOI] [PubMed] [Google Scholar]
- 38.Spicer AP, Duhig T, Chilton BS, Gendler SJ. Analysis of mammalian MUC1 genes reveals potential functionally important domains. Mamm Genome. 1995;6:885–8. doi: 10.1007/BF00292441. [DOI] [PubMed] [Google Scholar]
- 39.Julian J, Enders AC, Fazleabas AT, Carson DD. Compartmental distinctions in uterine Muc-1 expression during early pregnancy in cynomolgous macaque (Macaca fascicularis) and baboon (Papio anubis) Hum Reprod. 2005;20:1493–503. doi: 10.1093/humrep/deh801. [DOI] [PubMed] [Google Scholar]
- 40.Croce MV, Isla-Larrain MT, Rua CE, Rabassa ME, Gendler SJ, Segal-Eiras A. Patterns of MUC1 Tissue Expression Defined by an Anti-MUC1 Cytoplasmic Tail Monoclonal Antibody in Breast Cancer. J Histochem Cytochem. 2003;51:781–788. doi: 10.1177/002215540305100609. [DOI] [PubMed] [Google Scholar]
- 41.Grinstead JS, Koganty RR, Krantz MJ, Longenecker BM, Campbell AP. Effect of Glycosylation on MUC1 Humoral Immune Recognition: NMR Studies of MUC1 Glycopeptide-Antibody Interactions. Biochemistry (Mosc) 2002;41:9946–9961. doi: 10.1021/bi012176z. [DOI] [PubMed] [Google Scholar]
- 42.Thirkill TL, Lowe K, Vedagiri H, Blankenship TN, Barakat AI, Douglas GC. Macaque trophoblast migration is regulated by RANTES. Exp Cell Res. 2005;305:355–64. doi: 10.1016/j.yexcr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahn JJ, Shen Q, Mah BK, Hugh JC. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–90. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi T, Takahashi T, Motoya S, Ishida T, Itoh F, Adachi M, Hinoda Y, Imai K. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion. 2001;63(Suppl 1):87–92. doi: 10.1159/000051917. [DOI] [PubMed] [Google Scholar]
- 46.Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–83. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 47.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 48.Baldus SE, Hanisch FG, Kotlarek GM, Zirbes TK, Thiele J, Isenberg J, Karsten UR, Devine PL, Dienes HP. Coexpression of MUC1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer. 1998;82:1019–27. doi: 10.1002/(sici)1097-0142(19980315)82:6<1019::aid-cncr3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Bohm CM, Mulder MC, Zennadi R, Notter M, Schmitt-Graff A, Finn OJ, Taylor-Papadimitriou J, Stein H, Clausen H, Riecken EO, Hanski C. Carbohydrate recognition on MUC1-expressing targets enhances cytotoxicity of a T cell subpopulation. Scand J Immunol. 1997;46:27–34. doi: 10.1046/j.1365-3083.1996.d01-91.x. [DOI] [PubMed] [Google Scholar]
- 50.Flucke U, Zirbes TK, Schroder W, Monig SP, Koch V, Schmitz K, Thiele J, Dienes HP, Holscher AH, Baldus SE. Expression of mucin-associated carbohydrate core antigens in esophageal squamous cell carcinomas. Anticancer Res. 2001;21:2189–93. [PubMed] [Google Scholar]
- 51.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–7. [PubMed] [Google Scholar]
- 52.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–11. [PubMed] [Google Scholar]
- 53.Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;26:309–18. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]
- 54.Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–7. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]


