Summary
Although thymosins have been demonstrated to have immunomodulatory effects, it is still not clear whether they could affect dendritic cells (DCs), the most professional antigen-presenting cells. The objective of this study was to determine the effect and potential mechanisms of thymosin-α1 (Tα1) on DC differentiation and functional maturation. Human peripheral blood CD14+ monocytes were purified by using a magnetic separation column and cultured with GM-CSF and IL-4 to differentiate into immature DCs (iDCs). In the presence of Tα1, iDC surface markers CD40, CD80, MHC class I and class II molecules were significantly upregulated as measured by flow cytemotry analysis. However, Tβ4 or Tβ10 did not show these effects on iDCs. There was approximately a 30% reduction in antigen (FITC-conjugated dextran)-uptake by Tα1-treated iDCs as compared with non-Tα1-treated iDCs. In addition, Tα1-treated matured DCs (mDCs) showed an increased stimulation of allogeneic CD3+ T-cell proliferation as measured by a mixed-lymphocyte reaction assay. Tα1-treated mDCs also increased the production of several Th1- and Th2-type cytokines as measured by a Bio-Plex cytokine assay. Furthermore, rapid activation of p38 MAPK and NFκB was seen in Tα1-treated iDCs as measured by a Bio-Plex phosphoprotein assay. Tα1 significantly enhances on DC differentiation, activation and functions from human peripheral blood CD14+ monocytes possiblly through a mechanism of the activation of p38 MAPK and NFκB pathways. This study provides a basis to further evaluate Tα1 as a possible adjuvant for a DC-directed vaccine or therapy.
Keywords: thymosins, dendritic cell differentiation, dendritic cell activation, immature dendritic cells, mature dendritic cells, Ag-uptake, allogeneic T cell proliferation, cytokines, signal transduction
1. Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that are able to stimulate the immune response better than other types of APCs such as macrophages or B cells [1]. DCs are widely distributed at various sites throughout the body in immature forms and varying subsets, and each possesses different functions, phenotypes, and morphologies [2]. Immature DCs (iDCs) found in the periphery blood are primed for the acquisition and processing of antigens (Ags) and becoming activated in the process. Once activated, DCs are transformed into fully matured DCs (mDCs) while undergoing changes to their surface marker phenotypes and being primed for potent immune stimulation. When activated by pathogenic determinants, such as LPS or immune cytokines such as TNF-α and IL-1β, DCs up-regulate co-stimulatory molecules (CD40, CD86, CD80, and CD83) coupled to the increase in the secretion of IL-12 (IL-12 p70) [3], mDCs migrate to regional lymph nodes where they interact with greater numbers of naïve T cells and thereby initiate the immune response. This process involves the priming of both naïve CD4+ helper T cells [4] and CD8+ cytotoxic T cells [5]. Thus, activated DCs have a pivotal role in the efficient priming of the cellular and humoral immune responses. Understanding the DC activation process in order to manipulate the immune response is thus attractive in the ongoing effort to design effective vaccines and therapies for treating patients with cancer and infectious diseases.
Thymosin α1 (Tα1) is a naturally occurring 28-amino acid peptide that is widely distributed in many tissues and cells [6]. Synthetic Tα1, along with Tβ4 and Tβ10, have been observed to have positive immunomodulatory properties; in particular, Tα1 has the ability to induce CD4+ T cell activation and the humoral response [7,8]. Serum levels are particularly high in newborns but drop gradually throughout childhood to adult levels between 540–670 pg/mL, thus suggesting a role in early immune development [9]. Tα1 was observed to stimulate lymphokine production of IFN-γ, IFN-α, IL-2, and macrophage migration inhibitory factor (MIF) [10]. Further, Tα1 has been shown to induce a Th1-type response and antibody production [11]. Tα1 may also have positive roles in cytotoxic T cell and natural killer cell responses [12]. Because of its potential for positive immunomodulation, Tα1 is being tested in a number of diseases including HBV [13–16], HIV [17], cancer (head and neck squamous cancer) [18,19], or immunosuppressed patients [7,20–22] either in stand-alone therapy or in conjunction with IFN-α [23]. Although the observations of the Tα1 potential immune therapeutic effects are clearly evident, what is not clear is the mechanism of action on the immune system and through which cell type these disparate observations are exerted. There are reports of the involvement of mitogen-activated protein kinases (MAPKs) in the signal transduction pathway of bone marrow-derived macrophage activation in response to in vitro treatment with Tα1 [24] and enhancement of pathogen-activated mouse mDCs through the TLR/Myd88 signaling pathway via p38 MAPK [11]. Since in both previous studies MAPKs are implicated in Tα1 positive effects on immune cells, we thus hypothesized that Tα1 may exert a direct immunological effect by modulating the dendritic cells, the key facilitator of the immune system.
To investigate how thymosins might modulate the immune system via DCs, we studied the effects of Tα1, Tβ4, and Tβ10 on human CD14+ monocyte-derived DC differentiation, activation, functional maturation, and signaling pathway involvement. We determined and compared the effects of thymosins in the presence or absence of Tα1, Tβ4, or Tβ10 on iDC and mDC surface marker expression and endocytosis functions. Moreover, we characterized Tα1-treated and non-Tα1-treated mDC functions in the mixed lymphocyte reaction (MLR) as well as the cytokine production profile in different mDCs-activated T cells. Finally, possible signaling pathways including p38-MAPK, ERK1/2, JNK, and NF-κB phosphorylation stimulated by Tα1 were also investigated.
2. Materials and methods
2.1. Antibodies and reagents
FITC-labeled anti-CD14, CD40, CD83, CD86, HLA-A,B,C, HLA-DR,DP,DQ, CD205, CD206, and CD209 antibodies were obtained from BD PharMingen (San Diego, CA, USA). PE-labeled anti-CD8, CD4, CD11c, CD11b, CD80, and CD68 were obtained from BD PharMingen. FITC- and PE-labeled isotype control antibodies mIgG1 (κ) were obtained from BD PharMingen. FITC-labeled dextran (molecular mass 70 kDa) was obtained from Sigma-Aldrich (St. Louis, MO). rh-GM-CSF, rhIL-4, and rhTNF-α were obtained from R & D Systems Inc. (Minneapolis, MN, USA). Fetal calf serum was obtained from Mediatech Inc. (Herndon, VA, USA). [3H]-Thymidine was obtained from PerkinElmer Analytical and Life Sciences Inc. (Boston, MA, USA). Synthetic human Tα1, Tβ4, and Tβ10 peptides (>95% purity – HPLC) were obtained from Immundiagnostik AG (Bensheim, Germany).
2.2. DC generation
Fresh human buffy coat blood was obtained from the Gulf Coast Regional Blood Center (Houston, TX). Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation of the medium-diluted buffy coat over a layer of Lymphoprep (Axis-Shield Inc., Norway) at 1100 x g for 20 minutes. The phase containing white cells was removed and washed in RPMI-1640. For positive selection of CD14+ monocytes, PBMCs were mixed with anti-human CD14 antibody conjugated microbeads (BD Biosciences Inc.). CD14+ monocytes were separated from other cells by using a magnetic separation column over a magnetic field (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions and washed thoroughly to remove any unspecific binding to the beads. CD14+ cells were then eluted in PBS/2% FCS. Isolated cells were > 95% CD14+ as evaluated by FACS analyses. Cells were then incubated in the DC growth medium (RPMI 1640, 10% FCS, 2 mM L-glutamine, 10 mM HEPES, 50 μM β-ME, 1 mM sodium pyruvate, 100 I.U./mL Penicillin, 100 μg/mL Streptomycin, 500 U/mL of GM-CSF, and 500U/mL IL-4) and incubated for 5 days at 37°C to differentiate into iDCs, with a change of fresh medium on day 3. On day 5, cells were treated with 10 ng/mL of TNF-α in fresh medium for an additional 2 days to fully differentiate into activated and matured DCs.
2.3. Thymosins treatment
Where cells were treated with Tα1, the compound was included in the DC growth medium at 50 ng/mL and replaced with the fresh medium containing Tα1 every three days. Tβ4 and Tβ10 were also added to final concentrations of 50 ng/mL, which is at least 2-fold higher than normal adult serum levels. A dose dependent curve was generated previously (data not shown), and it was determined that optimal changes could be observed at this concentration.
2.4. Flow cytometry
Cells were harvested and washed with PBS/1% FCS. Subsequently, cells were stained with anti-human fluorescence-conjugated antibodies, which included the following DC markers: HLA-A,B,C; HLA-DR,DP,DQ; CD3, CD4, CD8, CD14, CD40, CD11c, CD11b, CD68, CD80, CD83, CD86, and CD209 in 100 μL of PBS/1% FCS for 45 minutes to 1 hour at 4°C. Isotype controls with mouse anti-human IgG1 FITC or PE-conjugated antibodies were included. Positive FITC and PE controls were also included in each analysis. Cells were washed in 3 mL of PBS/1% FBS and fixed with 2% paraformaldehyde for FACS analysis using the FACSCalibur (Becton Dickinson, San Jose, CA, USA) and CellQuest software.
2.5. Endocytosis assay
Approximately 5×104 to 1×105 DCs were incubated on ice for at least 30 minutes. DCs were then incubated with 200 μg/mL of FITC-conjugated dextran at 37°C for 30 minutes. Controls were conducted at 4°C to prevent endocytosis. Cells were then washed 3 times in cold PBS/1% FBS and finally fixed with 2% paraformaldehyde for FACS analysis.
2.6. MLR assay
CD3+ T cells were positively selected from fresh PBMCs using anti-human CD3-conjugated microbeads (BD Biosciences PharMingen Inc.). The purity of CD3+ T cells was more than 98% as determined by FACS. Prior to bead selection, cells were incubated overnight in 10% FCS/RPMI 1640 and removal of adherent cells. Purified T cells (responder cells) were added to each well of a U-bottom microtiter plate (1 × 105 cells/well). Stimulator cells (Tα1-treated or non-Tα1-treated DCs) were irradiated with a 6000 rad dose of gamma rays to render them unable to proliferate. The total incubation volume was 200 μl of growth medium in a U-bottom 96-well plate. The ratios of stimulator cells (DCs) to responder cells (T cell) were 1:40 and 1:200. Cells were cultured for 5 days at 37°C and added 0.5 μCi [3H]-thymidine per well during the last 18 hours. Cells were collected with a cell harvester and counted in a PerkinElmer TopCount NXT (Shelton, CT, USA). The incorporation of [3H]-thymidine into the proliferating T cells was used as the end-point of the assay, and data are expressed in counts per minute (cpm).
2.7. BioPlex cytokine assay
Supernatants were collected after 24 h of incubation of DCs and T cells (CD4+ or CD8+) in MLR assays. Cytokine concentrations of IL-2, IL-4, IL-5, IL-10, IL-12(p70), IL-13, GM-CSF, IFN-γ, and TNF-α were determined by using the Bioplex multiplex Human Cytokine Th1/Th2 Assay kit and the Cytokine Reagent kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. Briefly, 50 μL of culture supernatants or cytokine standards were plated in a 96 well filter plate coated with a multiplex of antibodies against the above mentioned cytokines and incubated overnight on a platform shaker at 300 rpm at room temperature. After a series of washes to remove unbound proteins, a mixture of biotinylated detection antibodies, each specific for a different epitope, was added to the reaction resulting in the formation of a complex of target-bound antibodies. Streptavidin-phycoerythrin (streptavidin-PE) was then added to bind to the biotinylated detection antibodies on the beads surface. Data from the reaction was then acquired and analyzed by using the Bio-Plex suspension array system (Luminex 100 system) from Bio-Rad Laboratories.
2.8. BioPlex phosphoprotein assay
Monocytes at concentrations of 1.5 × 105/mL were treated with Tα1 (50 ng/mL) for 10 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, and 24 hours. Protein lysates were prepared by using the Cell Lysis Kit (Bio-Rad, cat# 171-304011) on samples collected at each indicated time point. The presence of phosphorylated p38 (p-p38), p-IκBα, p-ERK1/2, and p-JNK was detected by using the BioPlex 4-Plex Phosphoprotein Assay Kit (Bio-Rad). The total protein levels for IκBα, ERK1/2, p38, and JNK were also determined by using the BioPlex 4-Plex Total Target Assay Kit (Bio-Rad) according to the manufacturer’s protocol. Briefly, 50 μL of cell lysate (adjusted to a final concentration of 100–450 μg/mL of protein) was plated in a 96 well filter plate coated with anti-phosphokinase antibody and incubated overnight on a platform shaker at 300 rpm at room temperature. Subsequent procedures were similar to those as described above for the cytokine assay. The data are presented as the ratio of phosphorylated protein and total protein for each molecule.
2.9. Statistical analysis
Experiments were conducted in triplicates and over three separate occasions. Student t-tests were calculated for the generated data to establish the degree of significance and expressed as p < 0.05 for greater than 95% confidence unless otherwise stated. Data were also calculated for standard deviation from the mean, either expressed as SD values or as equivalent error bars.
3. Results
3.1. Tα1 upregulates important cell surface markers on iDCs
We determined the ability of Tα1 to influence the expression level of cell-surface markers on iDCs by the addition of thymosin compounds into the CD14+ monocyte-differentiating medium. The optimal concentration of Tα1 at 50 ng/mL was determined by an initial dose-dependent analysis (data not shown). Changes in the expression level of a panel of surface molecules on Tα1-treated iDCs were monitored by FACS analysis and compared against day 0 CD14+ monocytes and day 5 untreated iDCs. We used mean fluorescence intensity (MFI) as an index to show the elevated expression level of the molecule. As shown in Fig. 1A, the CD40 expression level in Tα1-treated iDCs was higher (increased 11% in MFI) than that in untreated iDCs (p<0.01). MHC class I and class II molecules were also upregulated by 34% and 17% in MFI, respectively (p<0.01). Lastly, CD80 expression levels were increased by 28% in MFI (p<0.01). Therefore, Tα1 treatment could modulate the differentiation process of CD14+ monocytes to iDCs by moderately increasing MHC class I, MHC class II, CD40 and CD80 expression.
Figure 1.
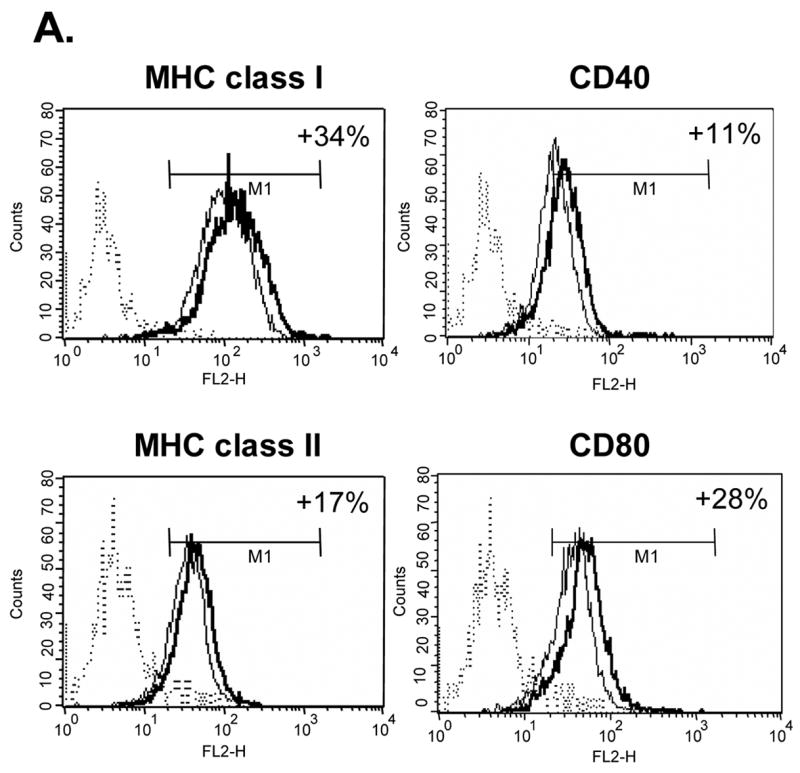
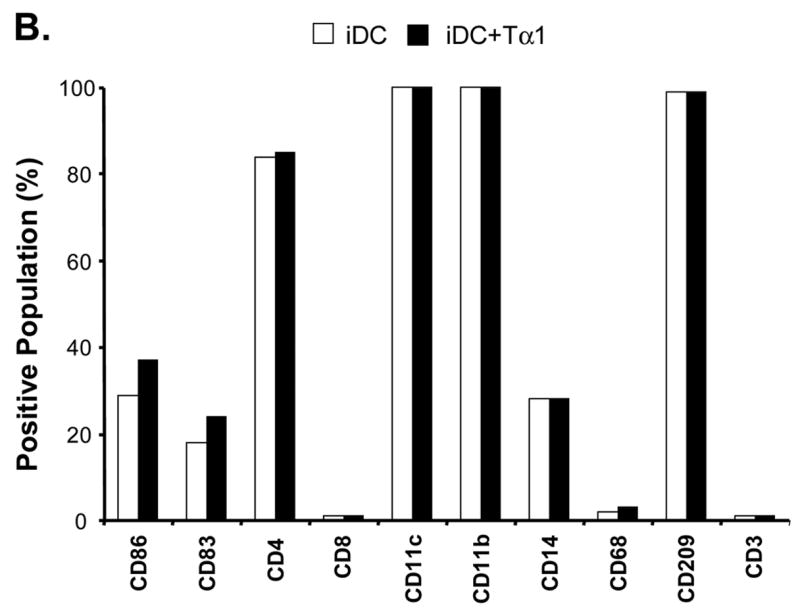
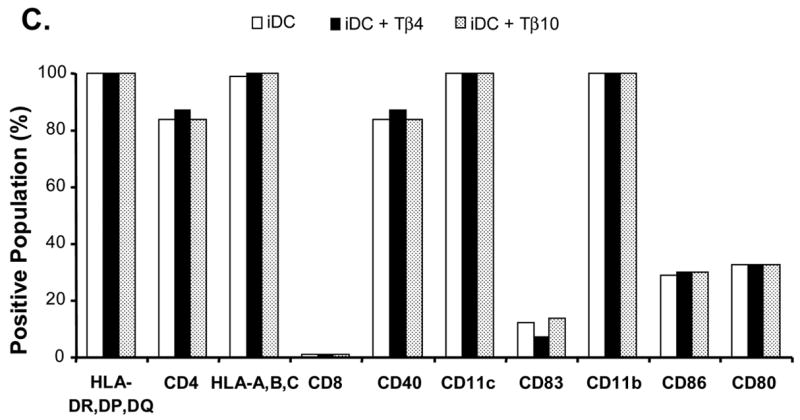
Effects of Tα1 on iDCs cell surface marker expression. CD14+ monocytes were induced to differentiate to iDCs in DC growth medium containing 500 U/mL rhGM-CSF and 500 U/mL rhIL-4 in the presence or absence of Tα1 (50 ng/mL), Tβ4 or Tβ10 (50 ng/mL) over the course of 5 days. Cell surface marker expression was stained for FACS analysis at day 5. iDC represents immature DC. IDC + Tα1 represents differentiation into immature DCs in the presence of Tα1 treatment. Data were statistically analyzed with student t-test. Data represented in this figure are representative of one from more than three experiments with DC preparations from different donors. (A) FACS histograms representing in percentages of changes in mean fluorescence intensity (MFI), relative to untreated controls, the elevated expression of cell surface markers MHC class I, MHC class II, CD40 and CD80 expression in the presence of Tα1 during DC differentiation. Dotted lines represent isotype controls. Solid lines represent untreated-controls. Bold lines represent Tα1-treated iDCs. Percentage values as denoted represent positive change of mean fluorescence intensity (MFI) after Tα1 treatment. Statistical t-test values for each marker are p < 0.01. (B) iDC surface markers that are not affected by Tα1 treatment. Y-axis represents percentage of marker staining positive cells. X-axis shows different markers determined. Data were statistically analyzed with student t-test values p > 0.05. (C) Beta- thymosin treatment does not affect iDCs surface markers expression. iDC + Tβ4 represents differentiation into immature DCs in the presence of Tβ4 treatment. iDC + Tβ10 represents differentiation into immature DCs in the presence of Tβ10 treatment. Y-axis represents percentage of marker staining positive cells. X-axis shows different markers determined. Data were statistically analyzed with student t-test values p > 0.05.
A number of other cell surface markers that are mostly associated with DC phenotypes were also tested but did not exhibit any statistically significant changes from those of controls (Fig. 1B). Results from the study showed that all Tα1-treated or non- Tα1-treated groups have almost 100% positive staining of cells that express CD11c, CD11b, and CD209. As consistent with normal monocyte differentiation into DCs, CD14 levels were down-regulated from high monocyte levels (at 97%) to approximately 30% in both Tα1-treated or non- Tα1-treated groups. Cells were negative for CD8 as well as CD3 (T cell marker) and CD68 (macrophage marker). Both CD205 and CD206 markers, which are known to be involved in receptor-mediated endocytosis, had no changes in the presence or absence of Tα1 treatment (data not shown). Therefore, these results showed the differentiation of CD14+ monocytes to DCs by day 5 with only surface markers CD40, CD80, MHC class I and MHC class II up-regulated by Tα1 treatment.
To determine whether the treatment of Tα1 could have any toxic effect on DCs, we compared the cell morphology, cell adhesion properties, and cell count at day 5 with or without Tα1 treatment. We found there was no significant difference of general cell properties between treated and non-treated groups (data not shown) during iDC differentiation.
3.2. Beta-thymosin treatment does not affect iDCs cell surface markers expression
The beta-thymosins have been suggested to have immunomodulatory properties. To explore the effect of these beta-thymosins on the phenotypic changes to CD14+ monocyte-differentiated iDCs, we carried out similar analyses of CD14+ monocyte-differentiated iDCs as had been carried out in Tα1 treatment, except with utilization of Tβ4 and Tβ10. The treatment concentration adopted for Tβ4 and Tβ10 represents a two-fold increase in normal physiological levels and is comparable to Tα1. As shown in Fig. 1C, there were no statistically significant differences between expression levels of a panel of surface markers from samples treated with 50 ng/mL of either Tβ4 or Tβ10 in comparison with the non-Tβ-treated control group. Furthermore, expression levels of CD40, CD80, MHC class I and MHC class II were not increased when treated with beta-thymosins. Expression levels of CD14, CD209, CD68, and CD3 were consistent with previous treatments (data not shown). Also, the beta-thymosins did not inhibit the expression of any studied surface markers in iDCs. Thus, the results from these experiments demonstrate that Tβ4 and Tβ10 may not have significant effects on CD14+ monocyte differentiation into DCs even at two-time normal physiological concentration of Tβ. This result could also serve as a random amino acid peptide control for Tα1 experiment described above. Therefore, it further indicates the specific effect of Tα1 on DCs.
3.3. Tα1 treatment reduces iDC endocytosis function
To investigate whether there is any functional change in Tα1-treated iDCs, we analyzed and compared the endocytosis ability of iDCs and Tα1-treated iDCs. The results indicated that Tα1-treated iDCs had a reduced ability to endocytose dextran (Fig. 2A). Shown in Fig. 2A, there were similar low levels of background endocytosis activity by both untreated and Tα1-treated iDCs when conducted at 4°C. However, there was about a 31% reduction in the mean fluorescent intensity (MFI) signals in Tα1-treated iDCs (400 MFI) as compared to untreated iDCs (583 MFI) at 37°C (Fig. 2B, p<0.05). Upon activation by TNF-α, both Tα1-treated and untreated mDCs were similarly inefficient in endocytosing dextran-FITC particles as shown by the reduced MFI values at approximately 300 MFI (Fig. 2B). Therefore, these data demonstrated that the addition of Tα1 in the iDC differentiation process resulted in iDCs with lowered endocytosis capacity similar to those of TNF-α activated mDCs.
Figure 2.
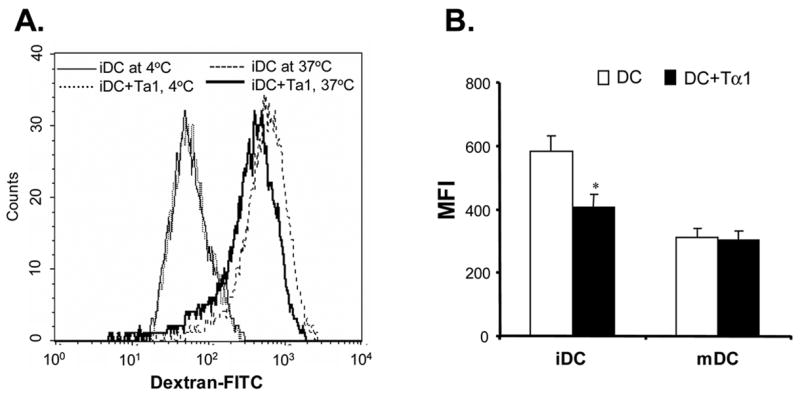
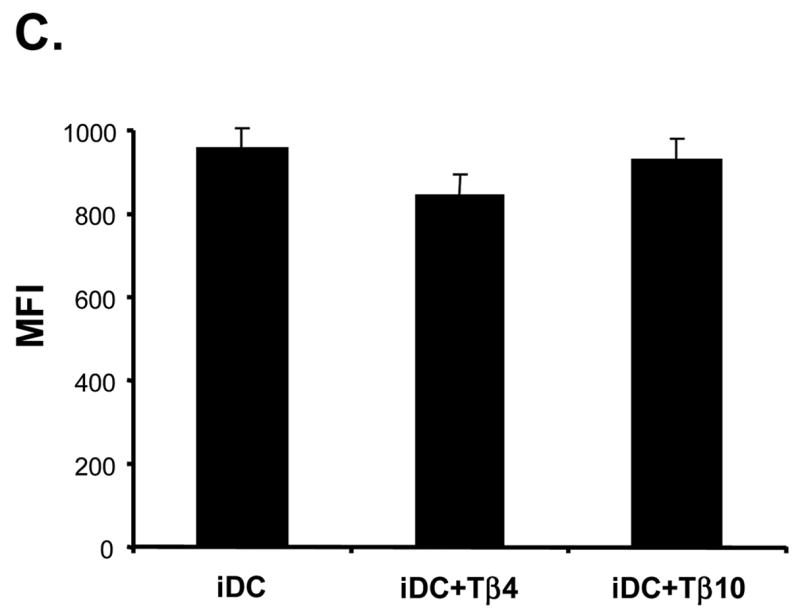
Effect of Tα1 treatment on iDC endocytosis function. (A) The function of dextran-FITC endocytosis by iDCs or Tα1-treated iDCs was analyzed by FACS. iDCs (1×105) were incubated at 37°C for 30 minutes in the presence of 200 μg/mL Dextran-FITC beads. Controls were respective iDCs incubated at 4°C. (B) Comparison of mean fluorescence intensity changes between iDCs and Tα1-treated iDCs or mDCs and Tα1-treated mDCs in Dextran-FITC endocytosis assay. mDCs were generated from iDCs by the addition of 10 ng/mL of TNF-α for 48 h. Error bars represent standard deviation of three separate experiments from one donor. * represents a student t-test value of p < 0.05. Data represented in this figure are representative of one from more than three experiments with DC preparations from different donors. (C) Beta-thymosin treatment does not alter iDC endocytosis function. No changes were apparent in the mean fluorescent intensity (MFI) between iDC and Tβ-treated iDC samples and their controls in the endocytosis assay. Error bars represent standard deviation of three separate experiments from one donor. Data were statistically analyzed with student t-test values p > 0.05. Data represented in this figure are representative of one from more than three experiments with DC preparations from different donors.
3.4. Beta-thymosin treatment does not alter iDC endocytosis function
We carried out similar functional endocytosis assays on iDCs treated with Tβ4 or Tβ10. Again, we intended to test whether the beta-thymosins might affect the ability of iDCs to endocytose dextran even though there were no phenotypic differences between beta-thymosin treated or untreated-iDCs. As shown in Fig. 2C, there were no endocytic functional changes brought about by either the treatment with Tβ4 or Tβ10. Similar to FACS analyses of surface markers expression, there were no significant changes between MFI levels in functional endocytosis of untreated iDCs and those treated with either Tβ4 or Tβ10. The fact that there is functional activity for these cells in the presence of the beta-thymosins suggests that the concentrations used in these studies were non-toxic to the cells. Further, there were no discernable differences in cell morphology when observed under light-microscope.
3.5. Tα1-treated mDCs have increased expression of key surface markers
In order to determine whether Tα1 by itself, in the absence of TNF-α, could induce DC maturation, DC cell surface markers were analyzed and found to be no difference in Tα1-treated verses non-Tα1-treated iDC differentiation. We then intended to determine whether Tα1 could help modulating the DC maturation effect by TNF-α treatment. After the 48-hour activation period induced by TNF-α in the presence or absence of Tα1, we found that the expression levels of four key markers were upregulated, in line with maturation phenotypes, but levels were further upregulated in cases where they were treated with Tα1 (Fig. 3). CD40 was upregulated by 26% (MFIs: iDC; 27, mDC; 46, mDC + Tα1; 58). CD80 was upregulated by 42% (MFIs: iDC; 39, mDC; 173, mDC + Tα1; 246). MHC class I was upregulated by 38% (MFIs: iDC; 94, mDC; 153, mDC + Tα1; 211). MHC class II was also upregulated by 38% (MFIs: iDC; 35, mDC; 111, mDC + Tα1; 153). A statistical t-test value for each marker mentioned above is p < 0.01. The expression levels of other markers including CD83, CD86 on mDCs treated with Tα1 were unchanged from those of untreated mDC controls (data not shown). But, the four markers that were upregulated coincided with those in iDCs. The results thus suggest that the addition of Tα1 may have an additive effect on TNF-α-induced DC maturation by upregulating CD40, CD80, MHC class I and MHC class II molecules on mDCs.
Figure 3.
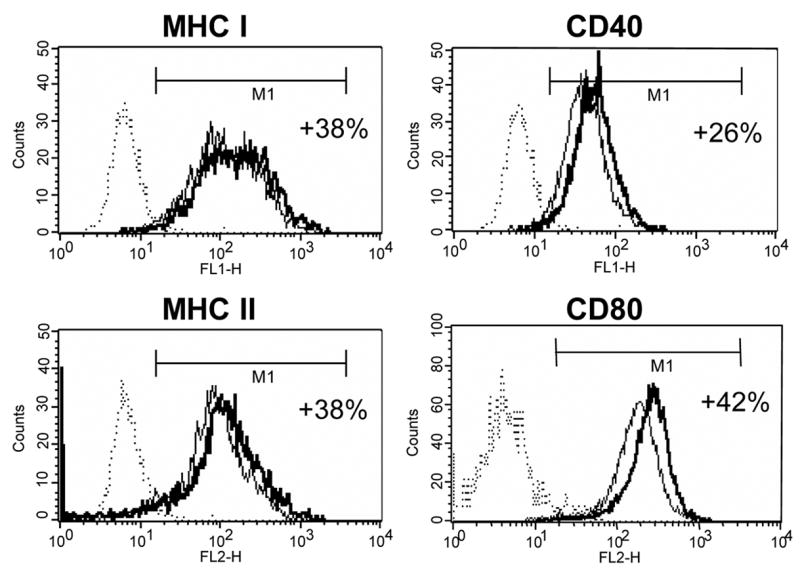
Effect of Tα1 on the expression of important cell surface markers on mDCs. iDCs on day 5 were induced to activate and mature in DC growth media containing 500 U/mL rhGM-CSF, 500 U/mL rhIL-4 and 10ng/mL of TNF-α in the presence or absence of Tα1 (50 ng/mL) over the course of 48 h. Cell surface marker expression was stained for FACS analysis at day 7. FACS histograms representing in percentages, relative to untreated controls, the elevated expression of cell surface markers MHC class I, MHC class II, CD40 and CD80 expression in the presence of Tα1 during DC maturation. Dotted lines represent isotype controls. Solid lines represent untreated-controls. Bold lines represent Tα1-treated iDCs. Statistical t-test values for each marker is p < 0.01. Data represented in this figure are representative of one from more than three experiments with DC preparations from different donors.
3.6. Tα1-treated mDCs enhance allogeneic T cell proliferation
We further tested the effects of Tα1 on the functional ability of mDCs to induce allogeneic T cells to proliferate in MLR assays. We conducted the assay by incubating mDCs with allogeneic CD3+ T cells at two DC to T cell ratios, 1:200 and 1:40, respectively. Results showed a clear and statistically significant increase in the level of [3H]-thymidine incorporation by T cells stimulated with Tα1-treated mDCs (Fig. 4). Increasing the ratio of mDCs in the incubation assay also increased the level of [3H]-thymidine incorporation for both controls and Tα1-treated mDCs. At 1:200 incubation ratio, Tα1-treated mDCs induced an increase in the cpm count by a factor of 2 from those of untreated controls (p<0.05). A similar factor difference could also be observed at 1:40 (p<0.05). MLR assays showed an increased ability of Tα1-treated mDCs to stimulate allogeneic T cell proliferation, indicating enhanced functional maturation of Tα1-treated mDCs. Furthermore, the upregulated surface marker expression in Tα1-treated mDCs may correlate with the enhanced MLR activity of Tα1-treated mDCs.
Figure 4.
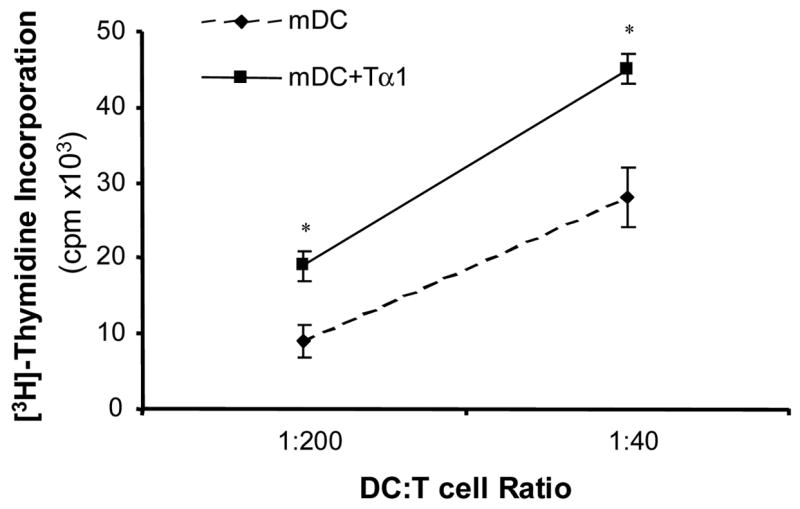
Effect of Tα1-treated mDCs on allogeneic T-cell proliferation. The allostimulatory activity of mDCs and Tα1-treated mDCs to T cells was measured by MLR assay at two different ratios of mDCs to CD3+ T cells. mDCs were irradiated, and allogeneic CD3+ T cells were positively selected using MACS microbeads. mDCs and T cells were mixed and incubated for 5 days at 37°C followed by a 16-hour incubation with 0.5 μCi of [3H]-thymidine. Radioactive thymidine incorporation into proliferating T cells were measured and determined in counts per minute. Error bars are standard deviation. * represents a student statistical t-test value of p < 0.05. Similar results were obtained in three separate experiments.
3.7. Tα1-treated mDCs enhance T cell cytokine release
To determine the nature of the immune responses induced by Tα1-treated mDCs, various cytokines released by mDC-stimulated allogeneic T cells were determined by using a multi-plex cytokine assay. We were able to show that Tα1-treated mDCs stimulated T cells to release a broad profile of cytokines, including Th1 (IFN-γ) and TNF-α (Fig. 5A), and Th2-type (IL-5, IL-10 and IL-13) cytokines (Fig. 5B). Both Th1 and Th2-type cytokine levels induced by Tα1-treated mDCs were consistently higher than those of untreated mDCs. Among them, TNF-α, IL-5, and IL-13 levels were almost doubled. Levels of IFN-γ and IL-10 were increased at least by 30% from Tα1-treated mDC cultures. Thus, results showed unambiguously that Tα1-treated mDCs were able to induce allogeneic T cells to release a broad array of cytokines consisting of both Th1 and Th2 associated types of response.
Figure 5.
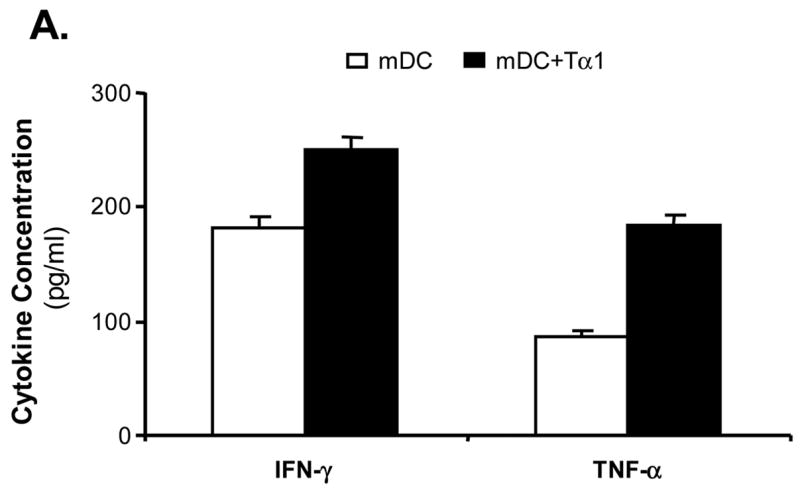
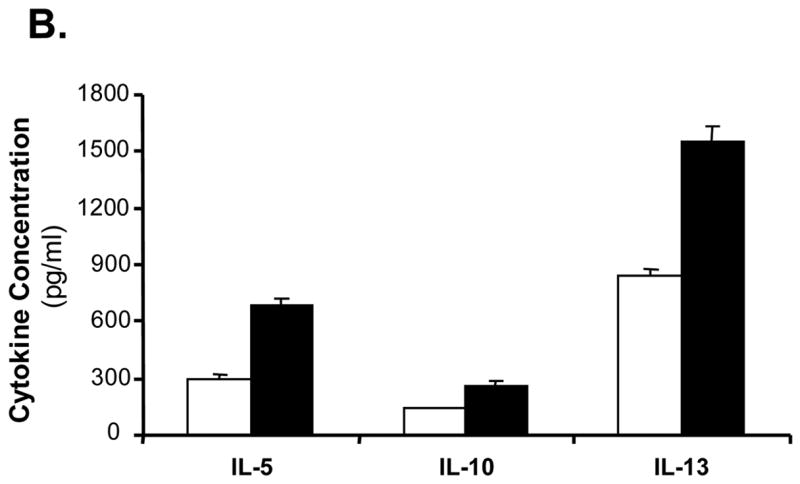
Effect of Tα1-treated mDCs on broad T-cell cytokines release. Supernatant of the incubation media from the MLR assay was collected 24-hour post incubation. Cytokines released from allogeneic T cells having been activated by mDCs or Tα1-treated mDCs were analyzed by the Bioplex multiplex Th1/Th2 cytokine assay. (A) Th1-type cytokines. (B) Th2-type cytokines. Supernatants were collected from three different MLR experiments. The cytokine levels shown are the average cytokine concentration from three different experiments. Error bars are standard deviation.
3.8. Tα1 induced activation of p38-MAPK and NFκB signaling pathways
We further investigated the possible signaling pathways through which Tα1 exerts its positive immunomodulatory activity on DC differentiation. Since there have been previous indications that Tα1 might act through either p38 [11] or JNK [24] kinases, the effects of Tα1 on the levels of p38, ERK, JNK, and NFκB phosphorylation in DC-differentiating monocytes were determined by using a multi-plex phosphoprotein assay. Monocytes were treated with or without Tα1, and cell lysates were collected at 0, 10, 30 and 60 minutes. Results demonstrated a clear and significant 3-fold increase in the level of p38 phosphorylation after 10 minutes of incubation (Fig. 6A). Similar results were obtained for IκBα, where the level of phosphorylated kinase was increased approximately 2-fold after 10 minutes (Fig. 6B). The level of phosphorylated p-38 and IκBα fell back to that of background levels beyond 30 minutes of treatment. However, the rising of p38 and IκBα phosphoproteins were not observed in the non- Tα1 treatment group, confirming the activation effect of Tα1 on p38 and NF-κB pathways. We did not observe evidence of significant effects on ERK1/2 or JNK phosphorylation by Tα1 treatment (data not shown). Thus, our results demonstrated that Tα1 induced the phosphorylation of at least two different kinases, p38 and IκBα, indicating the possible involvement of MAPK and NF-κB activation pathways in the Tα1 effect on DC differentiation.
Figure 6.
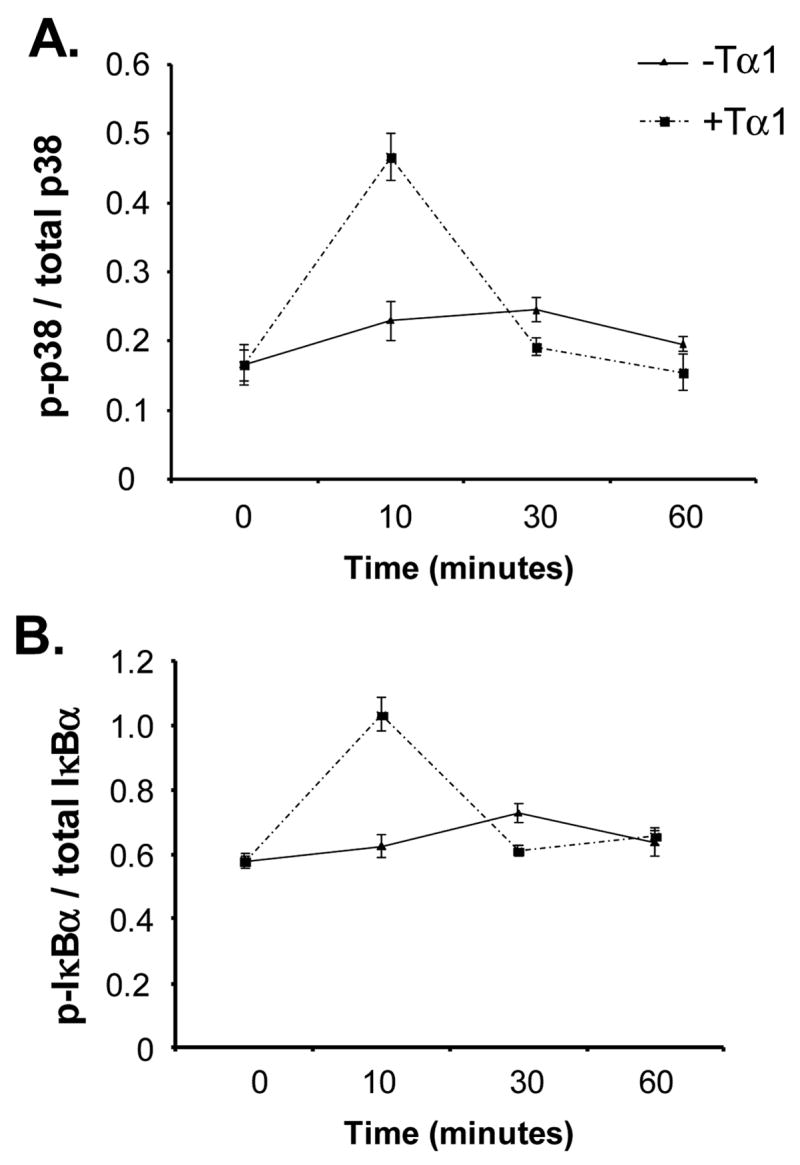
Effect of Tα1 on the activation of p38-MAPK and NFκB signaling pathways. Tα1-treated CD14+ monocytes were collected at the specified time points and lysed to release their protein contents. The levels of phosphorylation and totoal protein of p38-MAPK and IκBα were measured by the BioPlex immunoassay. The data are presented as the ratio of phosphorylated protein and total protein for each molecule. (A) Representation of the ratio of phosphorylated p38 kinase and total p38. (B) Representation of the ratio of phosphorylated IκBα kinase and total IκBα. Results are representative of triplicate assay points from 3 donors.
4. Discussion
This work has demonstrated the direct effects of Tα1 on human CD14+ monocyte to DC differentiation and activation by showing an increase in expression levels of several key DC surface markers on differentiated iDCs and activated mDCs, a reduction in the effective endocytosis function of these same iDCs, an enhancement in the activation of mDCs, and an elevated stimulation of allogeneic T cell proliferation. Furthermore, Tα1 induces the proliferating T cells to release a broad spectrum of Th1 and Th2-types of cytokines. Mechanistically, Tα1 may exert its action through the p38 MAPK and NFκB pathways, which were activated independently of externally introduced cytokines. These results suggest that Tα1 can exert its positive modulatory activity on the immune response by promoting a maturation of DC phenotype and function. This study is the first to show that human DCs could be modulated in this manner by Tα1 and thus articulated previous indications based on macrophage-derived dendritic cells [25] and Tα1 used in conjunction with pathogenic Aspergillus conidia in mice [11].
The effect of Tα1 on the surface markers of iDCs was to increase the expression levels of CD40, CD80, MHC class I and MHC class II. CD40 and CD80 are important upregulated surface markers in mDC, whereas MHC class I and class II are associated with antigen-presentation. CD40 is known to be upregulated once iDCs are activated and differentiated into mDCs. This process can be induced by the TLRs sensing of pathogens and the triggering of their signaling cascade, a process involving the activation of the NFκB pathway. The binding of CD40 to CD40L on activated T cells can also lead to further up-regulation of CD40 molecules on mDCs in an amplification of the signal mechanism. The binding and activation by TNF receptor of TNF-α and its signaling pathway will also trigger the up-regulation of CD40 molecules via a NF-κB-dependent mechanism. Thus, there can be numerous ways in which CD40 is up-regulated; however, CD40 plays an integral role in activated mDCs that function to facilitate an immune response. Therefore, the fact that CD40 is up-regulated during the differentiation of iDCs suggests that Tα1 can facilitate the condition for generating DCs with a matured phenotype. Concomitantly, similar arguments can be made for CD80, which is an essential co-stimulatory molecule for activated DCs. The increased expression of MHC class I and MHC class II molecules on iDCs further suggest that Tα1 enhanced the capacity for antigen-presentation. We did not observe any changes on surface phenotypes of iDCs treated by Tβ4 and Tβ10, which were indicated to have possible immunomodulatory roles [26,27]. We recognized the nature of DCs to function through TLRs as well as C-type lectin receptor (CLRs) in pattern recognition of foreign bodies. For an inclusion of a proper control in our test, it would be best to use similar class of molecules rather than scrambled peptides which may also act as stimulators. The fact that all thymosins are naturally synthesized by these cells gave us an opportunity to use three thymosins for our comparative studies. The fact that Tβ4 and Tβ10 did not have an effect at identical conditions strongly favors the argument that effect of Tα1 on DCs indeed is specific.
Our results are consistent with those of Romani et al. who showed similar up-regulation in CD40, and CD80 by Tα1 in mouse lung DCs, but which is only prominent in the presence of Aspergillus conidia [11]. Further, we showed increases in expression of the same four molecules in mDCs, confirming the potential of Tα1 to enhance firstly the co-stimulation and activation of immune cells and secondly antigen-presentation via MHC class I and MHC class II molecules. Thus, these results showed, for the first time, the effect of Tα1 on the independent and significant up-regulation of essential DC surface molecules on human CD14+ cells-derived iDCs.
Endocytosis is a critical function for circulating and peripheral iDCs to acquire and process antigens for presentation to the adaptive cells of the immune system. Endocytosis also starts the activation and maturation process for DCs. A distinguished characteristic of mDCs is that they lose their ability to acquire foreign bodies but possess highly developed abilities to present and activate the adaptive immune response. Our results demonstrated that Tα1 reduces the endocytosis function of iDCs. As yet, we do not know the precise mechanism behind the reduced endocytosis function but that it is independent of a CLR-mediated mechanism given that CD205, CD206, and CD209 remained unaffected. The fact that mature surface markers were observed to be more abundantly expressed in Tα1-treated iDCs, and since these iDCs resembled the mature surface phenotype, it is therefore logical to suggest that they would have a reduced capacity for iDC function, such as endocytosis. In contrast to Tα1-treated iDCs, Tβ4 and Tβ10-treated iDCs endocytosed dextran at similar levels to untreated controls, suggesting that their endocytosis functions are unchanged. This is consistent with the findings from surface markers expression of iDCs when treated with Tβ4 and Tβ10, which were found to be largely unaffected as determined by FACS. Tα1 does not seem to affect the TNF-α-induced mDC down-regulation of endocytosis function, suggesting the efficacy of the cytokine to induce DC maturation. However, Tα1-treated mDCs, in general, endocytose dextran more poorly than TNF-α treated DCs, although statistically we can not conclude that this is a significant reduction. The mDCs studied here do show a significantly reduced ability to endocytose foreign bodies. Romani et al. [11] have shown an increased phagocytosis ability in mouse iDCs and mDCs when treated with Tα1, indicating that the Aspergillus conidia and Tα1 combination can induce maturation of DCs. Our endocytosis results are explainable in association with the up-regulation of surface marker expression on iDCs since activated DCs show down-regulated endocytosis activity, which was detected in Tα1-treated iDCs.
We have also shown that Tα1-treated mDCs significantly increased the stimulation of allogeneic T cell proliferation by approximately two-fold. We have shown that the expression levels of four listed molecules on Tα1-treated DCs were all upregulated to nearly 50% as compared with the untreated DCs. This level of costimulatory molecules upregulation may count for the reasons of two-fold increase in alloantigen response. The increased allostimulatory activity of Tα1-treated mDCs was further supported by the broad release of Th1 and Th2-type cytokines by activated T cells, where a significant increase in IFN-γ (Th1) and TNF-α as well as IL5, IL10, and IL13 (Th2) were observed as compared to untreated controls. The elevated Th2 types of cytokine phenomenon may be due to the effect of IL-4 in the iDC differentiation process or it could be different responses between mouse and human cells as suggested by Romani et al. It was thought that unlike murine DCs producing IL-12 in response to stimuli, human DCs produced more IL-10 in response to stimuli [11]. Thus, here we showed unambiguously for the first time the allostimulatory activity of human-derived Tα1-treated mDCs to cause allogeneic T cell proliferation and induce the release of a broad array of both Th1 and Th2-type cytokines in comparison to normal mDCs. These results are supportive and consistent with previous sections, which showed Tα1-treated DCs expressed a mature surface phenotype as well as reduced endocytosis function when examined at the same stage of differentiation. It is reasonable and rather straightforward to suggest that iDCs that are primed for maturation by Tα1 treatment when further induced by TNF-α will subsequently lead to better allostimulatory activity than iDCs differentiated only in the presence of conventional GM-CSF and IL4 cytokines. Our results substantially expand previous observations in a mouse model that Tα1 could induce a Th1-type cytokine response [11].
In addressing the mechanistic role for Tα1 to exert its effect on DC differentiation, we found that Tα1 was able to activate p38 MAPK and IκBα rapidly over the first 10 minutes of a 60-minute time-course in human CD14+ monocytes. We also found that JNK and ERK1/2 were unaffected by Tα1 treatment. Both the NFκB pathway and the p38 MAPK pathway are important but distinct pathways in cellular activation processes, initiated by the ligation of a host of different receptors such as the TLRs, TNF family of receptors, TCRs, TGFRs, and other growth-factor receptors under a variety of pathogenic stresses, environmental stresses, and inflammatory cytokines. Tα1 is known to activate the MAPK-transduction pathways [24] and activates the MyD88-dependent pathway of TLR signaling in vitro and in vivo [11]. A recent report also showed that Tα1 could activate TRAF6 signal pathway by activating IKK [28]. Thus our results are consistent with previous observations and suggest that Tα1 by itself may activate DCs via a receptor-mediated signaling mechanism. Our results specifically indicate this effect through the rapid activation of both p38 MAPK and NFκB through the phosphorylation of IκBα. The fact that TLR/MyD88-dependent pathways and NF-κB pathways are known to lead to production of Th1-type cytokines, as found by Romani et al.,[11] whereas we further observed the release of Th2-type cytokines and p38 activation suggests that TLR/MyD88/NFκB-dependent pathway is not the only possible mechanism that can be facilitated by Tα1. Further, the study with p38 or NFκB inhibitor is warranted to determine the specific role the p38 or NF-kB play in DC differentiation in response to Tα1. A recent study has shown that Tα1 primes DCs through activation of immunosuppressive pathway of tryptophan catabolism via indoleamine 2,3-dioxygenase (IDO). Thus, Tα1 therapy initiates the balance between inflammation and tolerance [29].
In conclusion, this study has shown that Tα1 is a promising candidate as an adjuvant for immune therapy by positively altering the phenotype and presentation activity of mDCs as well as inducing a broad Th1 and Th2-type immune response. The study also highlights the fact that our understanding of the pathways involved that are targeted by the wide ranging effect of Tα1 are still limited. It is demonstrated in this study that activation through NF-κB and p38 MAPK-dependent pathways are involved. The findings from our study show for the first time the ability of Tα1 to influence the outcome of DC differentiation and functions and thus provide a basis to further evaluate Tα1 as a possible adjuvant for a DC-directed vaccine or therapy.
Table 1.
Effects of Tα1 on iDC and mDC surface molecules expression*
| IDC | iDC+Tα1 | mDC | mDC+Tα1 | |
|---|---|---|---|---|
| CD40 | 26.9±5.49 | 30.23±7.79 | 45.76±17.18 | 58.43±29.01 |
| CD80 | 39.24±16.86 | 50.48±25.47 | 173.09±51.23 | 245.82±47.01 |
| MHC I | 93.9±36.49 | 126.35±47.92 | 153.12±37.86 | 210.61±42.46 |
| MHC II | 35.23±17.71 | 41.05±27.53 | 110.74±55.18 | 152.92±64.24 |
Data are expressed as mean fluorescence intensity (MFI) for each molecule. Data are mean±standand deviation (SD) of triplicates from one experiment. Data are representative of three different donors DC differentiation and activation experiments.
Acknowledgments
This work is partially supported by research grants from the National Institutes of Health (Chen: HL65916, HL72716, EB-002436, and HL083471; and Yao: DE15543 and AT003094).
Abbreviations used in this paper
- iDC
immature dendritic cell
- mDC
mature dendritic cell
- Tα1
Thymosin-alpha 1
- Tβ4
Thymosin-beta 4
- Tβ10
Thymosin-beta 10
- MFI
mean fluorescent intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manca F, Li Pira G, Fenoglio D, Fang SP, Habeshaw A, Knight SC, et al. Dendritic cells are potent antigen-presenting cells for in vitro induction of primary human CD4+ T-cell lines specific for HIV gp120. J Acquir Immune Defic Syndr. 1994;7:15–23. [PubMed] [Google Scholar]
- 2.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Langhoff E, Steinman RM. Clonal expansion of human T lymphocytes initiated by dendritic cells. J Exp Med. 1989;169:315–20. doi: 10.1084/jem.169.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166:182–94. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach JF. Thymic hormones. J Immunopharmacol. 1979;1:277–310. doi: 10.3109/08923977909026377. [DOI] [PubMed] [Google Scholar]
- 7.Li CL, Zhang T, Saibara T, Nemoto Y, Ono M, Akisawa N, et al. Thymosin alpha1 accelerates restoration of T cell-mediated neutralizing antibody response in immunocompromised hosts. Int Immunopharmacol. 2002;2:39–46. doi: 10.1016/s1567-5769(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 8.Lau GK, Nanji A, Hou J, Fong DY, Au WS, Yuen ST, et al. Thymosin-alpha1 and famciclovir combination therapy activates T-cell response in patients with chronic hepatitis B virus infection in immune-tolerant phase. J Viral Hepat. 2002;9:280–87. doi: 10.1046/j.1365-2893.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- 9.Naylor PH, Friedman-Kien A, Hersh E, Erdos M, Goldstein AL. Thymosin alpha 1 and thymosin beta 4 in serum: comparison of normal, cord, homosexual and AIDS serum. Int J Immunopharmacol. 1986;8:667–76. doi: 10.1016/0192-0561(86)90001-9. [DOI] [PubMed] [Google Scholar]
- 10.Wolf GT, Hudson J, Peterson KA, Poore JA, McClatchey KD. Interleukin 2 receptor expression in patients with head and neck squamous carcinoma. Effects of thymosin alpha 1 in vitro. Arch Otolaryngol Head Neck Surg. 1989;115:1345–49. doi: 10.1001/archotol.1989.01860350079019. [DOI] [PubMed] [Google Scholar]
- 11.Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, et al. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232–39. doi: 10.1182/blood-2003-11-4036. [DOI] [PubMed] [Google Scholar]
- 12.Bistoni F, Baccarini M, Puccetti P, Marconi P, Garaci E. Enhancement of natural killer cell activity in mice by treatment with a thymic factor. Cancer Immunol Immunother. 1984;17:51–55. doi: 10.1007/BF00205497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung N. Treatment of chronic hepatitis B: case selection and duration of therapy. J Gastroenterol Hepatol. 2002;17:409–14. doi: 10.1046/j.1440-1746.2002.02767.x. [DOI] [PubMed] [Google Scholar]
- 14.Zavaglia C, Severini R, Tinelli C, Franzone JS, Airoldi A, Tempini S, et al. A randomized, controlled study of thymosin-alpha1 therapy in patients with anti-HBe, HBV-DNA-positive chronic hepatitis B. Dig Dis Sci. 2000;45:690–96. doi: 10.1023/a:1005431323945. [DOI] [PubMed] [Google Scholar]
- 15.Chien RN, Liaw YF, Chen TC, Yeh CT, Sheen IS. Efficacy of thymosin alpha1 in patients with chronic hepatitis B: a randomized, controlled trial. Hepatology. 1998;27:1383–87. doi: 10.1002/hep.510270527. [DOI] [PubMed] [Google Scholar]
- 16.Naylor PH, Mutchnick MG. Thymus-derived peptides in the treatment of viral chronic hepatitis. Dig Dis. 1996;14:362–70. doi: 10.1159/000171570. [DOI] [PubMed] [Google Scholar]
- 17.Chadwick D, Pido-Lopez J, Pires A, Imami N, Gotch F, Villacian JS, et al. A pilot study of the safety and efficacy of thymosin alpha 1 in augmenting immune reconstitution in HIV-infected patients with low CD4 counts taking highly active antiretroviral therapy. Clin Exp Immunol. 2003;134:477–81. doi: 10.1111/j.1365-2249.2003.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garaci E, Pica F, Rasi G, Palamara AT, Favalli C. Combination therapy with BRMs in cancer and infectious diseases. Mech Ageing Dev. 1997;96:103–16. doi: 10.1016/s0047-6374(97)01895-2. [DOI] [PubMed] [Google Scholar]
- 19.Garaci E, Pica F, Rasi G, Favalli C. Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application. Int J Immunopharmacol. 2000;22:1067–76. doi: 10.1016/s0192-0561(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 20.Christenson L, Aebischer P, Galletti PM. Encapsulated thymic epithelial cells as a potential treatment for immunodeficiencies. ASAIO Trans. 1988;34:681–86. [PubMed] [Google Scholar]
- 21.Ohmori H, Kamo M, Yamakoshi K, Nitta MH, Hikida M, Kanayama N. Restoration of immunocyte functions by thymosin alpha1 in cyclophosphamide-induced immunodeficient mice. Immunopharmacol Immunotoxicol. 2001;23:75–82. doi: 10.1081/iph-100102569. [DOI] [PubMed] [Google Scholar]
- 22.Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol. 2003;3:1061–71. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 23.Garaci E, Pica F, Sinibaldi-Vallebona P, Pierimarchi P, Mastino A, Matteucci C, et al. Thymosin alpha(1) in combination with cytokines and chemotherapy for the treatment of cancer. Int Immunopharmacol. 2003;3:1145–50. doi: 10.1016/S1567-5769(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 24.Sodhi A, Paul S. Involvement of mitogen-activated protein kinases in the signal transduction pathway of bone marrow-derived macrophage activation in response to in vitro treatment with thymosin alpha 1. Int Immunopharmacol. 2002;2:47–58. doi: 10.1016/s1567-5769(01)00139-4. [DOI] [PubMed] [Google Scholar]
- 25.Shrivastava P, Singh SM, Singh N. Effect of thymosin alpha 1 on the antitumor activity of tumor-associated macrophage-derived dendritic cells. J Biomed Sci. 2004;11:623–30. doi: 10.1007/BF02256128. [DOI] [PubMed] [Google Scholar]
- 26.Hannappel E, Huff T. The thymosins. Prothymosin alpha, parathymosin, and beta-thymosins: structure and function. Vitam Horm. 2003;66:257–96. doi: 10.1016/s0083-6729(03)01007-0. [DOI] [PubMed] [Google Scholar]
- 27.Low TL, Thurman GB, Chincarini C, McClure JE, Marshall GD, Hu SK, et al. Current status of thymosin research: evidence for the existence of a family of thymic factors that control T-cell maturation. Ann N Y Acad Sci. 1979;332:33–48. doi: 10.1111/j.1749-6632.1979.tb47095.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Chan J, Dragoi AM, Gong X, Ivanov S, Li ZW, et al. Activation of IKK by thymosin alpha1 requires the TRAF6 signalling pathway. EMBO Rep. 2005;6:531–37. doi: 10.1038/sj.embor.7400433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani L, Bistoni F, Perruccio K, Montagnoli C, Gaziano R, Bozza S, et al. Thymosin α1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–74. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]


