Abstract
We report here that human T lymphocytes have the capacity of acquiring large amounts of MHC class II molecules from various types of antigen-presenting cells (APC) in an antigen-independent manner. The transfer of MHC class II molecules from APC to T cell required direct cell-to-cell contact and appeared to involve the interaction of numerous adhesion molecules between these cells. Depletion of cholesterol from the plasma membrane reduced the amount of MHC class II transferred onto the T cells. Most significantly, the newly acquired MHC class II molecules were capable of efficiently presenting antigen to T helper cells. These results suggest that T cells are able to interact with other T cells to regulate immune responses by presenting MHC peptide complexes that have been snatched away from nearby APC.
Keywords: adhesion molecules, antigen presentation, MHC, T lymphocytes, trogocytosis
Introduction
T helper lymphocytes (HTL) recognize antigen in the form of peptide/MHC class II complexes, which are expressed on the surface of antigen-presenting cells (APC). In contrast to MHC class I molecules, which are expressed by the majority of cells in the organism, MHC class II molecules are only normally expressed on a subset of cells, most of them derived from the hematopoietic lineage, which are considered to function as APC (1,2). As a result of antigen presentation by APC, the HTL become activated and produce lymphokines that regulate most humoral and cellular immune responses. In addition, during antigen presentation the activated HTL are known to transmit signals to the APC, which can significantly modify the outcome of the immune response. When HTL recognize antigen on B lymphocytes, signals are generated resulting in B cell maturation and Ig class switching (3). In turn, when APC such as dendritic cells (DC) present antigen to HTL, signals are produced that result in the activation/maturation of the DC, enabling these APC to effectively present antigen to cytotoxic T lymphocytes (CTL) (4–6). In both of these cases CD40/CD40L interactions between APC and HTL are mainly responsible for the activation/maturation of the APC.
It is a well-known fact that human activated T lymphocytes express surface MHC class II molecules (7–9). These human activated T cells synthesize their own MHC class II molecules (10). This is because activation of human T cells results in the induction of expression of the PIII form of the class II transactivator (CIITA), which is the master regulator of the MHC class II antigen-presenting pathway (11). On the other hand, MHC class II molecules on activated mouse T cells are passively acquired from APC (12,13). In rats, activated T lymphocytes can both synthesize and passively acquire surface MHC class II molecules (14,15). Because passively acquired MHC molecules from APC to T lymphocytes creates the unique opportunity for T cells to present antigens to other T cells, we decided to examine whether this phenomenon can take place with human T lymphocytes and to study some of the mechanisms that would be involved in this process. Using MHC class II-deficient human T cells, we observed that significant amounts of MHC class II molecules could be transferred from APC onto the T cell surface. Such transfer required cell-to-cell contact, was independent of the presence of antigen and could be blocked by antibodies to adhesion molecules or by disrupting the membrane cholesterol rich regions. Most significantly, passively acquired MHC class II molecules were efficient in stimulating antigen-specific HTL. The results are discussed in the context of how antigen-specific T cell–T cell interactions may impact the regulation of immune responses.
Methods
Cells and cell cultures
Bare lymphocyte syndrome (BLS) CD8+ T cells, hereafter referred simply as BLS-CD8 T cells, were derived from a BLS patient who exhibited an alteration in the RFX5 gene resulting in deficient expression of MHC class II on APC and activated T cells (16). BLS-CD8 T cells were expanded in tissue culture using a modified protocol (17) originally developed by Riddell and Greenberg (18). Briefly, 3 × 105 BLS-CD8 T cells were incubated with 2.5 × 107 allogeneic irradiated (8000 rads) peripheral blood mononuclear cells (PBMC) from five normal donors, 5 × 106 irradiated (3300 rads) allogeneic Epstein–Barr virus (EBV) transformed B lymphoblastoid cells in the presence of 30 ng/ml monoclonal anti-CD3 antibody (OKT-3, Orthoclone; Ortho Biotech, Inc., Raritan, NJ) in a final volume of 20 ml RPMI-1640 medium containing 10% fetal calf serum (FCS). One day later and every 3 days thereafter, 50 IU/ml IL-2 was added to the expansion cultures. After 2 weeks, the BLS-CD8 T cells expanded approximately 200–500-fold and were ready to be used in the experiments. The Jurkat (clone Ju.1) T cells were provided by Dr D. McKean (Mayo Foundation, Rochester, MN). The MHC class II positive T cell leukemia line, TL-Hir was kindly provided by Dr H. Kobayashi (Department of Pathology, Asahikawa Medical College, Japan). Immortalized M35-EBV and M83-EBV lymphoblastoid B cell lines and the MHC class II-restricted helper Tcell line, M14-HTL, specific for peptide EBNA2280–290 were all prepared as described (19). X283 human CTL clone (CD8+) specific for melanoma-derived peptide gp100209–217 in the context of HLA-A2 was produced and maintained in tissue culture as described (17). DC were generated from adherent monocytes incubated for 1 week with GM-CSF and IL-4 as described (18). Non-transformed B cell lines were produced by incubating PBMC (2 × 106/ml) for 2 weeks in medium containing human recombinant CD40L trimer (3 μg/ml), IL-4 (10 ng/ml) and cyclosporin-A (10 μg/ml). Mouse B cell lymphoma LB27.4 cells, which express mouse MHC class II (IAb) was purchased from the American Type Culture Collection (Manassas, VA) and maintained as recommended by the vendor. Flow cytometry analysis revealed that these cells were >98% CD19+ and <2% CD3 positive.
Flow cytometry and fluorescent confocal microscopy
MHC class II molecule expression on T cells and APC was determined by flow cytometry using PE-conjugated anti-human CD8, FITC-conjugated anti-human HLA-DR (clone L243), FITC-conjugated anti-mouse IAb (clone AF6/20.1) and isotype control mouse IgG (Pharmingen BD, San Diego, CA). For the analysis of cell surface expression, 1 × 106 cells were stained with monoclonal antibodies for 30 min at 4°C in 100 μl of PBS containing 0.1% sodium azide and 2% FCS. Cells were then washed and fixed in 0.5% paraformaldehyde and analyzed for CD8 and HLA-DR expression on a Becton Dickinson FACScan (San Jose, CA) using CellQuest software for Macintosh. For confocal microscopy, BLS-CD8 T cells were incubated with M-35 cells (1:1) for 3 h. BLS-CD8 T cells and M35-EBV cells were stained with biotinylated anti-human CD8 and Avidin Texas red (Pharmingen BD, San Diego, CA) and FITC-conjugated anti-human HLA-DR (clone L243) and fixed in 0.5% paraformaldehyde. The fixed pelleted cells were mounted on a glass slide with Vectashield mounting medium with DAPI (Vector Labs Inc., Burlingame, CA) and allowed to dry at room temperature. The stained cells were observed under an LSM 510 fluorescent confocal microscope (Carl Zeiss).
Exosome preparation
Exosomes from 2-day old culture of M35-EBV cells were purified by differential centrifugation based on the protocol as described by Thery et al. (20). The final pellet containing the exosomes was washed one more time in PBS for 1 h at 110 000 g and resuspended in 100 μl of PBS with 0.01% sodium azide. The amount of recovered exosomal protein was measured by Bradford assay (Bio-Rad). For MHC class II transfer assays ~20 μg of the purified exosomal protein or 2-day old culture supernatant of M35-EBV cells were used. For transwell cultures, tissue culture inserts (0.4 mm/8 mm) were purchased from Nalge Nunc International, IL.
Cell purification by flow sorting
M35-EBV cells were stained with carboxy-fluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes, Eugene, OR). In brief, cells were resuspended in PBS at 2 × 107/ml. CFSE was added to the cell suspension at a final concentration of 0.5 μM and vortexed and incubated for 10 min at 37°C. Equal volume of FBS was added to quench the reaction. And then the cells were washed twice in RPMI 1640 with 10% FCS. CFSE-labeled M35-EBV cells were mixed with Tcells for the time indicated to allow transfer of MHC class II and subsequently the T cells were purified by flow sorting by eliminating the fluorescent cells. The resulting population was ~99% pure (CFSE negative).
Antibody blocking of MHC class II transfer
M35-EBV cells were incubated with 10 μg/ml of anti-human HLA-ABC (clone W6/32), anti-CD11a, anti-CD45 and anti-CD58 (Pharmingen BD, San Diego, CA) for 30 min at 37°C and co-cultured with BLS-CD8 T cells for 3 h. Alternatively, BLS-CD8 T cells were incubated with 10 μg/ml of anti-CD8 or mouse IgG1 (clone MOPC-315) as a control antibody (Pharmingen BD) for 30 min at 37°C and then mixed with M35-EBV cells for 3 h. HLA-DR expression was measured by gating on the CD8+ T cells by flow cytometric analysis at the end of the 3 h co-incubation period.
Plasma membrane cholesterol depletion
Methyl b cyclodextrin (MCD) was purchased from Sigma. BLS-CD8 T cells or Jurkat T cells were incubated with 10 mM MCD in culture medium for 10 min at room temperature, and then co-cultured with M35-EBV cells (1:1) for 3 h. HLA-DR expression was analyzed by flow cytometric analysis.
Antigen presentation assays
The capacity of T cells that acquired MHC class II to serve as APC was assessed by intracellular cytokine staining analysis. CFSE-labeled M35-EBV cells (HLA-DQ2) were pulsed with EBNA2280–290 peptide (10 μg/ml) for 4 h at 37°C and washed three times by centrifugation to remove unbound peptide. BLS-CD8 T cells were incubated with the peptide-pulsed CFSE labeled M83-EBV cells (HLA-DQ2+) at 1:1 ratio overnight. Next day, CFSE negative T cells were sorted from the M83-EBV cells. BLS-CD8 T cells were incubated with antigen specific DQ2 restricted M14-HTL at 1:1 ratio for 5 h in the presence of GolgiStop (Pharmingen BD). The cells were stained for FITC-conjugated anti-human CD4 (Pharmingen BD). The staining for intracellular IFN γ was done by using an intracellular IFN γ kit from Pharmingen BD. The percentage of IFN γ + cells gating on the CD4+ M14-HTL population was measured by flow cytometric analysis.
Results
To evaluate the possibility of transfer of MHC class II molecules from APC onto the surface of T cells in humans, we selected two model systems where activated T cells do not synthesize endogenous MHC class II. A CD8+ T cell line was prepared from a patient with BLS, which is a genetic disease resulting in the lack of transcription of the products of class II MHC genes (16,21). The RFX5-deficient BLS-CD8 Tcell line was generated by stimulating purified CD8+ T cells with anti-CD3 antibodies and irradiated allogeneic feeder cells as described in Methods. Analysis of the cell surface phenotype of this T cell line 2 weeks after anti-CD3 stimulation revealed that 100% of the cells were CD8+, MHC class II negative (data not shown). The second model system consisted of the acute T cell leukemia line Jurkat, which although it behaves as an activated CD4+ T cell, does not express surface MHC class II molecules because of lack of expression of CIITA (22). As APC we selected the EBV transformed B cell lymphoblastoid cell line M35-EBV, which expresses high levels of surface MHC class II as determined by flow cytometry (Fig. 1A). When BLS-CD8 T cells or Jurkat cells were incubated for 3 h with M35-EBV cells (at a 1:1 ratio), significant amounts of MHC class II molecules were transferred onto both types of T cells (Fig. 1B, D, E and G). In both cases, >80% of the T cells became positive for surface MHC class II. On the other hand, and as expected, the levels of surface MHC class II molecules expressed on the T cells not co-incubated with APC were low (Fig. 1C, D, F and G). To quantify the amount of MHC class II acquired by the T cells, we established a ‘HLA-DR transfer index’ (DRTI) by dividing the mean fluorescence intensity (MFI) of the T cells stained with anti-HLA-DR antibodies, which were co-incubated with APC, over the MFI of the T cells that were not co-incubated with APC, stained with the same antibody. In both cases, the DRTI values showed an increase between 30 and 45-fold in the amount of MHC class II of the T cells that were co-incubated with APC (Fig. 1D and G).
Fig. 1.
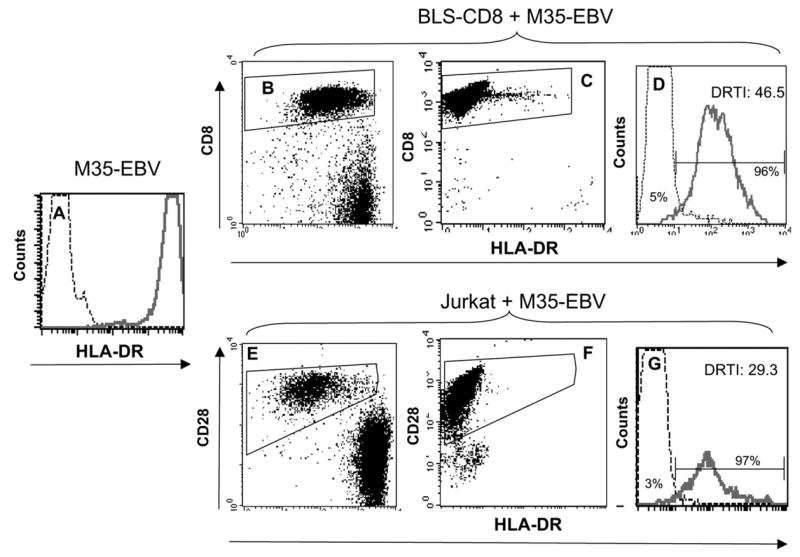
Transfer of MHC class II from APC to T cells. M35-EBV cells, which express high levels of HLA-DR, were used as APC. (A) Solid line corresponds to the staining of M35-EBV cells obtained with anti-HLA-DR antibody and the thin line represents the antibody isotype control. BLS-CD8 T cells (B–D) or CD28+ Jurkat T cells (E–G) were incubated with (or without) M35-EBV cells at 1:1 ratio for 3 h and HLA-DR expression on the gated Tcell populations was examined by flow cytometry. Dot plots in (B) and (E) correspond to cultures containing Tcells and APC, while dot plots in (C) and (F) are the cultures containing T cells alone. The data presented in the histograms (D) and (G) correspond to the cell populations gated on CD8+ T cells (B and C) or CD28+ Jurkat cells (E and F). Thin lines in the histograms (D) and (G) represent HLA-DR staining of BLS-CD8 T cells and Jurkat cells not incubated with M35-EBV (data gated from C and F). Solid lines in the histograms (D) and (G) correspond to the data gated on panels (B) and (E). The ‘DR transfer index’ (DRTI) was calculated by dividing the MFI of HLA-DR staining of the cells incubated with APC over the MFI of HLA-DR staining the control T cells (not incubated with APC). The percentage of HLA-DR+ cells for each population is shown for each histogram curve, calculated using the gates established with isotype controls. These results are representative of three independent experiments.
The transfer of MHC class II molecules from M35-EBV cells to the BLS-CD8 T cells was also evident by confocal microscopy (Fig. 2). Using this approach, we observed that the acquired MHC class II on the T cells appeared in the form of aggregates (example shown in Fig. 2C). In some instances, as shown in Fig. 2(D), the MHC class II molecules appeared to flow from the APC onto the T cell surface, when these cells were tightly engaged. Furthermore, the transfer of MHC class II from M35-EBV onto the surface of the T cells appeared to be independent of antigen recognition by the T cell receptor (TCR) since the BLS-CD8 T cells did not respond to antigens expressed by the M35-EBV cells as determined by cytotoxicity and lymphokine release assays (data not shown). To confirm that the transfer of MHC class II molecules from APC to T cells occurs in an antigen-independent fashion, we co-incubated a CD8+ CTL clone (HLA-A2 restricted) specific for a melanoma peptide (gp100209–217) with APC. Because these CTL express significant levels of human MHC class II molecules on their surface, as most previously activated T cells do (data not shown), we selected as APC a mouse B cell lymphoma (LB27.4) that expresses high levels of mouse MHC class II (IAb) molecules (Fig. 3A), which would allow specific measurement of the transfer of MHC class II using anti-IAb monoclonal antibodies. As shown in Fig. 3(B), significant amounts of mouse MHC class II were acquired by the CTL clone after co-incubation with the mouse B cell lymphoma. These results demonstrate that human T cells have the capacity to acquire substantial amounts of MHC class II molecules from APC onto their surface in an antigen-independent fashion.
Fig. 2.
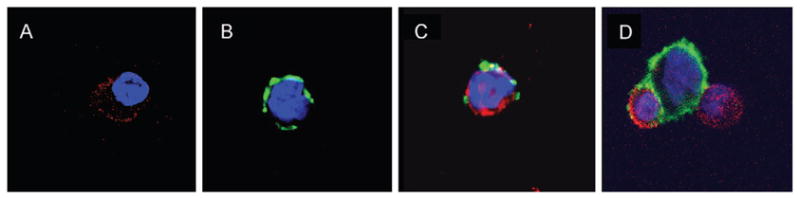
Transfer of MHC class II from APC to Tcells. BLS-CD8 Tcells were incubated with M35-EBV cells (at a 1:1 ratio) for 3 h and stained for CD8 (red) and HLA-DR (green) as described in Methods. (A) Control BLS-CD8 Tcells not incubated with APC. (B) Control M35-EBV cells not incubated with T cells. (C and D) Two representative examples of cells from mixtures of BLS-CD8 T cells with M35-EBV cells.
Fig. 3.
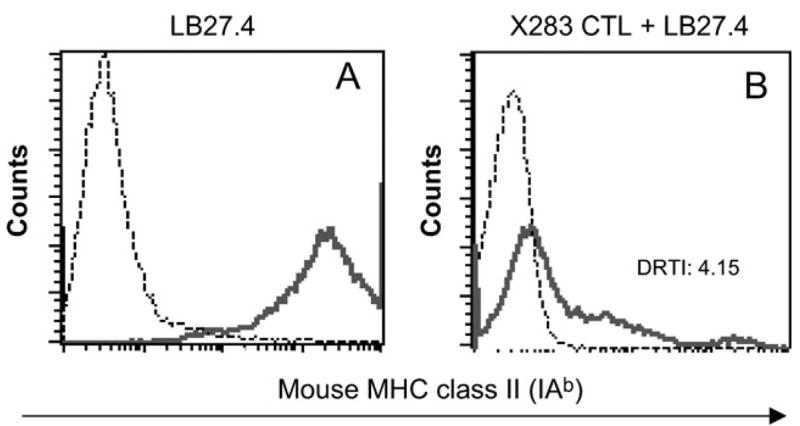
Antigen-independent transfer of MHC class II from APC to CD8+ T cell clone. The X283 CTL clone was co-cultured with mouse B cell lymphoma LB27.4 at a 1:1 ratio at 37°C overnight. (A) High level of mouse MHC II molecules (IAb) on the LB27.4 cells (solid line, anti-IAb antibody; dashed line, isotype control). (B) The amount of transferred mouse MHC class II molecules (IAb) was measured by flow cytometry using anti-IAb monoclonal antibody gating on human CD8+ T cells (solid line, X283 T cells co-incubated with LB27,4; dashed line, X283 T cells alone). This experiment was repeated twice with similar results.
Next, we examined the capacity of human T cells to procure MHC class II molecules from other types of APC. The results presented in Fig. 4 show that when BLS-CD8 T cells were incubated with DC, an MHC class II+ T cell leukemia or activated (non-transformed) B lymphoblasts, significant amounts of MHC class II were transferred onto the T cells. However, the levels of MHC class II acquired by the T cell from these APC, as estimated by the DRTI values, were lower than those observed when M35-EBV were used as APC (Fig. 1).
Fig. 4.
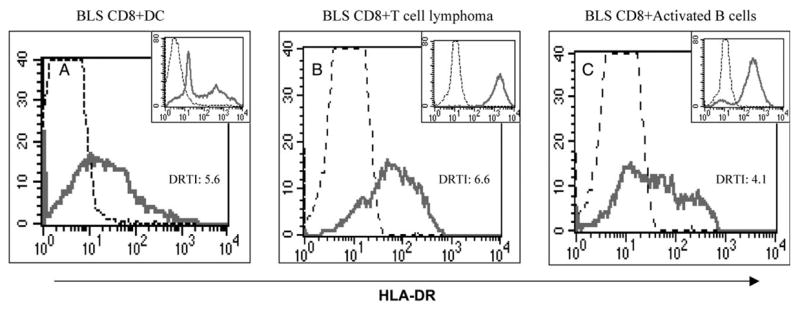
MHC class II transfer to Tcells by various APC. BLS-CD8 Tcells were co-cultured with human dendritic cells (A), with the TL-Hir HLA-DR+ T cell leukemia (B) or with normal (non-transformed) activated human B cells (C) for 3 h, and the HLA-DR expression on CD8+ T cells was analyzed by flow cytometry. Analysis was done as described in the legend to Fig. 1, and the DRTI is indicated. Dashed lines represent HLA-DR staining of BLS-CD8 Tcells not incubated with APC and solid lines the DR staining of the T cells co-incubated with APC. Histograms shown as inserts in each panel correspond to the levels of MHC class II found on each of the APC type used, where solid lines are the staining obtained with anti-HLA-DR antibody and the dashed lines are the isotype controls. These results are representative of three independent experiments.
To assess whether the transfer of MHC class II from APC to the T cells required direct contact between these cells or if this process could be mediated via the capture of cell debris or small membrane vesicles derived from the APC by the T cells, we studied this phenomenon in trans-well cultures. The BLS-CD8 T cells were separated from the M35-EBV APC by a semi-permeable membrane containing 0.4 μm diameter pores. The data presented in Fig. 5 illustrate that BLS-CD8 T cells were inefficient in capturing MHC class II from M35-EBV cells when these cells were separated by a semi-permeable membrane (Fig. 5A versus B). Identical results were obtained when trans-well culture dishes containing 8 μm diameter pore membranes were used (data not shown). The possibility that small subcellular particles, such as exosomes, could be involved in the acquisition of MHC class II by the T cells was explored by incubating the BLS-CD8 T cells with culture supernatant or a preparation containing an enriched exosome fraction derived from M35-EBV cells. The results from these experiments revealed that culture supernatant from the M35-EBV cells did not have the capacity to deliver MHC class II molecules to the T cells (Fig. 5D), while the exosome enriched preparation was able to supply some MHC class II to the BLS-CD8 T cells (Fig. 5E), but at a much lower efficiency as compared to the amount of MHC class II transferred in conditions where direct cell-to-cell contact takes place.
Fig. 5.
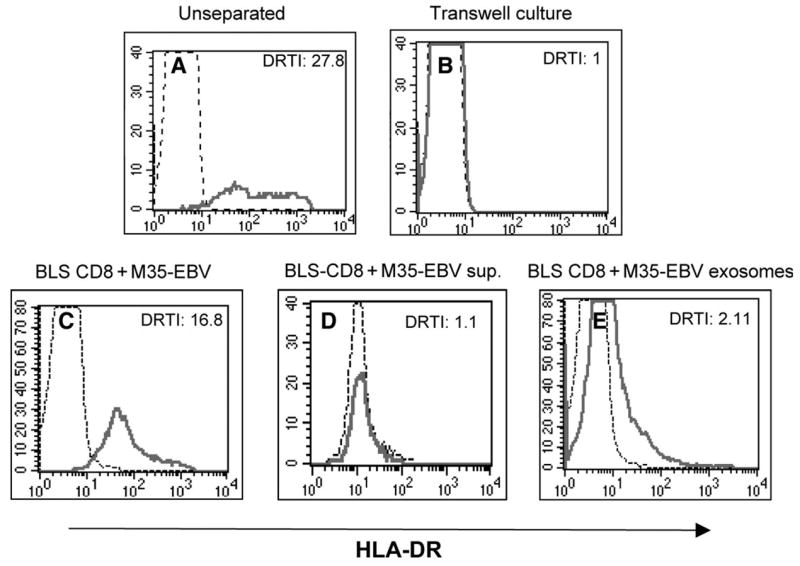
Direct cell–cell contact is required for MHC class II transfer. BLS-CD8 T cells and M-35 EBV cells (1:1) were co-cultured mixed (A) or separated by a semi-permeable membrane (0.4 μm) in a transwell dish (B) for 3 h. In a separate experiment, BLS-CD8 Tcells were co-cultured for 24 h with either M35-EBV cells (C), supernatant from a 2 day old M35-EBV cell culture (D) or exosomes purified from M35-EBV cells. The HLA-DR expression on CD8+ gated BLS-CD8 T cells was measured by flow cytometry and analyzed as described in the legend to Fig. 1, and the DRTI is indicated. Thin lines represent HLA-DR staining of the control BLS-CD8 T cells (not incubated with APC, supernatant or exosomes). These results are representative of three independent experiments.
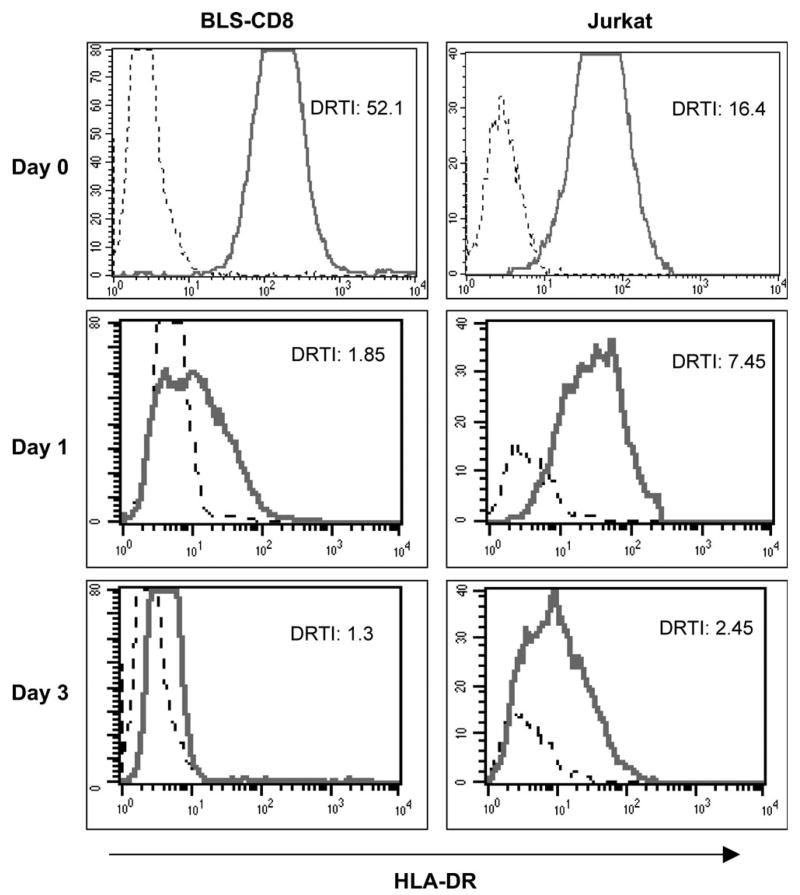
Next, we studied the persistence of the acquired MHC class II molecules on the surface of the T cells. In the first series of experiments, BLS-CD8 T cells were co-incubated with irradiated M35-EBV cells for various time periods and the levels of MHC class II on the surface of the T cells were measured daily. Under these conditions, the amounts of MHC class II on BLS-CD8 T cells remained constant for at least 12 days (data not presented). However, since irradiation of the M35-EBV cells did not actually kill the cells, but only suppressed their growth during the duration of the experiment, the possibility existed that the T cells could be able to continuously procure the MHC class II molecules from the APC, replenishing those molecules that would be shed or internalized. Thus, to assess more precisely the half-life of the acquired MHC class II, the T cells were purified from the APC (by flow sorting) after an overnight co-incubation period. The results shown in Fig. 6 illustrate that the levels of acquired surface MHC class II rapidly decreased in the absence of APC. However, significant amounts of MHC class II could still be detected 24 h after the T cells had acquired this material from the APC.
Fig. 6.
Kinetics of MHC class II transfer. BLS-CD8 (left panels) or Jurkat T cells (right panels) were incubated overnight with CFSE-labeled M35-EBV cells. Next day, the CFSE negative T cells were separated by flow sorting, placed on fresh medium and analyzed immediately (Day 0) and at various time points (Days 1 and 3) for HLA-DR expression by flow cytometry.
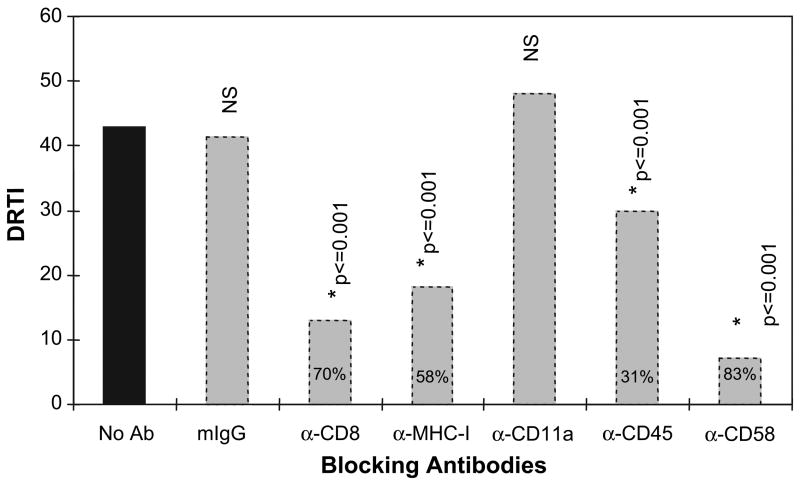
Since the transfer of MHC class II from APC to Tcells did not appear to involve the specific recognition of antigen by the T cells and required cell-to-cell contact by these cells, we hypothesized that adhesion molecule interactions between APC and T cells would be involved in this process. Thus, we determined the capacity of antibodies specific for various adhesion molecules to inhibit the transfer of MHC class II from APC to the BLS-CD8 T cells. The first usual suspect we considered was CD28, since it has been reported to play a role in the transfer of MHC class I from APC to CTL. However, the BLS-CD8 Tcells, as most of our long-term cultured CD8+ Tcell lines, have lost expression of this cell surface marker (data not shown). On the other hand, since the Jurkat cells do express significant levels of surface CD28 (Fig. 1E and F) we studied the capacity of soluble anti-CD28 monoclonal antibodies to inhibit the transfer of MHC class II molecules from M35-EBV cells to the Jurkat cells. The results showed that the presence of anti-CD28 antibodies (used at 10 μg/ml added to the Jurkat cells 1 h prior to co-incubation with the M35-EBV and maintained throughout the co-incubation cultures) did not substantially decrease the transfer of MHC class II (DRTI = 8.3 in the absence of antibody versus DRTI = 7.8 with anti-CD28 antibody; flow cytometry data not shown). Next, we proceeded to evaluate the ability of antibodies to CD8, MHC class I, LFA-1 (CD11a), CD45 and CD58 to block the acquisition of MHC class II by the Tcells when co-cultured with M35-EBV APC. The results shown in Fig. 7 indicate that >50% of the transfer of MHC class II from APC to T cells was inhibited by anti-CD8, anti-MHC class I and anti-CD58 antibodies. While antibodies specific for CD45 inhibited about one third of the transfer of class II to the T cells, anti-CD11a antibodies did not have any effect. These results indicate that some adhesion molecule pairs play an important role in the transfer of class II from APC to T lymphocytes.
Fig. 7.
Antibodies to adhesion molecules block MHC class II transfer to Tcells. BLS CD8 T cells and M35-EBV APC were co-incubated (1:1) in the presence of blocking antibodies at 10 μg/ml for 3 h and HLA-DR expression on BLS-CD8 Tcells was determined by flow cytometry, calculating the DRTI as explained in Methods. The numbers inside the bars indicate % inhibition of HLA-DR transfer. *P-values were derived by comparing the no antibody-treated sample (black bar) with each antibody treated sample (gray bars). mIg = normal mouse immunoglobulin control. These experiments were repeated three times with nearly identical results.
Because cholesterol rich areas (e.g. lipid rafts) on the plasma membrane play important roles in T-cell activation and in the interaction between immune cells (23–25), we examined the effect of the cholesterol depleting agent, MCD, on the transfer of MHC class II molecules from APC to T lymphocytes. BLS-CD8 T cells and Jurkat T cells were incubated with MCD for 10 min before they were mixed with M35-EBV APC and the amount of MHC class II transferred onto the T cells was measured 3 h later. The viability of the T cells and APC in these experiments, as determined by trypan blue exclusion, was not affected by the presence of MCD (data not shown). The data presented in Fig. 8 illustrate that plasma membrane cholesterol depletion with MCD inhibited the acquisition of MHC class II by 71% and 60% by the BLS-CD8 and Jurkat cells, respectively. Treatment of the M35-EBV APC with MCD also resulted in diminished transfer of MHC class II molecules to the T cells, but the effect was of a lesser magnitude (~30% inhibition, data not shown).
Fig. 8.
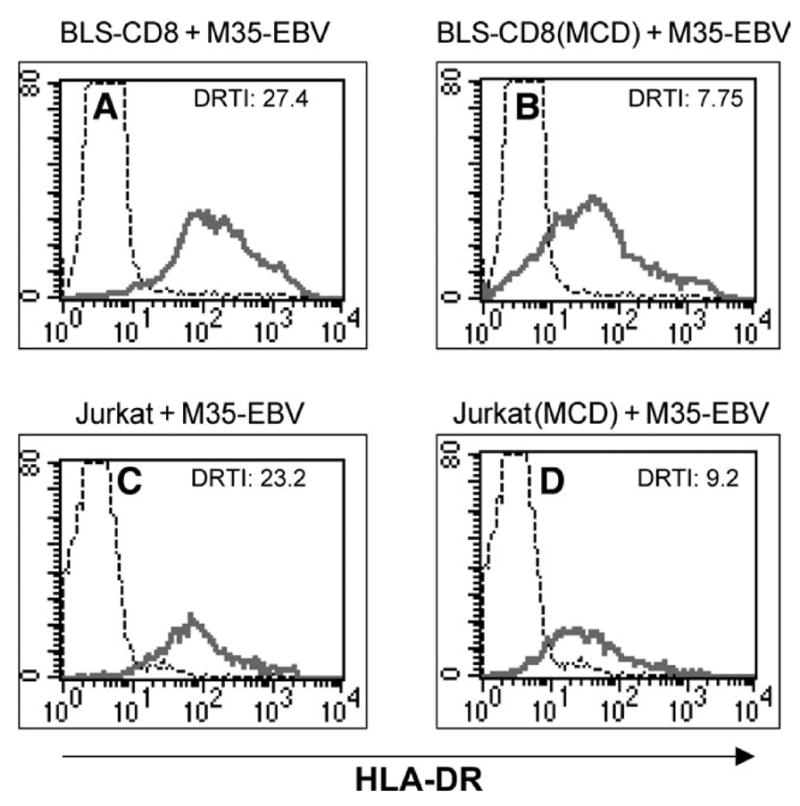
Cholesterol depletion inhibits MHC class-II transfer to T cells. BLS-CD8 T cells (A and B) or Jurkat T cells (C and D) were incubated with 10 mM MCD (B and D) or in medium alone (A and C) for 10 min at room temperature. After this incubation, the T cells were co-cultured with M35-EBV cells (at 1:1 ratio) for 3 h and HLA-DR expression was analyzed by flow cytometry as described in previous figure legends.
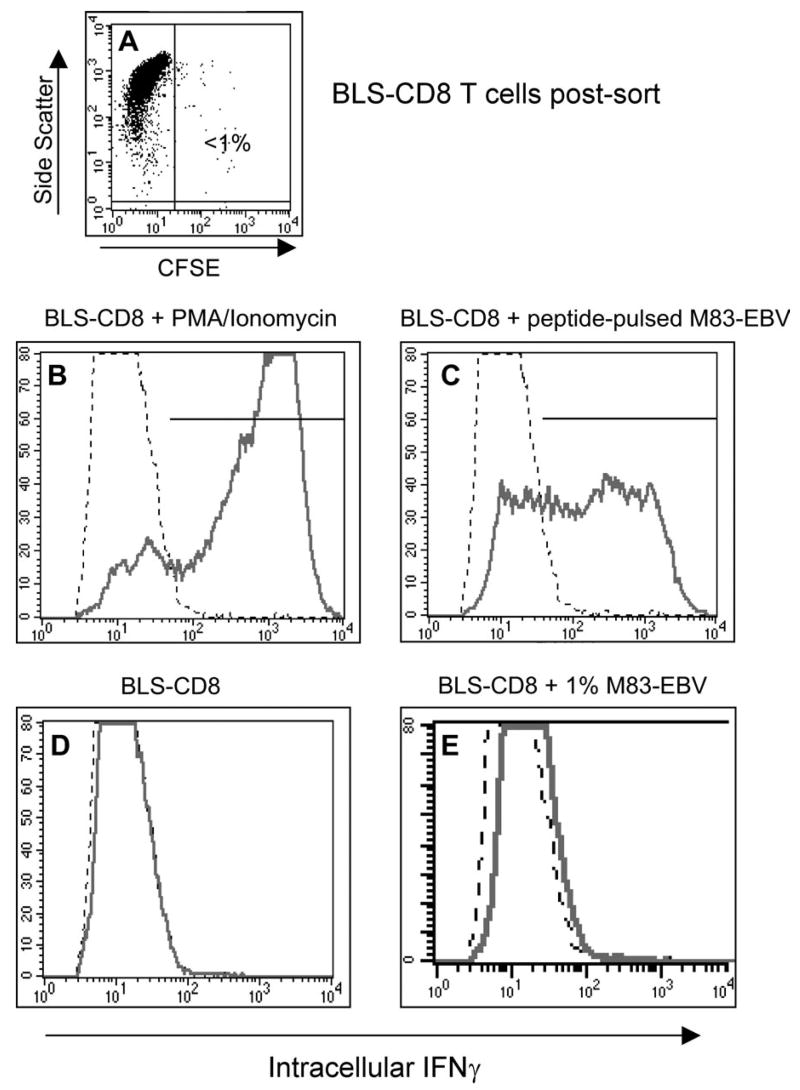
The capacity of the acquired MHC class II molecules on the surface of T cells to react with antibodies suggests that these molecules maintain to some extent their natural conformational structure. Nevertheless, we wished to determine whether the transferred MHC class II on T lymphocytes would be able to present peptide to, and activate, antigen-specific CD4+ T helper cells. For these experiments we utilized a CD4+ helper T cell clone that recognizes peptide EBNA2280–290 in the context of HLA-DQ2 (19). CFSE-labeled M83-EBV cells (HLA-DQ2) were pulsed with 10 μg/ml of peptide EBNA2280–290 and after extensively washing out the unbound peptide, BLS-CD8 Tcells were added to the co-incubation cultures. The following day the BLS-CD8 T cells were purified by flow sorting to ~99% purity (Fig. 9A) and were used as APC to stimulate the HLA-DQ2-restricted CD4+ T cell clone. The data in Fig. 9 demonstrate that the BLS-CD8 T cells that acquired MHC class II molecules from M83-EBV were quite effective in stimulating the production of IFN-γ by the antigen-specific CD4+ T lymphocytes. The possibility that the magnitude of this response was due to the ~1% contaminating M83-EBV cells in the flow sorted BLS-CD8 T cells was eliminated, since purposely spiking BLS-CD8 T cells that had not acquired MHC class II with ~1% M83-EBV cells failed to stimulate the CD4+ T cell clone (Fig. 9E). These results demonstrate that passively acquired MHC class II molecules on T lymphocytes retain the capacity to present peptide to MHC class II-restricted T cells.
Fig. 9.
T cells with acquired MHC class II can function as APC for helper T cells. BLS-CD8 T cells were incubated with CFSE labeled, peptide-pulsed (10 μg/ml EBNA2280–290 peptide for 4 h) M83-EBV cells (HLA-DQ2+) at a 1:1 ratio overnight. Next day, the CFSE negative T cells were purified by flow sorting (>98% purity, panel A) and were co-incubated with antigen specific HLA-DQ2 restricted M14-HTL at 1:1 ratio for 5 h. Antigen response of the M14-HTL was measured by intracellular staining for IFNγ. The percentage of IFNγ positive M14-HTL was detected by flow cytometric analysis by gating on the CD4+ Tcell population. As a positive control, M14-HTL were activated by 50 ng/ml PMA plus 1 μg/ml calcium ionophore (B). IFNγ expression by M14-HTL (gated on CD4+ cells) incubated with BLS-CD8 cells that acquired MHC class II (C). IFNγ expression of M14-HTL incubated with control BLS-CD8 T cells, without M83-EBV pre-incubation (D). Since the contamination of the flow sorted BLS-CD8 Tcells was ~1% (A), as an additional control BLS-CD8 Tcells were mixed with 1% peptide pulsed M83-EBV cells and incubated with M14-HTL (E), demonstrating that this level of contamination was not sufficient to activate the M14-HTL. This experiment was repeated twice with similar results.
Discussion
We have shown here that human T lymphocytes are capable of acquiring large amounts of MHC class II from various classes of APC in an antigen-independent manner. The acquisition of MHC class II required cell-to-cell contact between T cells and APC, and appeared to be mediated by the interaction of various adhesion molecule pairs such as CD8/MHC class I, CD2/CD58 and required the presence of cholesterol in the plasma membrane of the acceptor cell. Although the amount of passively acquired MHC class II rapidly decreased with time, detectable levels remained on the surface of the T cells for at least 72 h. Most significantly, our results indicate that the acquired MHC class II molecules had retained the necessary conformation to present peptide antigen to CD4+ T lymphocytes.
There are some reports describing the acquisition of cell surface molecules by T lymphocytes from APC. This phenomenon, which has been named ‘trogocytosis’, is postulated to serve an important biological function in lymphocytes by facilitating intercellular communication (26,27). To our knowledge, the first indication of MHC class II uptake by human T lymphocytes from other cells was reported more than 20 years ago by Kunkel and co-workers (28). Since activated human T cells make their own MHC class II, these authors were able to detect the exchange of MHC class II molecules between two allogeneic T cell blast lines using antibodies specific for HLA-DR allogeneic determinants. A few years later, Fitch and coworkers described, using allogeneic mixed lymphocyte cultures, that mouse T cells passively acquired cell surface MHC class II molecules from other cells (12). In the rat system, Mannie and co-workers reported that membrane vesicles produced by APC (presumably exosomes) were able to load MHC class II molecules onto the surface of T cells (14,15). The same group described that activated rat CD4+ T cells are capable of acquiring MHC class II molecules in an antigen-independent fashion, even from APC derived from other species (29). Similarly, Tsang and collaborators reported that previously activated mouse CD4+ Tcells were shown to acquire MHC class II molecules and present antigen to themselves, resulting in an increase in apoptosis and in the induction of hyporesponsiveness (30). Our results show that although activated human CD8+ T cells appear to function in a similar manner, they are able to effectively activate CD4+ Tcells by the presentation of antigen. It has been reported that T lymphocytes can also acquire CD80 (B7-1) from APC and incorporate it into their own surface, enabling these cells to function as APC for other T cells (31).
Recent experiments by Sprent’s group described in the mouse system the transfer of MHC class I molecules from APC to CD8+ T cells, which also requires the recognition of antigen by the CTL, or alternatively, the interaction CD28 with B7 between these cell types (32,33). As a result of this type of transfer, the CTL were able to present antigen to themselves, which led to fratricide. In our experience with human CTL acquiring MHC class II from APC, the transfer did not involve the recognition of antigen or CD28/B7 interactions. In the case of Jurkat T cells, which express CD28, antibodies to this surface marker had little effect in blocking transfer of MHC class II from APC to the T cells. The BLS-CD8 T cells used in this study did not react with the APC (as determined by cytotoxicity and lymphokine-release assays) and similarly to many chronically stimulated CTL, they do not express surface CD28 (data not shown) (34–37). In our system, the interaction between other receptor/ligand-pairs such as CD8/MHC class I and CD2/CD58 appeared to play an important role in the transfer of MHC class II from APC to T lymphocytes. These molecules as well as MHC class II tend to localize in cholesterol-rich areas such as lipid rafts or in tetraspan microdomains on the plasma membrane (38–40). Our results indicate that disrupting the normal architecture of the plasma membrane by the sequestration of cholesterol using MCD leads to a substantial decrease of the transfer of class II to the T cells.
At present we do not know the mechanism by which T cells are able to retain cell surface molecules such as MHC class II from the APC. It appears too far-fetched that lipid rafts or membrane protrusions such as filipodia (which are rich in rafts) would be simply ripped off the plasma membrane of the APC. A more likely explanation is that the T cells ‘harvest’ MHC class II-rich membrane vesicles that already exist on the surface of APC. In agreement with this alternative explanation are the observations that large numbers of exosomes often remain attached to the surface of APC (41). Regardless of the exact mechanism operating on the acquisition of MHC class II by T cells, the important question remaining unanswered is whether there is any biological significance to this phenomenon. In the case of CTL presenting antigen in the context of MHC class II molecules to HTL it becomes tantalizing to speculate that delivery of helper signals in the form of lymphokines and costimulation through an immunological synapse would be a highly effective process leading to increased survival, cell division and function of the CTL. Whether this type of interaction takes place in vivo during the course of an immune response remains to be determined.
Acknowledgments
This work was supported by NIH grants R01CA80782, P50CA91956, M01-RR00585 and CA15083.
Abbreviations
- APC
antigen-presenting cell
- BLS
bare lymphocyte syndrome
- CFSA
carboxy-fluorescein diacetate succinimidyl ester
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DRTI
DR transfer index
- EBV
Epstein-Barr virus
- FCS
fetal calf serum
- HTL
helper T lymphocyte
- MCD
methyl b cyclodextrin
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- TCR
T cell receptor
Footnotes
Transmitting editor: T. Hünig
References
- 1.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 2.McDevitt HO. Discovering the role of the major histocompatibility complex in the immune response. Annu Rev Immunol. 2000;18:1. doi: 10.1146/annurev.immunol.18.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 4.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 5.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 6.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 7.Fu SM, Chiorazzi N, Wang CY, Montrazeri G, Kunkel HG, Ko HS, Gottlieb AB. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978;148:1423. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves MF, Verbi W, Festenstein H, Papasteriadis C, Jaraquemada D, Hayward A. ‘Ia-like’ antigens on human T cells. Eur J Immunol. 1979;9:356. doi: 10.1002/eji.1830090504. [DOI] [PubMed] [Google Scholar]
- 9.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979;150:246. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima S, Eckels DD. Selective signal transduction through the CD3 or CD2 complex is required for class II MHC expression by human T cells. J Immunol. 1990;145:4018. [PubMed] [Google Scholar]
- 11.Holling TM, van der Stoep N, Quinten E, Van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II trans-activator promoter III. J Immunol. 2002;168:763. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- 12.Lorber MI, Loken MR, Stall AM, Fitch FW. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol. 1982;128:2798. [PubMed] [Google Scholar]
- 13.Swartz TJ, Evavold B, Suzuki H, Yokoyama A, Quintans J. Antigenicity of passively acquired major histocompatibility antigens on T cells. Transplantation. 1988;46:137. doi: 10.1097/00007890-198807000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by Tcell responders during specific antigen recognition. J Immunol. 1999;163:5201. [PubMed] [Google Scholar]
- 15.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29:1363. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Peijnenburg A, Van Eggermond MJ, Gobin SJ, Van den Berg R, Godthelp BC, Vossen JM, Van den Elsen PJ. Discoordinate expression of invariant chain and MHC class II genes in class II transactivator-transfected fibroblasts defective for RFX5. J Immunol. 1999;163:794. [PubMed] [Google Scholar]
- 17.Tsai V, Kawashima I, Keogh E, Daly K, Sette A, Celis E. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit Rev Immunol. 1998;18:65. doi: 10.1615/critrevimmunol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 18.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 19.Omiya R, Buteau C, Kobayashi H, Paya CV, Celis E. Inhibition of EBV-induced lymphoproliferation by CD4(+) T cells specific for an MHC class II promiscuous epitope. J Immunol. 2002;169:2172. doi: 10.4049/jimmunol.169.4.2172. [DOI] [PubMed] [Google Scholar]
- 20.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 22.Holling TM, Schooten E, Langerak AW, Van den Elsen PJ. Regulation of MHC class II expression in human T cell malignancies. Blood. 2004;103:1438. doi: 10.1182/blood-2003-05-1491. [DOI] [PubMed] [Google Scholar]
- 23.Alonso MA, Millan J. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J Cell Sci. 2001;114:3957. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- 24.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 25.Dykstra M, Cherukuri A, Pierce SK. Rafts and synapses in the spatial organization of immune cell signaling receptors. J Leukoc Biol. 2001;70:699. [PubMed] [Google Scholar]
- 26.Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 2002;16:477. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- 27.Jolly E, Hudrisier D. What is trogocytosis and what is its purpose? Nature Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 28.Yu DT, McCune JM, Fu SM, Winchester RJ, Kunkel HG. Two types of Ia-positive T cells. Synthesis and exchange of Ia antigens. J Exp Med. 1980;152:89S. [PubMed] [Google Scholar]
- 29.Patel DM, Dudek RW, Mannie MD. Intercellular exchange of class II MHC complexes: ultrastructural localization and functional presentation of adsorbed I-A/peptide complexes. Cell Immunol. 2001;214:21. doi: 10.1006/cimm.2002.1887. [DOI] [PubMed] [Google Scholar]
- 30.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 31.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 32.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. Tcells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 34.Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, MacDonald HR, Cerottini JC, Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28− cytotoxic effector clones. Int Immunol. 1999;11:229. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 36.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunol. 1996;88:501. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28− CD8+ T-cell population. Immunol. 1999;98:443. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becart S, Setterblad N, Ostrand-Rosenberg S, Ono SJ, Charron D, Mooney N. Intracytoplasmic domains of MHC class II molecules are essential for lipid-raft-dependent signaling. J Cell Science. 2003;116:2526. doi: 10.1242/jcs.00449. [DOI] [PubMed] [Google Scholar]
- 39.Hiltbold EM, Poloso NJ, Roche PA. MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse. J Immunol. 2003;170:1329. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 40.Kropshofer H, Spindeldreher S, Rohn TA, et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nature Immunol. 2002;3:61. doi: 10.1038/ni750. [DOI] [PubMed] [Google Scholar]
- 41.Mobius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T, Heijnen HF, Geuze HJ, Slot JW. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50:43. doi: 10.1177/002215540205000105. [DOI] [PubMed] [Google Scholar]