Abstract
Rationale
In some rats, the hormone corticosterone is reinforcing. High novelty-seeking rats (high responders, HR) self-administered corticosterone at a much higher rate than low novelty-seeking rats (low responders, LR) do [24]. While previous studies demonstrated that corticosterone reinforces nose poking in a self-administration paradigm, no studies to date have examined whether corticosterone is rewarding.
Objective
Using the conditioned place preference (CPP) paradigm, we examined the rewarding effects of corticosterone in HR and LR rats.
Methods
Male Sprague-Dawley rats were classified into HR and LR groups based on their locomotor activity in a novel environment. Subsequently, independent groups of HR and LR rats underwent CPP for corticosterone (0; 2.5 or 10 mg/kg; i.p.) or cocaine (12 mg/kg; i.p). CPP for Cocaine was used as a positive control.
Results
While cocaine produced a strong CPP in both HR and LR rats, corticosterone failed to produce either preference or aversion in both phenotypes.
Conclusion
Corticosterone is neither rewarding nor aversive in either behavioral phenotype.
Keywords: reward, reinforcement, glucocorticoids, corticosterone, individual differences
In humans, novelty-seeking is a trait that reliably predicts drug abuse. Novelty seekers report higher and more frequent use of illicit substances [35,36]see [4]. To further investigate this phenomenon, a model of individual differences in novelty-seeking was developed in rats. Outbred rats can be classified as high responders (HR) or low responders (LR) based on their locomotor activity in a novel environment. An important difference between HR and LR rats is their drug-taking behavior, as HR rats readily self-administer psychostimulants when compared to LR rats [19,22,23]. This animal model is therefore widely used to assess the neurobiological and environmental factors that predict the susceptibility and behavioral responses to various substances of abuse [11,17,19,21,23].
Glucocorticoids increase the appetitive properties of numerous stimuli [25], including drugs of abuse [8,14]. For example, adrenalectomy attenuates the reinforcing properties of psychostimulants [8,14] and this effect is reversed in a dose-dependent manner by administration of corticosterone (CORT) [8]. In a study evaluating individual differences in the reinforcing properties of CORT, rats performed an operant task to self-administer this hormone [24], [34]. CORT increased operant responding in HR rats compared to LR rats, suggesting that the hormone’s strength as a reinforcer depends on these behavioral phenotypes. Furthermore, when compared to LR rats, HR rats exhibited an exaggerated CORT response following mild stressors, which was associated with a reduction in anxiety-like behaviors in these rats [7,18]. Taken together, these data suggest that HR rats may actively seek stress-induced increases in plasma CORT levels, which these animals experience as reinforcing [18].
Clinical observations have shown that chronic glucocorticoid treatment induces negative symptoms (including asthenia, fatigue, reduced concentration, apathy, depression, and increased irritability) in some individuals [13,16,28] and positive symptoms (such as euphoria) in others [13,16,28]. These findings led us to hypothesize that there might be individual differences in the rewarding properties of CORT [2]. In particular, HR rats may find CORT more rewarding than LR rats. Accordingly, the aim of the present study was to evaluate the rewarding properties of CORT (0, 2.5, and 10 mg/kg) and cocaine (12 mg/kg) using a CPP paradigm. Cocaine was used as a positive control since it has been shown to produce strong CPP in both HR and LR rats [15].
Seventy-four male Sprague–Dawley rats from Charles River (Wilmington, MA, USA), weighing 250–275g on arrival, were used in this study. Forty-eight of these rats were used to test CORT (0, 2.5, and 10 mg/kg, i.p.) CPP in HR and LR rats. Fourteen rats were used to test cocaine CPP in HR and LR rats. The remaining fourteen rats were used to examine the effects of CORT injections (0, 2.5, 10 mg/kg; i.p.) on plasma CORT levels. These rats were injected with either the vehicle or the CORT and then were terminated 30 minutes after exposure to the white or to the dark compartments of the CPP apparatus. The CORT doses were chosen based on previous reports showing that these doses do not induce seizures and can alter different behaviors including CPP, drug self-administration, and spatial memory [1,3,30,31]. CORT and cocaine were purchased from Sigma-Aldrich, St. Louis MO USA.
In all experiments, the rats were housed 2 per cage in clear Plexiglas cages (19"×10.5"×8") and were kept in a 12 hour light cycle (lights on at 7am). All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Florida State University.
Rats were allowed to habituate to the housing conditions for 5 days. Subsequently, their locomotor response to novelty was tested in circular activity chambers (Med Associates Inc, St. Albans, Vermont) for one hour. Four photobeam sensors at equal distances recorded each rats’ crossings between adjacent quadrants. The rats that exhibited locomotor counts in the highest 50 percent of the sample were classified as HR, and the rats that exhibited locomotor counts in the lowest 50 percent were classified as LR.
Place conditioning started two days after the novelty response testing and was conducted in shuttle boxes (Med Associates Inc, St. Albans, Vermont) consisting of 3 distinct compartments operated by automatic doors. The overall inside dimensions of the apparatus were 21 × 21 × 68 cm. The center compartment was gray with a smooth PVC floor. The choice compartments were 28 cm long. One compartment was all black with a stainless steel grid rod floor consisting of 4.8 mm rods, placed 16 mm on center, while the other was all white with a 0.25 × 1.25 cm stainless steel mesh floor. The location of each rat was recorded during test sessions using fifteen infrared photobeam detectors.
On day one (pre-conditioning), each rat was placed in the neutral gray compartment for 30 seconds. After this delay, the automatic guillotine doors opened and the rat had free access to the entire apparatus for a total of 20 minutes. The amount of time spent in each compartment was recorded. In this experiment, the rats tended to have a slight bias for the black compartment. On average, the rats spent 138 seconds more in the black compartment than in the white compartment. Therefore, injections of CORT and cocaine were paired with the least-preferred white side.
On the following four days of conditioning, two groups of rats were injected with CORT at 2.5 mg/kg (HR n=8; LR=8) or 10 mg/kg (HR n=8; LR=8). Injections were performed twice a day with one pairing of CORT in the white chamber and one pairing of vehicle in the black chamber. The injections were counterbalanced between morning and afternoon with at least 4 hours between each session. A separate control group of rats (HR n=8; LR=8) was injected only with the vehicle (45% hydroxypropyl-beta-cyclodextrin) in both compartments twice daily for all four days of conditioning. As a positive control for our CPP procedures, a separate group of HR (n=5) and LR (n=9) rats were conditioned with cocaine (12mg/kg i.p.) in the white chamber or saline (1 ml/kg; i.p.) in the black chamber twice daily for all four conditioning days. In this case also, the injections were counterbalanced between morning and afternoon with at least 4 hours between each session. All conditioning sessions lasted 30 minutes.
On the day following the last conditioning, the rats were tested for CPP in a drug-free state. The experimental conditions used during the test were exactly the same as those used during the pretest. The amount of time the rats spent in each compartment was recorded.
In an additional experiment, we sought to determine the levels of plasma CORT induced by the intraperitoneal injections of CORT or its vehicle. For this purpose, 14 male Sprague-Dawley rats were injected with CORT at 2.5 mg/kg (n=5) and CORT at 10 mg/kg (n=4) or with vehicle (n=5). After the injection of CORT or vehicle, each rat was placed in the white chamber (for CORT-injected rats) or in the black chamber (for vehicle-injected rats) of the CPP apparatus. The rats were then terminated 30 minutes later. Their trunk blood was collected in 7ml blood collection tubes coated with EDTA (Becton Dickinson, Franklin Lakes NJ USA). The collection tubes were centrifuged at 2500 rpm at 4°C and plasma was collected and frozen at −20°C until used for CORT analysis. Plasma CORT levels were determined using the Coat-A-Count corticosterone radioimmunoassay kit according to the manufacturer’s instructions (DPC Los Angeles, CA, USA).
Our results show that locomotor activity in a novel environment does not predict individual differences in place conditioning to CORT (figure 1). Unlike CORT, cocaine induced an equally strong place preference in both HR and LR rats (figure 2). Our results are in agreement with the results of Gong et al., [15] and confirm that response to novelty does not predict the rewarding properties of cocaine. Since we were able to induce a robust conditioned place preference to cocaine but not to CORT using exactly the same CPP procedure, we conclude that CORT is not rewarding.
Figure 1.
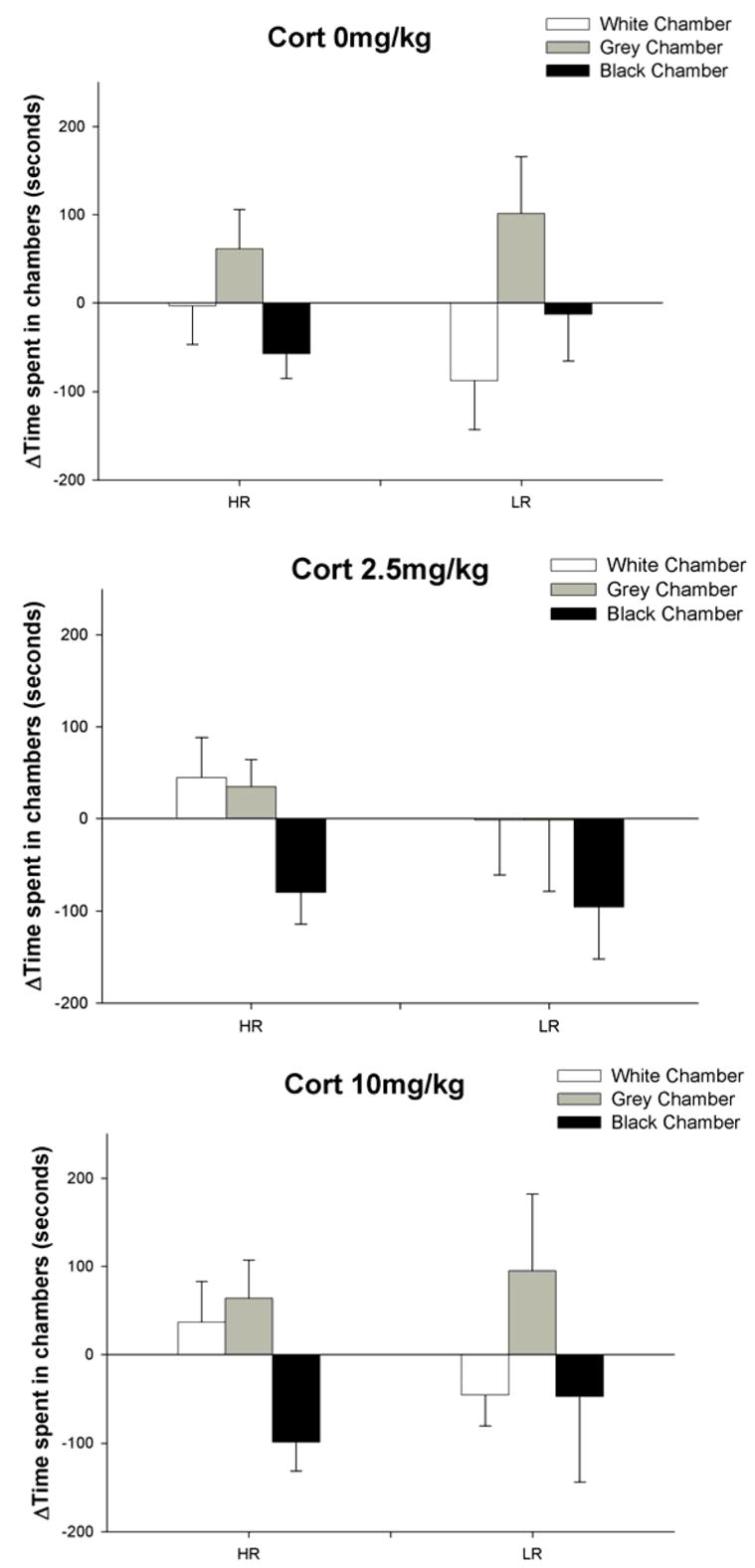
Response to novelty did not predict conditioned place preference or conditioned place aversion to CORT. In the white CORT paired chambers, there was no effect of individual differences (HR/LR) [F(1,42)=3.426; p>0.05] nor CORT doses [F(2,42)=1.026; p>0.05] on time spent in the white chamber on test day compared to pretest day. There was also no interaction between individual differences and CORT doses [F(2,27)=0.32;p>0.05]. Furthermore, there was no main effect of individual differences [F(1,42)=0.360; p>0.05] nor a main effect of CORT doses [F =0.521; p>0.05] on time spent in the vehicle-paired black chamber. There was also no interaction between individual differences and CORT doses for the black chamber [F(2,27)=0.20; p>0.05]. Finally, there was no main effect of individual differences [F(1,42)=0.938; p>0.05] nor CORT dose effects [F(2,42)=0.022; p>0.05] for the time spent in the gray chamber. There was also no interaction between individual differences and CORT doses in the gray chamber [F(2,27)=0.23; p>0.05]. Individual differences in CPP were analyzed using two-way Analysis of Variance (ANOVA). HR/LR and CORT doses were the two independent factors and the delta time spent in the white conditioning chamber, gray start box, or black vehicle-paired chamber were the dependent variables.
Figure 2.
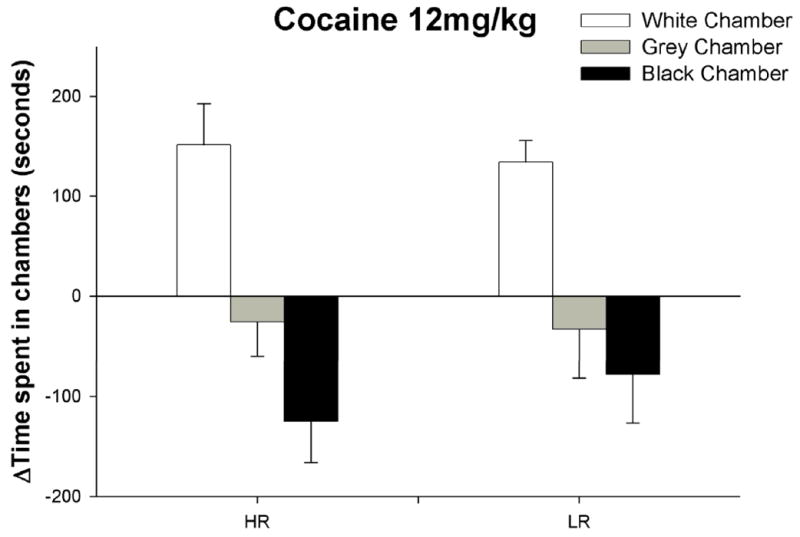
Cocaine induced a conditioned place preference, as demonstrated by a significant increase in the time spent by rats in the white chamber [F(1,12)=43.12; p<0.001] and a significant decrease in time spent by rats in the vehicle-paired black chamber [F(1,12)=7.16; p<0.05]. There were no effects of individual differences on the time spent in the white chamber [F(1,12)=.132; p>0.05] or the black chamber [F(1,12)=.579; p>0.05]. Finally, there was no effect of cocaine-conditioning on the time spent in the gray start box [F(1,12)=0.623;P>0.05], nor was there an effect of individual differences [F(1,12)=.003;P>0.05]. Individual differences in cocaine-induced CPP were analyzed using one-way repeated measures ANOVA. HR/LR was the independent factor and the delta time spent in the white conditioning chamber, gray start box, or black vehicle paired chamber were the dependent variables.
In order to ascertain whether or not the CORT treatment produced physiological levels of the hormone we carried out an experiment in which rats were injected with either the vehicle or CORT and the levels of the hormone was determined in the plasma. Acute injection of CORT at 10 mg/kg produced plasma CORT levels above the physiological range produced by a stress exposure. However, the injection of CORT at 2.5 mg/kg produced physiological levels of the hormone ( figure 3) that are in the range of the physiological plasma levels that were shown in a previous study to be reinforcing [24]. Accordingly, pharmacological as well as the reinforcing physiological plasma levels of CORT fail to be rewarding or aversive.
Figure 3.
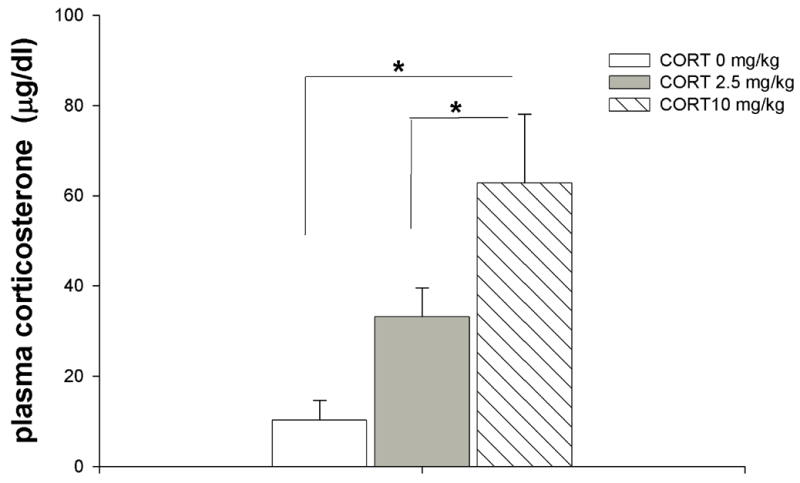
Plasma CORT concentration in rats injected with vehicle, 2.5 and 10mg/kg of CORT. There was a significant main effect of the CORT doses [F(2,11)=8.41; P=0.006]. Fisher’s post-hoc analysis revealed that there was significant increase in plasma levels of CORT in the rats injected with 10mg/kg as compared to vehicle-injected rats (p=0.0018). Rats injected with CORT at 10 mg/kg exhibited also higher plasma CORT when compared to rats injected with CORT at 2.5 mg/kg (p=0.040). Rats injected with CORT at 2.5mg/kg exhibited a nonsignificant increase in plasma CORT when compared to vehicle-treated rats (p=0.08). Plasma CORT levels were analyzed using one-way ANOVA followed by Fisher’s Post-hoc test.
The rewarding properties of glucocorticoids in a CPP paradigm have been examined in two studies. In one study, high dose of dexamethasone appeared to induce place preference [20]. In the second study, CORT (10 mg/kg) failed to produce CPP [3]. Our results confirm with the finding of Brooks et al., but extend them to lower concentration of the hormone and extend them by demonstrating that even HR rats that are known to self administer CORT do not form place preference for a CORT-associated environment. It is likely that some of the discrepancies between the dexamethasone and the CORT studies are due to methodological issues. The most likely issue is the choice of the glucocorticoid, as dexamethasone and CORT diffuse across the blood brain barrier with different efficacies and bind with different affinities to glucocorticoid receptors [6,27].
One might ask why HR and LR rats exhibit differences in the expression of behavioural reinforcement for response-contingent injections of CORT but fail to develop a CPP to the same hormone. This is not the first time that such dissociation between the reinforcing and rewarding properties of a substance in HR and LR rats was reported. Indeed, response to novelty failed to predict individual differences in the formation of preferences for psychostimulant-associated environments [12,15], despite the fact that HR and LR rats clearly exhibit different patterns of psychostimulant self-administration [19,22,23,26]. The seemingly contradictory observations that rats will self administer CORT [9,24] but will not develop CORT CPP is consistent with the notion that several classes of drugs are self-administered but do not support CPP [2]. Although self-administration and CPP data usually concur, these mismatches suggest that these two paradigms are measuring drug actions that are at least partially dissociable [33,34]. For example, CPP is entirely dependent on a bolus injection by the experimenter whereas in drug self–administration, rats receive their injections at a much lower rate and they have the choice to either take the drug or not. Past reports have clearly show that dopamine transmission is attenuated in animals receiving yoked infusions when compared to rats actively self-administering amphetamine [10,29].
It should be noted that the CPP paradigm differs from the self-administration paradigm in that it is dependent on contextual learning and temporal associations [2]. Highly elevated circulating CORT concentrations may actually interfere with contextual learning, since adrenalectomized rats injected with a high dose of CORT (40mg/kg) were impaired in a Y maze test [5]. However, in our study, an impairment of contextual learning cannot explain the lack of preference for CORT for at least 3 reasons: 1) the doses of CORT that we used are much lower than the doses that have been shown to impair learning. CORT at 2.5 and 10 mg/kg were even shown to enhance learning [1,32] 2) rats injected with CORT at low and high doses exhibit similar behavior in the CPP paradigm; 3) rats conditioned with CORT and rats conditioned with the vehicle exhibit similar behavior in the CPP paradigm.
Further experimentation will be necessary to understand how CORT appears to have strong reinforcing properties in some rats and not others, even in the absence of marked conditioned reward effects. As shown in humans, it is possible that longer exposure to CORT may reveal the existence of individual differences in the rewarding/aversive properties of this hormone.
Acknowledgments
This work was supported by NIDA (1RO1 DA019627–01 to Dr. Zuoxin Wang and Dr. Kabbaj; 1RO3 DA021554–01A1 to Dr. Kabbaj and 1F31DA019745–01A1 to David Dietz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SP, Hennebry G, Croft AP, Thomas A, Little HJ. Effects of corticosterone on place conditioning to ethanol. Psychopharmacology (Berl) 2004;174:291–299. doi: 10.1007/s00213-003-1745-y. [DOI] [PubMed] [Google Scholar]
- 4.Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- 5.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 7.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 8.Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- 9.Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Res. 1993;622:315–320. doi: 10.1016/0006-8993(93)90837-d. [DOI] [PubMed] [Google Scholar]
- 10.Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine oxidation currents in the nucleus accumbens during unlimited-access self-administration of d-amphetamine by rats. Behav Pharmacol. 1996;7:714–729. doi: 10.1097/00008877-199611000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86:347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 13.Fox HM, Gifford S. Psychological responses to ACTH and cortisone; a preliminary theoretical formulation. Psychosom Med. 1953;15:614–627. doi: 10.1097/00006842-195311000-00005. discussion, 627–631. [DOI] [PubMed] [Google Scholar]
- 14.Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- 15.Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- 16.Goolker P, Schein J. Psychic effects of ACTH and cortisone. Psychosom Med. 1953;15:589–612. doi: 10.1097/00006842-195311000-00004. discussion, 612–583. [DOI] [PubMed] [Google Scholar]
- 17.Hooks MS, Jones GH, Neill DB, Justice JB., Jr Individual differences in amphetamine sensitization: dose-dependent effects. Pharmacol Biochem Behav. 1992;41:203–210. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- 18.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- 20.Lebedev AA, Gurkovskaya OV, Nozdrachev AD, Shabanov PD. Role of the dopaminergic system of the brain in the effects of glucocorticoid hormones. Neurosci Behav Physiol. 2003;33:231–236. doi: 10.1023/a:1022195113607. [DOI] [PubMed] [Google Scholar]
- 21.Lucas LR, Angulo JA, Le Moal M, McEwen BS, Piazza PV. Neurochemical characterization of individual vulnerability to addictive drugs in rats. Eur J Neurosci. 1998;10:3153–3163. doi: 10.1046/j.1460-9568.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 22.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 24.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- 26.Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- 27.Reul JM, Gesing A, Droste S, Stec IS, Weber A, Bachmann C, Bilang-Bleuel A, Holsboer F, Linthorst AC. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol. 2000;405:235–249. doi: 10.1016/s0014-2999(00)00677-4. [DOI] [PubMed] [Google Scholar]
- 28.Rome HP, Braceland FJ. The psychological response to ACTH, cortisone, hydrocortisone, and related steroid substances. Am J Psychiatry. 1952;108:641–651. doi: 10.1176/ajp.108.9.641. [DOI] [PubMed] [Google Scholar]
- 29.Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 30.Steketee JD, Goeders NE. Pretreatment with corticosterone attenuates the nucleus accumbens dopamine response but not the stimulant response to cocaine in rats. Behav Pharmacol. 2002;13:593–601. doi: 10.1097/00008877-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Sze PY. Glucocorticoids antagonize the sedative action of ethanol in mice. Pharmacol Biochem Behav. 1993;45:991–993. doi: 10.1016/0091-3057(93)90154-l. [DOI] [PubMed] [Google Scholar]
- 32.Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- 33.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 35.Zuckerman M. Sensation seeking and behavior disorders [letter] Arch Gen Psychiatry. 1988;45:502–504. doi: 10.1001/archpsyc.1988.01800290124017. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]


