Abstract
DNA vaccines were introduced less than a decade ago but have already been applied to a wide range of infectious and malignant diseases. Here we review the current understanding of the mechanisms underlying the activities of these new vaccines. We focus on recent strategies designed to enhance their function including the use of immunostimulatory (CpG) sequences, dendritic cells (DC), co-stimulatory molecules and cytokine- and chemokine-adjuvants. Although genetic vaccines have been significantly improved, they may not be sufficiently immunogenic for the therapeutic vaccination of patients with infectious diseases or cancer in clinical trials. One promising approach aimed at dramatically increasing the immunogenicity of genetic vaccines involves making them ‘self-replicating’. This can be accomplished by using a gene encoding RNA replicase, a polyprotein derived from alphaviruses, such as Sindbis virus. Replicase-containing RNA vectors are significantly more immunogenic than conventional plasmids, immunizing mice at doses as low as 0.1 μg of nucleic acid injected once intramuscularly. Cells transfected with ‘self-replicating’ vectors briefly produce large amounts of antigen before undergoing apoptotic death. This death is a likely result of requisite double-stranded (ds) RNA intermediates, which also have been shown to super-activate DC. Thus, the enhanced immunogenicity of ‘self-replicating’ genetic vaccines may be a result of the production of pro-inflammatory dsRNA, which mimics an RNA-virus infection of host cells.
Keywords: Genetic vaccines, Dendritic cells, Replicase
1. Why use genetic vaccines?
DNA vaccination has become the fastest growing field in vaccine technology following reports at the beginning of the 90’s that plasmid DNA induces an immune response to the plasmid-encoded antigen [1,2]. This unexpectedly successful new method is considered by some to be one of the most important discoveries in the history of vaccinology [3,4]. However, DNA vaccination in many cases is hampered by poor efficacy. Thus, as discussed later, various strategies are being developed to improve immune responses induced by genetic vaccines.
In contrast to vaccines that employ recombinant bacteria or viruses, genetic vaccines consist only of DNA (as plasmids) or RNA (as mRNA), which is taken up by cells and translated into protein. In case of gene-gun delivery, plasmid DNA is precipitated on to an inert particle (generally gold beads) and forced into the cells with a helium blast. Transfected cells then express the antigen encoded on the plasmid resulting in an immune response. Like live or attenuated viruses, DNA vaccines effectively engage both MHC-I and MHC-II pathways allowing for the induction of CD8+ and CD4+ T cells (reviewed in [5]) whereas antigen present in soluble form, such as recombinant protein, generally induces only antibody responses.
Because genetic vaccines are relatively inexpensive and easy to manufacture and use, their immunogenicity and efficacy have been analyzed in a large number of systems and results from preclinical studies have supported human clinical trials [6,7]. Studies have rapidly moved from small laboratory animals to primates and clinical trials are currently being conducted for diseases such as cancer [8], HIV-infection [9], or malaria [10,11]. DNA vaccine studies for disease-models have intensively been reviewed elsewhere [12-15]. The focus of this review will be on experimental strategies aimed at improving genetic vaccines and in particular on those capable of self-replication.
2. A variety of factors determine the success of genetic vaccination
The quick acceptance of genetic vaccines in experimental settings is due to the many advantages this strategy has over traditional vaccines. However, the efficacy of genetic vaccines in many systems has not proven to be satisfactory, leading some to conclude that genetic vaccines are not a viable alternative to conventional vaccines and will never replace them (reviewed in [16]). Some studies, however, purport that DNA vaccines are more efficacious than some established vaccines based on recombinant proteins [17], recombinant viruses [18], or both [19]. Indeed, DNA vaccines can circumvent many of the problems associated with recombinant protein-based vaccines, such as high costs of production, difficulties in purification, incorrect folding of antigen and poor induction of CD8+ T cells. DNA also has clear advantages over recombinant viruses, which are plagued with the problems of pre-existing immunity, risk of insertion-muta-genesis, loss of attenuation or spread of inadvertent infection [8,20]. Perhaps, the primary goal of genetic vaccines should not be to replace well established conventional vaccines with a good track record, instead the focus should be on diseases for which conventional vaccine approaches are ineffective.
Genetic vaccines can be delivered into the host by several routes and methods. Needle-injection into muscle tissue and into the skin is the most commonly used method [1,21,22]. Also the spleen and a variety of mucosal surfaces [23-25] including those of the nose [26] and gut [27] have been targeted. Scale-up from small rodents to larger animals and humans may not be an obstacle: A given DNA-dose may effectively induce an immune response regardless of body size [28,29]. Despite the large number of genetic vaccine-studies conducted so far, many of the results are difficult to compare and inconsistent. A number of factors determine the magnitude and type of immune response induced by plasmid DNA (summarized in Table 1).
Table 1.
Factors determining the immunogenicity of genetic vaccines
| Factors affecting the immunogenicity of genetic vaccines | Comments and conclusions | References |
|---|---|---|
| Structure of the plasmid backbone | Presence of immunostimulatory sequences, introns, poly-A-sequence | [60,67,69,98,99,131-133] |
| Amount of plasmid delivered | More is better | [28,61,134-136] |
| Expression levels of the antigen | More antigen correlates with stronger response, but not necessarily linearly | [61,63] |
| Immunization schedule | Increasing interval between immunizations can strongly enhance the response | [63,137,138] |
| Route of immunization | Intramuscular, intradermal (needle), epidermal (gene gun), mucosal | [61,63,136,139,140] |
| Target-tissue | Including what muscle is injected or what section of the skin | [39,136] |
| Number of immunizations | DNA-induced immune response can effectively be boosted with DNA | [63,141,142] |
| Presence or absence of introns in front of the gene | Introns increase efficacy | [61,103] |
| Strain of the particular species | Different mouse strains show qualitative and quantitative differences in DNA-induced immune responses | [61] (and Leitner WW, Lyon JA, unpublished) |
| Age of animals | Stronger response the younger the mice | [61,68] |
| Toxicity of the antigen for transfected host cell | High expression undesirable for toxic antigens | [61] |
Genetic vaccines may mimic some aspects of the natural infection of host-cells. However, microorganisms contain surface-molecules such as LPS and a variety of soluble factors that function as adjuvants, alerting the immune system to ‘danger’ by inducing inflammation. The potency of genetic vaccines may be significantly enhanced by mimicking these signals with synthetic adjuvants such as QS21 [26] or monophosphoryl lipid A (MPL) [30,31]. However, DNA plasmids without adjuvant are able to induce remarkably strong immune responses to the encoded antigen. In part, this may be due to immunostimulatory sequences within the DNA itself [32,33].
3. Myocytes likely play a secondary role in successful vaccination
Because myocytes are able to take up some of the plasmid injected into the muscle the mechanism of intramuscular immunization seemed very straightforward (Fig. 1). Some reported that this uptake was enhanced when muscle fibrils were recovering from tissue damage induced by the injection of bupivacaine [34] or cardiotoxin [35,36], although such pre-treatment may not be beneficial for all systems [16]. However, muscle is not considered an immunologically relevant tissue as myocytes lack the characteristics of antigen-presenting cells (APC) such as MHC-II expression, costimulatory molecules or marked cytokine-secretion. Even the co-expression of costimulatory molecules or cytokines like GM-CSF or IL-12 is insufficient to turn non-hematopoetic cells into efficient APC [37]. Successful antigen presentation to naive T cells in vivo by ‘non-professional’ APC like fibroblasts may be restricted to some viral antigens that are immunogenic enough to overcome costimulatory requirements [38]. The insignificant role of muscle cells in intramuscular DNA-immunization is further supported by a study, in which the surgical removal of the injected muscle within 10 min of injection of DNA-plasmid did not affect the magnitude or longevity of the antibody response to the encoded antigen [39]. Thus, it seems unlikely that muscle tissue is the immune activating component. Indeed, there is little or no local inflammatory infiltrate at the DNA injection-site, especially after the acute effects of the vaccination have disappeared [40].
Fig. 1. Transfection of host cells with plasmid DNA.
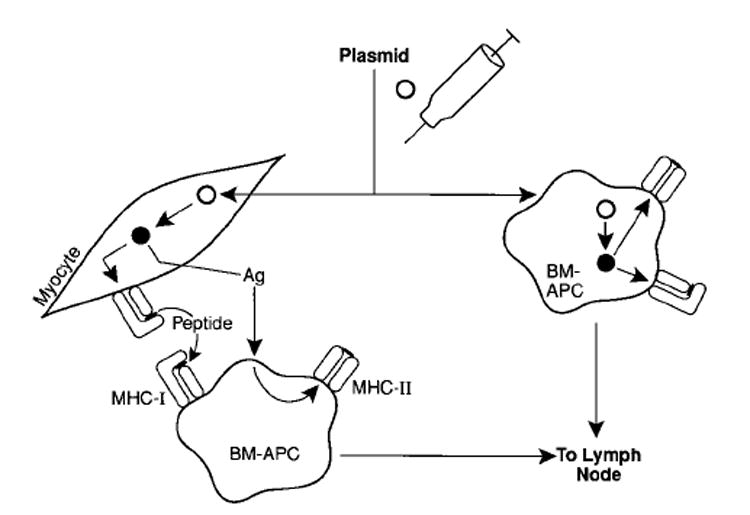
Plasmid (◯) is actively or passively taken up by host cells. Antigen (ν) produced by transfected myocytes can be taken up by bone marrow (BM)-derived APCs. Alternatively, BM-APC can be transfected directly. Antigen-bearing APC then can process and present peptides complexed with MHC-molecules to the immune system after migrating to lymphoid tissue.
Two possible scenarios could explain the mechanism of immune-priming by intramuscularly injected genetic vaccines (Fig. 1). First, myocytes are the antigen-factories that supply professional APCs with antigen for the induction of an immune response in the form of full-length protein or peptides [41,42]. Alternatively, resident APCs may be transfected directly and the antigen that is expressed by transfected myocytes is an irrelevant side-product. In either case, the antigen-expressing bone marrow-derived APCs then migrate to lymph nodes where they activate the T and B lymphocytes. Transfected myocytes may also serve as plasmid-depots for continued APC-transfection. Because myocytes expressing the antigen are subject to CTL-lysis [43], plasmid released from these myocytes may be picked up by monocytes migrating through the muscle.
4. Bone marrow derived APC are required for the induction of an immune response after DNA-vaccination
The crucial role of bone marrow-derived APC (BM-APC) has been well established by using BM-reconstituted chimeras [42,44-46]. The migration of plasmid-carrying macrophages from the injected muscle to lymph nodes has been observed. Minute amounts of the immunizing plasmid have been demonstrated in regional lymph nodes and the spleen [47]. In the case of gene gun delivery, only a small number of transfected dendritic cells (DC) can be found in local lymph nodes after the immunization leading some to question whether these few cells are sufficient to induce an immune response [48]. Alternative explanations such as antigen-transfer to DC or antigen-presentation by non-hematopoetic cells are not consistent with data from experiments in which the transfected cells were selectively depleted in vitro, severely impairing the ability of the remaining non-transfected APCs from immunized animals to induce T cells [48]. The crucial role of DC is also supported by the observation that subcutaneous DNA-injection is very inefficient since this tissue lacks Langerhans cells [49].
The role of DC is not unique to DNA-based vaccines. In case of recombinant vaccinia viruses, the strongest immune response is not induced by the construct with a promoter that showed the highest in vitro expression but the construct using a promoter that was active in DC [50]. Likewise, the function of DNA immunogens should be optimized in DC.
5. Intramuscular and intradermal genetic vaccination may employ different mechanisms in inducing immune responses
In contrast to muscle, skin has important immunological functions as it represents the ‘first line of defence’ of the immune system. Throughout the epidermis, specialized DC form a 3-dimensional network to assure tight immune-surveillance of the skin. Infections agents stimulate DC to pick up antigen and after migrating to the local lymph nodes initiate an immune response [51,52]. The main methods of plasmid-DNA delivery to the skin, by needle injection or by gene-gun, differ in several respects. While needle injection requires relatively large amounts of plasmid (similar to the 50–100 μg dose used in intramuscular immunization), the amount of plasmid required for gene-gun immunization has been titrated down to a few nanogram [53,54]. As in myocyte-transfection after intramuscular immunization, plasmid can be actively taken up by skin cells [55] but only few cells are transfected after intradermal injection [22].
When delivered by gene-gun, the plasmid solubilizes when the plasmid-coated gold bullet penetrates the cells in the skin. Thus, plasmid is directly deposited into cells transfecting up to 20% of the cells in the target-area [56]. Tissue stress resulting from the blast may contribute to the activation of DC. Indeed, the total number of DC in the skin-draining lymphoid tissue increases enormously after gene-gun immunization, although the majority of these cells do not carry the plasmid. Such massive DC-immigration into lymph nodes could be induced simply with plasmidfree gold bullets demonstrating the ‘adjuvant effect’ of gene-gun immunization [48]. In case of gene gun immunization, an antibody-response to the plasmidencoded antigen has been reported not to occur if the target area is excised within 24 h. There is little or no effect when the excision occurs later [39,57]. This time period may coincide with the time it takes an activated DC to migrate from the skin to the lymph nodes. In contrast to these findings, the removal of the gene-gun immunized mouse-ear immediately after immunization does not abrogate the immune response (Johnston SA, personal communication) most likely because some plasmid can rapidly leave the tissue after it is delivered directly into the bloodstream, possibly transfecting cells in blood vessels close to the surface of the skin.
Larger amounts of plasmid delivered by needle-injection together with increased quantities of immunostimulatory DNA sequences may steer the immune system towards a Th1-type response [58-60]. Indeed, the intramuscular injection of relatively small amounts of plasmid elicits Th2-type responses that are characterized by IgG1 [61]. The small amounts of immunostimulatory DNA delivered with the gene gun may not be sufficient to mediate a Th1-type response allowing Th2-type responses to emerge (reviewed in [61]). It is important to note that needle immunization with large amounts of DNA does not always induce Th1-type responses and gene gun immunization does not always yield a Th2-response [62,63]. By modifying the immunization-regimen, we have shown that either IgG1 or mixed IgG1/IgG2-responses can be induced by gene-gun immunization [63]. Furthermore, a gene gun-induced Th2-type response can be switched to a Th1-type response by co-delivering the genes for IL-2, -7 or -12 [64] (Fig. 2).
Fig. 2. ‘Super-activation’ of APC after vaccination with genetic vaccines.
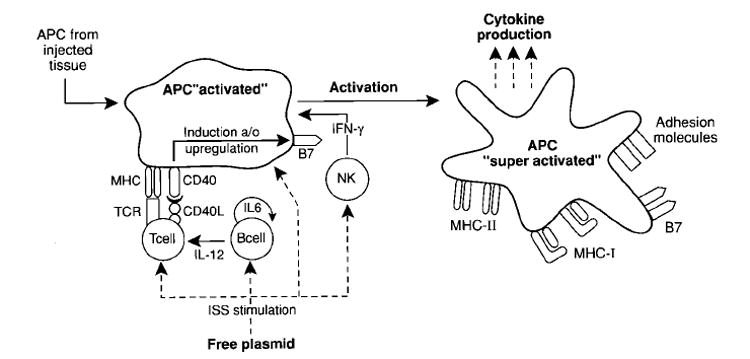
APC are initially stimulated in the injected tissue by either needle or gene-gun delivery of plasmid. Immunostimulatory sequences (ISS) stimulate host APCs, T cells and NK cells. APC interact with T cells through both TCR/MHC and CD40/CD40L, triggering the upregulation of B7 expression on the APC. ISS also polyclonally stimulate B-cells and induce the release of IL-6 and IL-12, further promoting a Th1 response.
6. Enhancing the efficacy of genetic vaccines
A large number of approaches have been used in an attempt to improve the often poor efficacy of DNA vaccines. Because the efficacy of DNA vaccines in many systems has not been satisfactory, the most simple and unexpectedly effective strategy is increasing the intervals between immunizations and, thereby, the ‘rest-period’ of the immune system [63,65]. In addition, many elements of the plasmid can be optimized for use of the vector as a DNA vaccine [66]. Based on the idea that more antigen is better, most DNA vaccines use strong viral promoters and are geared towards maximum expression. Other sequences that can be optimized in a plasmid include introns, enhancers and poly-adenylation signals [67-69].
6.1. Antigens can be modified to make them better immunogens
To improve the immunogenicity of an antigen encoded by a genetic vaccine, functional sequences like the intracellular domain or the trans-membrane sequence can be eliminated [70]. Antigens can be targeted to the Class-I or Class-II processing pathways with the addition of sequences designed to direct intracellular trafficking. Finally, immunodominant epitopes from antigens can be expressed as minigenes, or they can be buried within unrelated, but highly immunogenic core-sequences [71]. This may be especially useful in cases where ‘full-length’ proteins are not suitable as vaccine candidates, because they are toxic for the host [61] or immunosuppressive [72]. Antigenic proteins can be maximally truncated, leaving only defined epitopes for B or T cells. Antigens consisting of CD8+ T cell-epitopes alone are sufficient to induce CTLs as shown with an ova-epitope in vaccinia [73] and this approach has also successfully been used for CD4+-T cell epitopes [55]. To overcome MHC-restriction of individual epitopes or to induce a broader range of effector-cells, it is possible to deliver multiple contiguous minimal epitopes in form of a ‘polytope’. This approach has successfully been used for vaccinia as a carrier [74] as well as for an analogue DNA-construct [75]. To improve MHC-I-loading, endoplasmic reticulum (ER) insertion signal sequences can be attached to minigenes. These sequences can facilitate the targeting of the antigen to the ER, where MHC-I molecules are complexed with antigen. This approach was pioneered in the vaccinia system [76] and also works for peptide-immunization [77]. An adenovirus leader-sequences has successfully been used to target DNA vaccine encoded CD8+ T cell epitopes to the ER [71].
Helper epitopes, such as the hepatitis B core-antigen, can activate B cells and elicit strong T cells responses adding significantly to DNA-based vaccines against hepatitis [78,79]. In another example, a short sequence of a malaria antigen encoding a known helper-epitope (P. berghei CSP57-70) with a nested and previously unidentified H2-Kd CD8+ T cell epitope (PbCSP58-67) was engineered into a DNA plasmid together with the hepatitis core antigen-sequence. When this plasmid was delivered as a DNA vaccine by gene gun, we were able to induce the highest level of protection seen with any PbCSP-DNA vaccine tested (Lyon JA, Leitner WW, unpublished observation).
6.2. Exogenous cytokines can enhance or direct the immune response
Cytokines can significantly improve vaccine-induced immune responses, accelerating and augmenting it as well as directing it, for example, towards a Th1- or Th2-type response [80,81]. In the case of cancer, adjuvant cytokines are especially important, because tumor-associated-antigens are generally of poor immunogenicity. Irvine et al. demonstrated that gene gun delivery of a model antigen was protective by itself, but was only therapeutic when co-delivered with IL-2, IL-6, IL-7 or especially IL-12 [82]. Other useful cytokine adjuvants include GM-CSF, a cytokine thought to recruit and mature dendritic cells [83]. Besides using exogenous factors, cytokines [84] as well as chemokines [85,86] encoded on plasmid DNA or as cDNA have been used to study, modulate or enhance a DNA vaccine induced immune response. One study shows the conversion of a non-immunogenic antigen into a DNA vaccine by fusing it to the genes for chemokines [87].
6.3. DNA-encoded costimulatory molecules can enhance APC-functions
To develop a T cell response, APCs have to deliver two signals to the T cell: one signal is from the MHC/peptide complex to the T cell receptor, the second is from a costimulatory molecule, of which B7 is perhaps the most important and best characterized. In the absence of costimulation, T cells may become anergic preventing self-reactive cells from producing auto-reactivity. B7.1 and B7.2 are expressed on professional APC and on a variety of other tissues after exposure to inflammatory cytokines [88]. Transfection of tumor cells with either isoform has successfully been used to overcome the poor immunogenicity of tumor cells [89-91]. Both molecules bind CD28, the constitutively expressed T cell ligand, as well as CTLA-4, an inducible ligand expressed upon T cell activation. There is an ongoing controversy over how these two B7-molecules differ functionally. Blockade of B7.1 has been shown to abrogate primary and secondary antibody responses, while B7.2 blockade suppressed only primary responses [92]. The differential expression of B7.1 and B7.2 on APC could result in differences in the T helper cell phenotype [93]. Alternatively, some investigators challenge the role of B7 as a costimulatory molecule altogether and describe B7.1 and B7.2 as ‘late’ triggers of IL-2 production and T cell survival that are not involved in the initial T cells stimulation [94,95].
The use of B7 for DNA-immunization has shed some light on the differential roles of the two isoforms. Interestingly, co-immunization with the B7.2-gene yielded better results in some studies while B7.1 codelivery had little beneficial effect [37,96]. We, however, observed that co-delivery of B7.1 with a malaria antigen (Pb-CSP) by gene gun significantly increased the protective effect of a low-expressing plasmid, but not of a high expresser plasmid (Lyon JA, Leitner WW, unpublished observations). The enhanced efficacy was strongly dependent on the immunization interval and, surprisingly, repeated B7.1 co-immunization was suppressive when used with longer intervals. Importantly, enhanced immune responses are observed in some studies only when the two genes are encoded on the same plasmid, suggesting that the antigen-specific and costimulatory signals must emanate from the same cell [84].
6.4. Bacterial DNA-sequences called immunostimulatory sequences can be potent adjuvants
Non-methylated, palindromic DNA-sequences containing CpG-oligodinucleotides (CpG-ODN) can activate an ‘innate’ immune response by activating monocytes, NK cells, dendritic cells and B-cells in an antigen-independent manner (immunostimulatory DNA sequences, ISS). Indeed, methylation of the CpG-ODN reportedly abrogates the immunogenicity of the DNA vaccine [97]. Thus, the use of large amounts of plasmid for immunization may not only overcome the low transfection eficiency in vivo, but may also serve as an adjuvant, driving a Th1-type response (reviewed elsewhere [98]). Immunostimulatory DNA sequences may activate skin-derived DC in vitro and in vivo [99]. Because resting DC are weak APCs, this activation may be critical for successful plasmid-vaccination [100,101]. CpG-ODN have been reported by one group to be as effective an adjuvant as Complete Freund’s Adjuvant and to be without significant toxicity [33]. In the case of DNA vaccines they can either be co-administered with plasmid-DNA in the form of oligonucleotides or the number of ISS on the plasmid-backbone can be increased. Limited information is available so far about the species-specificity and restrictions of the DNA-sequences outside the central CpG-ODN [97]. Nevertheless, the study of the immunostimulatory properties of DNA may be an important area of research because of the limited number of adjuvants for use in humans (reviewed in [102]).
6.5. Heterologous prime-boost-regimens can enhance the efficacy of genetic vaccines
The delivery of the same antigen multiple times using carriers with little or no immunogenic crossreactivity (heterologous prime-boost-regimen) provides several advantages over the repeated delivery of an antigen with the same carrier (homologous boosting). The repeated use of any given recombinant virus-based vaccine may be impaired by anamnestic responses to the carrier itself [8]. Including DNA in these regimens may also shift the response towards Th1, even when a Th2-type response was initiated with recombinant protein [103]. Heterologous boosting yielded full protection in the P.berghei malaria model when plasmid immunization was followed by administration of recombinant vaccinia virus. Homologous boosting was weak or ineffective in this model [104,105]. Similarly, Irvine et al, reported greatly improved immune responses and tumor treatment when delivering a model tumor antigen by gene gun, cowpox or fowlpox in a heterologous prime-boost-regimen [106].
The order of carriers used in a heterologous primeboost-regimen may be important. Going from the antigenically simpler vector in the priming, to the antigenically more complex (and potentially more powerful) vector in the boosting may help the immunotherapist to focus the immune response. For example, priming with DNA-priming and boosting with recombinant vaccinia virus was the only effective regimen in some studies [104,105]. This might be due to viral products that are immunosuppressive such as soluble, secreted cytokine receptor homologues, the expression of which could interfere with immune priming, especially to weak antigens [107-110]. Furthermore, epitopes derived from viral vectors can be immunodominant over weak antigens. Such interference might have less impact on a secondary response that had been primed with a DNA vaccine. Finally, in humans, pre-existing immunity to some viral vectors might add another level of complexity to the outcome of heterologous prime-boost strategies.
7. Self-replicating genetic vaccines have some clear advantages over conventional vectors
‘Self-replicating’ genetic vaccines are designed to overcome the poor efficacy of some current DNA-based and RNA-based genetic vaccines. The idea and the elements for this new generation of vaccines come from members of the Alphavirus genus, which includes Sindbis virus, Semliki Forest virus (SFV) and Venezuelan equine encephalitis (VEE) virus. These RNA viruses contain a single copy of positive-stranded RNA encapsidated by a protein/lipid envelope. The viral RNA encodes its own RNA replicase, an autoproteolytic polyprotein that cleaves itself into four non-structural protein components (nsP1-4) [111,112]. Upon infecting a cell, the viral RNA first translates the replicase complex, which in turn drives its own RNA replication. The replicase complex then synthesizes a genomic negative-strand (anti-sense RNA), which is used as a template for the synthesis of the genomic positive-stand RNA as well as a subgenomic RNA encoding the structural viral proteins (Fig. 3). The genes for structural proteins can be replaced with the gene for the antigen of interest to construct powerful replicase-based vaccines [113].
Fig. 3. Self-replicating genetic vaccines.
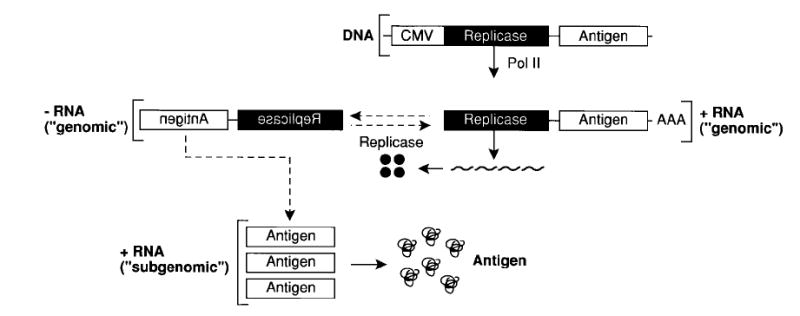
The first product of the self-replicating RNA is a four-subunit-replicase which uses the (+) strand RNA as a template to make (−) strand RNA and more copies of full length (+) strand ‘genomic’ RNA and (+) strand ‘subgenomic’ mRNA for the encoded antigen. Due to the high number of RNA-copies, the main product of the transfected cells becomes the encoded antigen. The host cell eventually undergoes apoptosis.
Theoretically up to 200,000 copies of RNA can be produced in a single cell within 4 h and expression of the encoded antigen can be as much as 25% of total cell protein [114]. The alphavirus replicase functions in a broad range of host cells (mammalian, avian, reptilian, amphibian and insect cells) [115]. Replication takes place in the cytoplasm of the host cell and, therefore, is independent of the host’s replication system. All the above features, i.e. high level expression, broad host range and cytoplasmic replication, are useful features in genetic vaccine development.
To facilitate vaccine production, genomic alphavirus RNA alone can be used as a vaccine vehicle. The in vitro transcribed self-replicating RNA contains sequences coding for the SFV replicase and a model antigen. A single intramuscular injection of a self-replicating RNA elicited antigen-specific antibody and CD8+ T cell responses and was shown to be significantly more effective than non-replicating RNA [115,116]. DNA-based vaccines can also be constructed by inserting a strong promoter like the human CMV immediate promoter/enhancer element to initiate the transcription of the full length ‘genomic’ RNA in the nucleus [112,117-119]. Replicase-based DNA vaccines may be significantly more immunogenic and efficacious than conventional DNA-plasmid vaccines when low doses of the vaccine are given. Indeed, nanogram amounts of replicase-based vaccine can induce antigen-specific antibody and CD8+ T cell responses [118-120] (and Leitner WW et al., in preparation).
8. Mechanisms that could account for the high efficacy of self-replicating genetic vaccines
A major rationale for putting antigen-coding genes under the control of the alphaviral RNA replicase was to enhance antigen expression and presentation. Unexpectedly, the level of antigen expression of replicase-based constructs in vitro was not necessarily higher than that obtained with conventional DNA or RNA-vectors [118,120] (and Leitner WW et al., manuscript in preparation). The discordance between antigen expression level and the increase in immunogenicity suggests that other mechanisms are involved such as those illustrated in Fig. 4. A fundamental difference between replicase-based DNA vaccines and conventional DNA vaccines is the virus-like RNA-replication inside transfected host. Transfection of host cells with replicase-based genetic vaccines could trigger a series of ‘danger signals’[121].
Fig. 4. Potential factors contributing to the high immunogenicity of self-replicating genetic vaccines.
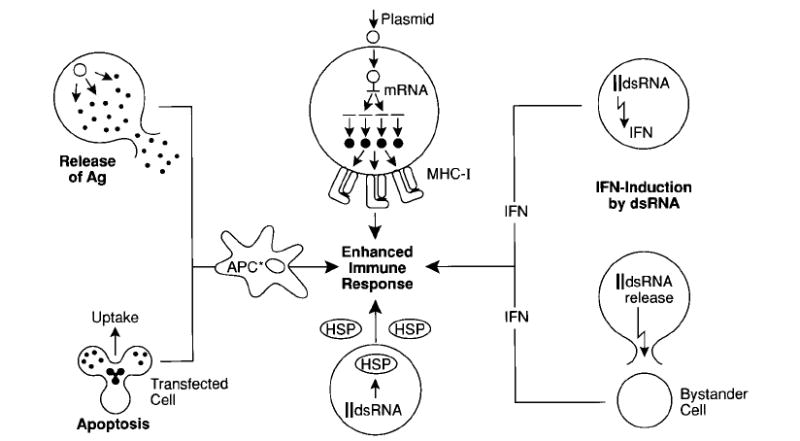
(Starting in the upper centre and moving clockwise): Accumulation of antigen in the transfected cell can result in highly efficient MHC-I-loading. A number of ‘danger signals’ may be generated such as interferon production and interferon release from infected cells resulting from the presence of dsRNA. Interferon may also be produced by bystander cells in response to dsRNA released from dead and lysed transfected cells. Heat shock proteins (HSP) have also been shown to be produced in response to the presence of dsRNA in the cells. Ingestion of antigen-loaded apoptotic cells by APCs can also result in the elicitation of powerful immune responses. Finally, the local release of large amounts of antigen at the site of injection by transfected cells may be fed into resident APC.
Replicase-based DNA or RNA induce apoptotic death of the host cell in vitro just as alphaviral infection induces apoptosis in host cells [116,122]. These apoptotic cells may be picked up by dendritic cells for presentation to the immune system [123]. Transfection with self-replicating genetic vaccines may also cause the production of heat shock proteins in transfected or bystander cells [124]. The activity of the viral replicase may provide a powerful adjuvant-effect because of the requisite production of double stranded RNA (dsRNA) intermediates (Fig. 5). dsRNA itself is a potent inducer of the interferons and virus-derived dsRNA can function as a strong adjuvant for cellular and humoral immune responses [125]. Several molecules are known to bind to and can be activated by dsRNA. The best characterized are 2′–5′ oligoadeny-late (2-5A) synthetase and protein kinase-RNA activated (PKR). The 2-5A system contributes to the antiviral effect of the interferons through the synthesis of 2-5A and its activation of RNAse, which degrades both viral and cellular RNA. PKR-expression both induces and is induced by the interferons. PKR is then activated by dsRNA to phosphorylate its substrates, including eIF2. This results in the inhibition of translation, further diminishing viral replication. The cellular death observed in response to dsRNA is likely to be mediated by both the 2-5A system-induced RNAse as well as some substrates of PKR [126,127]. INF-γ potentiates the apoptotic effects of dsRNA [128].
Fig. 5. dsRNA might be a central element in the immunogenicity of replicase-based genetic vaccines.
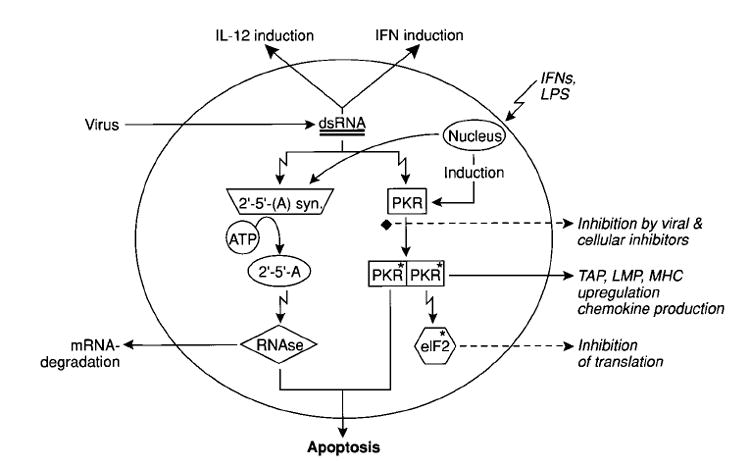
dsRNA is produced in cells infected with RNA-viruses, but is also expected to be an intermediate in the replicase-mediated duplication of mRNA after delivery of ‘self-replicating’ genetic vaccines. dsRNA is a potent inducer of interferon, which in turn induces the expression of PKR and 2-5A synthetase. These enzymes are activated by dsRNA and mediate at least some of the cellular effects associated with viral infection, including apoptotic cell death, aimed at preventing viral spread. The ‘danger signals’ resulting from the activation of these two enzymes could account for the increased immunogenicity of replicase-based genetic vaccines, providing an adjuvant-effect.
The mediators involved in the apoptosis of virally infected cells are subject to viral and cellular inhibitors, like vaccinia E3L [127], HIV-1 Tat protein [129] or the cellular P85IPK [130]. Inhibitors encoded by viruses allow efficient viral replication despite the host cells defense mechanisms. Although apoptosis of cells transfected with self-replicating genetic vaccines might contribute to their enhanced immunogenicity, their efficacy might be limited by the rapid death of host cells due to the absence of vaccine encoded apoptosis-inhibitors that delay the apoptotic death. The manipulation of apoptosis is likely to be a rich area of exploration for vaccinologists.
9. Conclusion
Ideally, a vaccine should be: safe, highly immunogenic, non-integrating, easy to manipulate, genetically stable and inexpensive to produce. In addition to these features, a therapeutic vaccine must not be compromised by pre-existing immunity of the patient against the vaccine vehicle. While ‘conventional’ DNA vaccines are frequently hampered by low efficacy, replicase-based vaccines may significantly improve efficacy. ‘self-replicating’ genetic vaccines may be effective in the fight against diseases that have so far successfully resisted conventional vaccination strategies using recombinant proteins, viruses or bacteria.
References
- 1.Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 2.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 3.Mor G. Plasmid DNA: a new era in vaccinology. Biochem Pharmacol. 1998;55:1151–3. doi: 10.1016/s0006-2952(97)00529-7. [DOI] [PubMed] [Google Scholar]
- 4.Whalen RG. DNA vaccines, cyberspace and self-help programs. Intervirology. 1996;39:120–5. doi: 10.1159/000150483. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Godillot AP, Madaio MP, Weiner DB, Williams WV. Vaccination against pathogenic cells by DNA inoculation. Curr Top Microbiol Immunol. 1998;226:21–35. doi: 10.1007/978-3-642-80475-5_2. [DOI] [PubMed] [Google Scholar]
- 6.Mor G, Singla M, Steinberg AD, Hoffman SL, Okuda K, Klinman DM. Do DNA vaccines induce autoimmune disease? Hum Gene Ther. 1997;8:293–300. doi: 10.1089/hum.1997.8.3-293. [DOI] [PubMed] [Google Scholar]
- 7.Nichols WW, Ledwith BJ, Manam SV, Troilo PJ. Potential DNA vaccine integration into host cell genome. Ann NY Acad Sci. 1995;772:30–9. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 8.Restifo N, Rosenberg S. The development of recombinant and synthetic cancer vaccines. Curr Opin Oncol. 1999;11:50–7. doi: 10.1097/00001622-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calarota S, Bratt G, Nordlund S, et al. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet. 1998;351:1320–5. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman SL, Doolan DL, Sedegah M, et al. Strategy for development of a pre-erythrocytic Plasmodium falciparum DNA vaccine for human use. Vaccine. 1997;15:842–5. doi: 10.1016/s0264-410x(96)00273-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Doolan DL, Le T, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–80. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 12.Wahren B. Gene vaccines. Immunotech. 1996;2:77–83. doi: 10.1016/1380-2933(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Robinson HL. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–7. doi: 10.1016/s0264-410x(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 14.Mahvi DM, Burkholder JK, Turner J, et al. Particle-mediated gene transfer of granulocyte-macrophage colony-stimulating factor cDNA to tumor cells: implications for a clinically relevant tumor vaccine. Hum Gene Ther. 1996;7:1535–43. doi: 10.1089/hum.1996.7.13-1535. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery DL, Ulmer JB, Donnelly JJ, Liu MA. DNA vaccines. Pharmacol Therapeut. 1997;74:195–205. doi: 10.1016/s0163-7258(97)82003-7. [DOI] [PubMed] [Google Scholar]
- 16.Manickan E, Karem KL, Rouse BT. DNA vaccines — a modern gimmick or a boon to vaccinology? Crit Rev Immunol. 1997;17:139–54. doi: 10.1615/critrevimmunol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 17.Davis HL, Demeneix BA, Quantin B, Coulombe J, Whalen RG. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum Gene Ther. 1993;4:733–40. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- 18.Hurpin C, Rotarioa C, Bisceglia H, Chevalier M, Tartaglia J, Erdile L. The mode of presentation and route of administration are critical for the induction of immune responses to p53 and antitumor immunity. Vaccine. 1998;16:208–15. doi: 10.1016/s0264-410x(97)00190-4. [DOI] [PubMed] [Google Scholar]
- 19.Schirmbeck R, Bohm W, Reimann J. DNA vaccination primes MHC class I-restricted, simian virus 40 large tumor antigen-specific CTL in H-2d mice that reject syngeneic tumors. J Immunol. 1996;157:3550–8. [PubMed] [Google Scholar]
- 20.Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1999;90:1894–900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao S, Williams P, Berg RK, et al. Direct gene transfer into nonhuman primate myofibers in vivo. Hum Gene Ther. 1992;3:21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 22.Raz E, Carson DA, Parker SE, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–23. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SC, Jones DH, Fynan EF, et al. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J Virol. 1998;72:5757–61. doi: 10.1128/jvi.72.7.5757-5761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etchart N, Buckland R, Liu MA, Wild TF, Kaiserlian D. Class I-restricted CTL induction by mucosal immunization with naked DNA encoding measles virus haemagglutinin. J Gen Virol. 1997;78:1577–80. doi: 10.1099/0022-1317-78-7-1577. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Dang K, Agadjanyan MG, et al. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine. 1997;15:821–5. doi: 10.1016/s0264-410x(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki S, Sumino K, Hamajima K, et al. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol. 1998;72:4931–9. doi: 10.1128/jvi.72.6.4931-4939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones DH, Clegg JC, Farrar GH. Oral delivery of micro-encapsulated DNA vaccines. Developments in Biological Standardization. 1998;92:149–55. [PubMed] [Google Scholar]
- 28.Cox GJ, Zamb TJ, Babiuk LA. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J Virol. 1993;67:5664–7. doi: 10.1128/jvi.67.9.5664-5667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis HL, McCluskie MJ, Gerin JL, Purcell RH. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–8. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki S, Tsuji T, Hamajima K, et al. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–8. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray NB, Ewalt LC, Lodmell DL. Nanogram quantities of plasmid DNA encoding the rabies virus glycoprotein protect mice against lethal rabies virus infection. Arch Virol. 1997;142:1011–9. doi: 10.1016/s0264-410x(96)00281-2. [DOI] [PubMed] [Google Scholar]
- 32.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 33.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–7. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis HL, Michel ML, Whalen RG. Use of plasmid DNA for direct gene transfer and immunization. Ann NY Acad Sci. 1995;772:21–9. doi: 10.1111/j.1749-6632.1995.tb44728.x. [DOI] [PubMed] [Google Scholar]
- 35.Davis HL, Whalen RG, Demeneix BA. Direct gene transfer into skeletal muscle in vivo: factors a3ecting eficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151–9. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 36.Le Borgne S, Mancini M, Le Grand R, et al. In vivo induction of specific cytotoxic T lymphocytes in mice and rhesus macaques immunized with DNA vector encoding an HIV epitope fused with hepatitis B surface antigen. Virology. 1998;240:304– 15. doi: 10.1006/viro.1997.8942. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–601. [PubMed] [Google Scholar]
- 38.Sprent J. Antigen-presenting cells. Professionals and amateurs. Curr Biol. 1995;5:1095–7. doi: 10.1016/s0960-9822(95)00219-3. [DOI] [PubMed] [Google Scholar]
- 39.Torres CA, Iwasaki A, Barber BH, Robinson HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–32. [PubMed] [Google Scholar]
- 40.Levitsky HI. Accessories for naked DNA vaccines. Nat Biotech. 1997;15:619–20. doi: 10.1038/nbt0797-619. [DOI] [PubMed] [Google Scholar]
- 41.Spier RE. International meeting on the nucleic acid vaccines for the prevention of infectious disease and regulating nucleic acid (DNA) vaccines. Vaccine; Natcher Conference Center NIH; Bethesda, MD. 5-8 February, 1996; 1996. pp. 1285–8. [DOI] [PubMed] [Google Scholar]
- 42.Fu TM, Ulmer JB, Caulfield MJ, et al. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–71. [PMC free article] [PubMed] [Google Scholar]
- 43.Davis HL, Millan CL, Watkins SC. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181–8. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 44.Doe B, Selby M, Barnett S, Baenziger J, Walker C. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci USA. 1996;93:8578–83. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–60. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwasaki A, Torres CA, Ohashi PS, Robinson HL, Barber BH. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–4. [PubMed] [Google Scholar]
- 47.Chattergoon MA, Robinson TM, Boyer JD, Weiner DB. Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells. J Immunol. 1998;160:5707–18. [PubMed] [Google Scholar]
- 48.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 50.Bronte V, Carroll MW, Goletz TJ, et al. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–8. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107:6–8. [PubMed] [Google Scholar]
- 52.Bos JD. The skin as an organ of immunity. Clin Exp Immunol. 1997;107:3–5. [PubMed] [Google Scholar]
- 53.Degano P, Sarphie DF, Bangham CR. Intradermal DNA immunization of mice against influenza A virus using the novel PowderJect system. Vaccine. 1998;16:394–8. doi: 10.1016/s0264-410x(97)00202-8. [DOI] [PubMed] [Google Scholar]
- 54.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–30. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 55.Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997;186:1481–6. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams RS, Johnston SA, Riedy M, DeVit MJ, McElligott SG, Sanford JC. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci USA. 1991;88:2726–30. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klinman DM, Sechler JM, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–92. [PubMed] [Google Scholar]
- 58.Krieg AM, Yi AK, Schorr J, Davis HL. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 1998;6:23–7. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 59.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 61.Barry MA, Johnston SA. Biological features of genetic immunization. Vaccine. 1997;15:788–91. doi: 10.1016/s0264-410x(96)00265-4. [DOI] [PubMed] [Google Scholar]
- 62.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–84. [PubMed] [Google Scholar]
- 63.Leitner WW, Seguin MC, Ballou WR, et al. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159:6112–9. [PubMed] [Google Scholar]
- 64.Prayaga SK, Ford MJ, Haynes JR. Manipulation of HIV-1 gp120-specific immune responses elicited via gene gun-based DNA immunization. Vaccine. 1997;15:1349–52. doi: 10.1016/s0264-410x(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 65.Fuller DH, Corb MM, Barnett S, Steimer K, Haynes JR. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 1997;15:924–6. doi: 10.1016/s0264-410x(96)00271-x. [DOI] [PubMed] [Google Scholar]
- 66.Norman JA, Hobart P, Manthorpe M, Felgner P, Wheeler C. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine. 1997;15:801–3. doi: 10.1016/s0264-410x(96)00247-2. [DOI] [PubMed] [Google Scholar]
- 67.Bohm W, Kuhrober A, Paier T, Mertens T, Reimann J, Schirmbeck R. DNA vector constructs that prime hepatitis B surface antigen-specific cytotoxic T lymphocyte and antibody responses in mice after intramuscular injection. J Immunol Methods. 1996;193:29–40. doi: 10.1016/0022-1759(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 68.Danko I, Williams P, Herweijer H, et al. High expression of naked plasmid DNA in muscles of young rodents. Hum Mol Genet. 1997;6:1435–43. doi: 10.1093/hmg/6.9.1435. [DOI] [PubMed] [Google Scholar]
- 69.Montgomery DL, Shiver JW, Leander KR, et al. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–83. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Hu D, Eling DJ, Robbins J, Kipps TJ. DNA vaccines encoding full-length or truncated Neu induce protective immunity against Neu-expressing mammary tumors. Cancer Res. 1998;58:1965–71. [PubMed] [Google Scholar]
- 71.Ciernik IF, Berzofsky JA, Carbone DP. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–75. [PubMed] [Google Scholar]
- 72.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCabe BJ, Irvine KR, Nishimura MI, et al. Minimal determinant expressed by a recombinant vaccinia virus elicits therapeutic antitumor cytolytic T lymphocyte responses. Cancer Res. 1995;55:1741–7. [PMC free article] [PubMed] [Google Scholar]
- 74.Thomson SA, Elliott SL, Sherritt MA, et al. Recombinant polyepitope vaccines for the delivery of multiple CD8 cytotoxic T cell epitopes. J Immunol. 1996;157:822–6. [PubMed] [Google Scholar]
- 75.Thomson SA, Sherritt MA, Medveczky J, et al. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–23. [PubMed] [Google Scholar]
- 76.Restifo NP, Bacik I, Irvine KR, et al. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–22. [PMC free article] [PubMed] [Google Scholar]
- 77.Minev BR, McFarland BJ, Spiess PJ, Rosenberg SA, Restifo NP. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolongs survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–61. [PMC free article] [PubMed] [Google Scholar]
- 78.Kuhrober A, Wild J, Pudollek HP, Chisari FV, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–12. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 79.Wild J, Gruner B, Metzger K, et al. Polyvalent vaccination against hepatitis B surface and core antigen using a dicistronic expression plasmid. Vaccine. 1998;16:353–60. doi: 10.1016/s0264-410x(97)80913-9. [DOI] [PubMed] [Google Scholar]
- 80.Maecker HT, Umetsu DT, DeKruyff RH, Levy S. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine. 1997;15:1687–96. doi: 10.1016/s0264-410x(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 81.Chow YH, Chiang BL, Lee YL, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–9. [PubMed] [Google Scholar]
- 82.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–45. [PMC free article] [PubMed] [Google Scholar]
- 83.Mellstedt H, Fagerberg J, Frodin JE, et al. Augmentation of the immune response with granulocyte-macrophage colony-stimulating factor and other hematopoietic growth factors [In Process Citation] Curr Opin Hematol. 1999;6:169–75. doi: 10.1097/00062752-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Conry RM, Widera G, LoBuglio AF, et al. Selected strategies to augment polynucleotide immunization. Gene Ther. 1996;3:67–74. [PubMed] [Google Scholar]
- 85.Lu Y, Xin KQ, Hamajima K, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) expression plasmid enhances DNA vaccine-induced immune response against HIV-1. Clin Exp Immunol. 1999;115:335–41. doi: 10.1046/j.1365-2249.1999.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JJ, Nottingham LK, Sin JI, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–24. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–8. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 88.Pechhold K, Patterson NB, Craighead N, Lee KP, June CH, Harlan DM. Inflammatory cytokines IFN-gamma plus TNF-alpha induce regulated expression of CD80 (B7-1) but not CD86 (B7-2) on murine fibroblasts. J Immunol. 1997;158:4921–9. [PubMed] [Google Scholar]
- 89.Townsend SE, Su FW, Atherton JM, Allison JP. Specificity and longevity of antitumor immune responses induced by B7-transfected tumors. Cancer Res. 1994;54:6477–83. [PubMed] [Google Scholar]
- 90.Chamberlain RS, Carroll MW, Bronte V, et al. Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines. Cancer Res. 1996;56:2832–6. [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CT, Ciernik IF, Wu S, et al. Increased immunogenicity of tumors bearing mutant p53 and P1A epitopes after transduction of B7-1 via recombinant adenovirus. Cancer Gene Ther. 1996;3:238–44. [PubMed] [Google Scholar]
- 92.Horspool JH, Perrin PJ, Woodcock JB, et al. Nucleic acid vaccine-induced immune responses require CD28 costimulation and are regulated by CTLA4. J Immunol. 1998;160:2706–14. [PubMed] [Google Scholar]
- 93.Lanier LL, O’Fallon S, Somoza C, et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 94.Zhang YQ, van Neerven RJ, Van Gool SW, Coorevits L, de Boer M, Ceuppens JL. B7-CD28 interaction is a late acting co-stimulatory signal for human T cell responses. Int Immunol. 1997;9:1095–102. doi: 10.1093/intimm/9.8.1095. [DOI] [PubMed] [Google Scholar]
- 95.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–17. [PubMed] [Google Scholar]
- 96.Kim JJ, Bagarazzi ML, Trivedi N, et al. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotech. 1997;15:641–6. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 97.Lipford GB, Sparwasser T, Bauer M, et al. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420–6. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 98.Krieg AM. Lymphocyte activation by CpG dinucleotide motifs in prokaryotic DNA. Trends Microbiol. 1996;4:73–6. doi: 10.1016/0966-842X(96)81515-0. [DOI] [PubMed] [Google Scholar]
- 99.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–4. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 100.Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–98. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700–5. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leclerc C, Ronco J. New approaches in vaccine development. Immunol Today. 1998;19:300–2. doi: 10.1016/s0167-5699(98)01282-1. [DOI] [PubMed] [Google Scholar]
- 103.Li X, Sambhara S, Li CX, et al. Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med. 1998;188:681–8. doi: 10.1084/jem.188.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schneider J, Gilbert SC, Blanchard TJ, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 105.Sedegah M, Jones TR, Kaur M, et al. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–53. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SA, Restifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J Natl Cancer I. 1997;89:1595–601. doi: 10.1093/jnci/89.21.1595. [DOI] [PubMed] [Google Scholar]
- 107.Bell AF, Burns JB, Fujinami RS. Measles virus infection of human T cells modulates cytokine generation and IL-2 receptor alpha chain expression. Virology. 1997;232:241–7. doi: 10.1006/viro.1997.8577. [DOI] [PubMed] [Google Scholar]
- 108.Borrow P, Evans CF, Oldstone MB. Virus-induced immuno-suppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–70. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–21. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–8. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 111.Berglund P, Tubulekas I, Liljestrom P. Alphaviruses as vectors for gene delivery. Trends Biotechnol. 1996;14:130–4. doi: 10.1016/0167-7799(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 112.Tubulekas I, Berglund P, Fleeton M, Liljestrom P. Alphavirus expression vectors and their use as recombinant vaccines: a minireview. Gene. 1997;190:191–5. doi: 10.1016/s0378-1119(96)00679-8. [DOI] [PubMed] [Google Scholar]
- 113.Caley IJ, Betts MR, Irlbeck DM, et al. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031– 8. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rolls MM, Webster P, Balba NH, Rose JK. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 115.Zhou X, Berglund P, Rhodes G, Parker SE, Jondal M, Liljestrom P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12:1510–4. doi: 10.1016/0264-410x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 116.Ying H, Zaks T, Wang RF, et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5(7):823–7. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herweijer H, Latendresse JS, Williams P, et al. A plasmid-based self-amplifying Sindbis virus vector. Hum Gene Ther. 1995;6:1161–7. doi: 10.1089/hum.1995.6.9-1161. [DOI] [PubMed] [Google Scholar]
- 118.Dubensky TW, Jr, Driver DA, Polo JM, et al. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70:508–19. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hariharan MJ, Driver DA, Townsend K, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–8. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljestrom P. Enhancing immune responses using suicidal DNA vaccines. Nat Biotech. 1998;16:562–5. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 121.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 122.Glasgow GM, McGee MM, Sheahan BJ, Atkins GJ. Death mechanisms in cultured cells infected by Semliki Forest virus. J Gen Virol. 1997;78:1559–63. doi: 10.1099/0022-1317-78-7-1559. [DOI] [PubMed] [Google Scholar]
- 123.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 124.Chelbi-Alix MK, Sripati CE. Ability of insulin and dsRNA to induce interferon system and Hsp 70 in fibroblast and epithelial cells in relation to their effects on cell growth. Exp Cell Res. 1994;213:383–90. doi: 10.1006/excr.1994.1213. [DOI] [PubMed] [Google Scholar]
- 125.Wright CL, Alder-Moore JP. The adjuvant effects of mycoviral dsRNA and polyinosinic:polycytidylic acid on the murine immune response. Biochem Biophys Res Commun. 1985;131:949–55. doi: 10.1016/0006-291x(85)91331-2. [DOI] [PubMed] [Google Scholar]
- 126.Castelli JC, Hassel BA, Wood KA, et al. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J Exp Med. 1997;186:967–72. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rivas C, Gil J, Melkova Z, Esteban M, Diaz-Guerra M. Vaccinia virus E3L protein is an inhibitor of the interferon (ifn)-induced 2-5A synthetase enzyme. Virology. 1998;243:406–14. doi: 10.1006/viro.1998.9072. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka N, Sato M, Lamphier MS, et al. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998;3:29–37. doi: 10.1046/j.1365-2443.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 129.Brand SR, Kobayashi R, Mathews MB. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem. 1997;272:8388–95. doi: 10.1074/jbc.272.13.8388. [DOI] [PubMed] [Google Scholar]
- 130.Tan SL, Gale MJ, Jr, Katze MG. Double-stranded RNA-independent dimerization of interferon-induced protein kinase PKR and inhibition of dimerization by the cellular P58IPK inhibitor. Mol Cell Biol. 1998;18:2431–43. doi: 10.1128/mcb.18.5.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–6. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 132.Leclerc C, Deriaud E, Rojas M, Whalen RG. The preferential induction of a Th1 immune response by DNA-based immunization is mediated by the immunostimulatory effect of plasmid DNA. Cell Immunol. 1997;179:97–106. doi: 10.1006/cimm.1997.1161. [DOI] [PubMed] [Google Scholar]
- 133.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 134.Ulmer JB, Deck RR, DeWitt CM, Friedman A, Donnelly JJ, Liu MA. Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine. 1994;12:1541–4. doi: 10.1016/0264-410x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 135.Deck RR, DeWitt CM, Donnelly JJ, Liu MA, Ulmer JB. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine. 1997;15:71–8. doi: 10.1016/s0264-410x(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 136.Yokoyama M, Hassett DE, Zhang J, Whitton JL. DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine. 1997;15:553–60. doi: 10.1016/s0264-410x(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 137.Davis HL, Mancini M, Michel ML, Whalen RG. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–5. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 138.Olsen CW, McGregor MW, Dybdahl-Sissoko N, et al. Immunogenicity and efficacy of baculovirus-expressed and DNA-based equine influenza virus hemagglutinin vaccines in mice. Vaccine. 1997;15:1149–56. doi: 10.1016/s0264-410x(96)00309-x. [DOI] [PubMed] [Google Scholar]
- 139.Gramzinski RA, Millan CL, Obaldia N, Hoffman SL, Davis HL. Immune response to a hepatitis B DNA vaccine in Aotus monkeys: a comparison of vaccine formulation, route, and method of administration. Mol Med. 1998;4:109–18. [PMC free article] [PubMed] [Google Scholar]
- 140.Choi AH, Knowlton DR, McNeal MM, Ward RL. Particle bombardment-mediated DNA vaccination with rotavirus VP6 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1997;232:129–38. doi: 10.1006/viro.1997.8552. [DOI] [PubMed] [Google Scholar]
- 141.Lu S, Santoro JC, Fuller DH, Haynes JR, Robinson HL. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–54. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 142.Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–25. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


