Abstract
Background
Osteopontin is over expressed in metastatic uveal melanoma (UM). S-100β and melanoma-inhibitory activity (MIA) serum levels are elevated in metastatic cutaneous melanoma. The ability of osteopontin, S-100β and MIA serum levels to be used as non-invasive markers for detecting metastatic UM was tested.
Materials and Methods
Osteopontin, S-100β, and MIA levels were measured by ELISA assays in 18 patients with metastatic UM and in 38 patients who were disease-free (DF) for at least 10 years after treatment of the primary tumor. Paired serum samples from 8 patients before and after development of metastasis were analyzed. Forty-four healthy controls (C) were compared to the other two groups.
Results
Serum osteopontin, MIA, and S-100β levels were significantly higher in patients with metastatic UM as compared to patients who were DF for at least 10 years after treatment (p=0.0001) or with age matched controls. Serum osteopontin, MIA, and S-100β levels were significantly higher (p<0.005) after metastasis formation than before the clinical detection of metastasis in the 8 patients. Receiver operator characteristic analysis was performed for metastatic patients vs. DF and vs. C and the area under the curve was calculated for each marker and for the combination of the 3 markers which was 91%.
Conclusion
Elevated serum osteopontin, MIA and S-100β levels correlate with metastatic UM to the liver. When used in combination, these markers provide a highly sensitive and specific method to detect hepatic metastases and therefore provide for earlier therapeutic intervention that can prolong survival.
Keywords: Uveal melanoma, metastasis, markers, osteopontin, S-100, MIA
Uveal melanoma (UM) is the most frequent primary intraocular tumor in adults. It tends to spread first and preferentially to the liver (1, 2). The survival rate of UM patients has changed little over the past few decades in spite of a better local treatment of the intraocular tumor. Currently, there is no effective treatment for metastatic UM, in part because the metastatic tumor burden is typically high when liver metastases are detected by abnormalities in liver function tests or imaging studies (3, 4). Therefore, there is a need to develop sensitive biomarkers to detect early metastatic UM..
A broad variety of molecules synthesized, secreted or shed into the blood, have been proposed as markers of cutaneous melanoma tumor progression (5). These include melanin-related metabolites and enzymes (Alpha-MSH, tyrosinase ), adhesion molecules (ICAM-1, PECAM-1, CD-44) and cytokines (IL-8, IL-10, IL-2R). The currently available diagnostic tests to detect cutaneous melanoma- associated molecules include LDH as introduced by the AJCC (American Joint Committee on Cancer), S-100β (6), NSE (neuron specific enolase ), LASA-P (lipid bound sialic acid) and melanoma-inhibitory activity (MIA) (7).
In cutaneous melanoma, a comparative study showed serum S-100β to be an independent prognostic maker for Stage II and II disease (8, 9). S-100β serum level changes have been reported to reflect the increase or decrease of tumor burden and were of predictive value for a positive response to chemotherapy and immunotherapy (8, 9). Much less is known about the clinical value of S-100β in managing patients with UM. In one study, serum levels of S100β in primary UM patients were not predictive of outcome. However, in this study, the serum levels of S100β were not tested as a marker to detect the presence of hepatic metastases (10).
Recently, two serum markers were reported to correlate with the presence of hepatic metastases from uveal melanoma: the tumor-associated antigen known as MIA was detected in histological sections of primary and metastatic UM to the liver by immunohistochemistry. In addition, serum levels of MIA increased significantly when patients developed metastases to the liver with a lead time of a few months (7).
Elevated serum osteopontin levels in the blood were observed in patients with a variety of metastatic cancers (11, 12) and we recently demonstrated the overexpression of osteopontin in metastatic UM (13).
In this study, high serum levels of S-100β are demonstrated for the first time to be associated with hepatic metastases, similar to MIA and osteopontin. By combining the results of serum levels of S100β, MIA and osteopontin, it is possible to detect hepatic metastases with a high level of sensitivity and specificity.
Materials and Methods
Patient serum samples
One hundred twenty-four serum samples were obtained from the Ocular Oncology Serum Bank at the Hadassah University Hospital. The use of patient sera was approved by the Helsinki Committee of the Hadassah University Hospital. Forty-two samples from 18 patients with metastatic UM, 38 samples from UM patients who were disease-free (DF) for at last 10 years following treatment of the primary tumor, and 1 sample from each of 44 age and sex-matched controls were used in this study. Among the 18 patients with metastatic uveal melanoma, 8 paired samples were available for the comparison in patients before and after the detection of metastasis by ultrasonography and CT scan, followed by tissue confirmation by hepatic biopsy.
Blood (7 cc) was drawn from patients at the time of diagnosis of the primary tumor before and at least every 6 months after treatment. After collection, blood was centrifuged for 10 min at 1200 rpm and serum was stored at −20°C.
Serum assays
Serum levels of osteopontin (R&D Systems, Minneapolis, MN, USA), S-100β (DiaSorin, Stillwater, MN, USA) and MIA – (Roche Diagnostics, Mannheim, Germany) were evaluated with enzyme linked immunosorbent assays (ELISA) kits, following the manufacturers’ instructions.
Statistics
The statistical methods included the Student’s t-test to compare marker serum levels between the 3 groups and the Sign test for comparing serum levels before and after the development of metastatic UM, with a p-value less than 0.05 considered significant. In addition, receiver operator characteristic (ROC) analysis was performed (14) and the area under the curve (AUC) was calculated separately for each of the 3 markers of interest and for the combinations of markers.
Results
There was no significant difference between levels of the 3 markers in the control group and in patients with primary UM who remained DF for at least 10 years. However, serum levels of osteopontin –OPN- (Figure 1A), MIA (Figure 1B) and S-100 β (Figure 1C) were significantly higher in patients with metastatic UM compared with patients who were DF for 10 years (osteopontin, p = 0.0037; S-100β, p = 0.0111; MIA, p = 0.0005,). Similarly, levels of metastatic patients were also significantly higher than those of the controls (osteopontin, p = 0.0039, MIA, p = 0.018 and S100, p = 0.009 ) The development of hepatic metastases was accompanied by significant elevations in serum levels of all 3 markers: as shown in Figure 2 : osteopontin (Figure 2A, p = 0.002 ), S-100 β (Figure 2B, p = 0.046 ), and MIA (Figure 2C, p = 0.045 )
Figure 1.
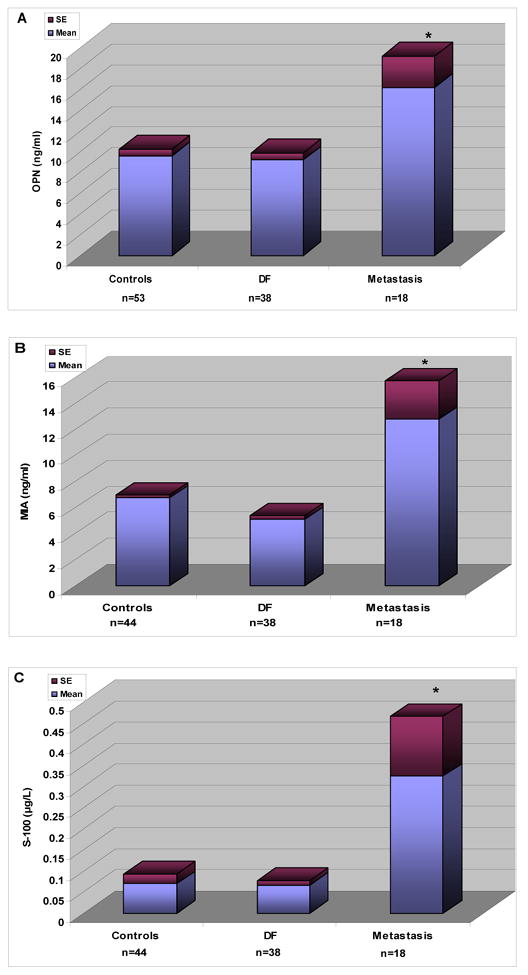
Serum levels of osteopontin –OPN (A), S-100β (B), and MIA (C) in gender and age-matched controls, patients who remained disease free ( DF) for at least 10 years after treatment for primary uveal melanoma, and patients who developed metastatic uveal melanoma to the liver.
* Represents a statistically different level of marker as compared to the other groups.
Figure 2.
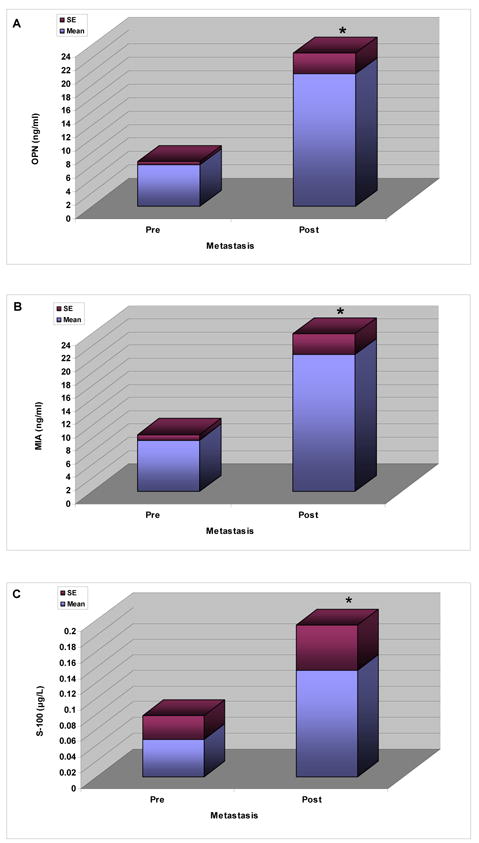
Serum levels of osteopontin –OPN (A), S-100β (B), and MIA (C) before and after the detection of metastasis to the liver.
* Represents a statistically different level of marker as compared to the other groups.
The changes in serum maker levels for two patients who developed hepatic metastases and were treated by partial hepatectomy, are illustrated in Figures 3A and 3B.
Figure 3.
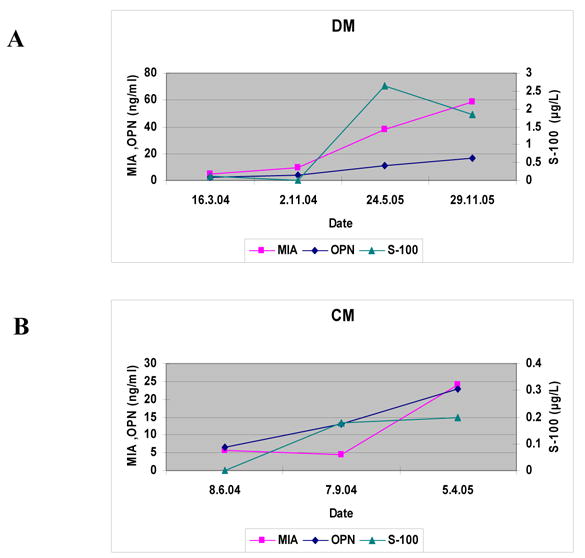
Serum levels of osteopontin- OPN , S100b and MIA recorded every 6 months on 2 patients (named DM, CM ) with uveal melanoma.
ROC analysis was performed for the marker levels of metastatic patients compared to controls: the AUC for all three markers combined was 87%. ROC analysis was also performed for marker levels of metastatic patients compared to patients with treated primary UM who had survived DF for at least 10 years: the AUC for all 3 markers combined was 91 %. The AUC for each marker and for all the combinations of markers are summarized, for both comparisons, in Table I and are shown in Figures 4A and 4B.
Table I.
Receiver operator characteristic (ROC) curve analyses for patients with metastatic uveal melanoma to the liver compared with controls and patients with treated primary uveal melanomas disease-free for 10 years
| Osteopontin | S-100β | MIA | % area under the curve (AUC) | |
|---|---|---|---|---|
| Metastases vs. Controls | Metastases vs. Disease-Free | |||
| - | 70 | 73 | ||
| - | 85 | 77 | ||
| - | 76 | 88 | ||
| - | - | 83 | 85 | |
| - | - | 81 | 82 | |
| - | - | 84 | 84 | |
| - | - | - | 87 | 91 |
Figure 4.
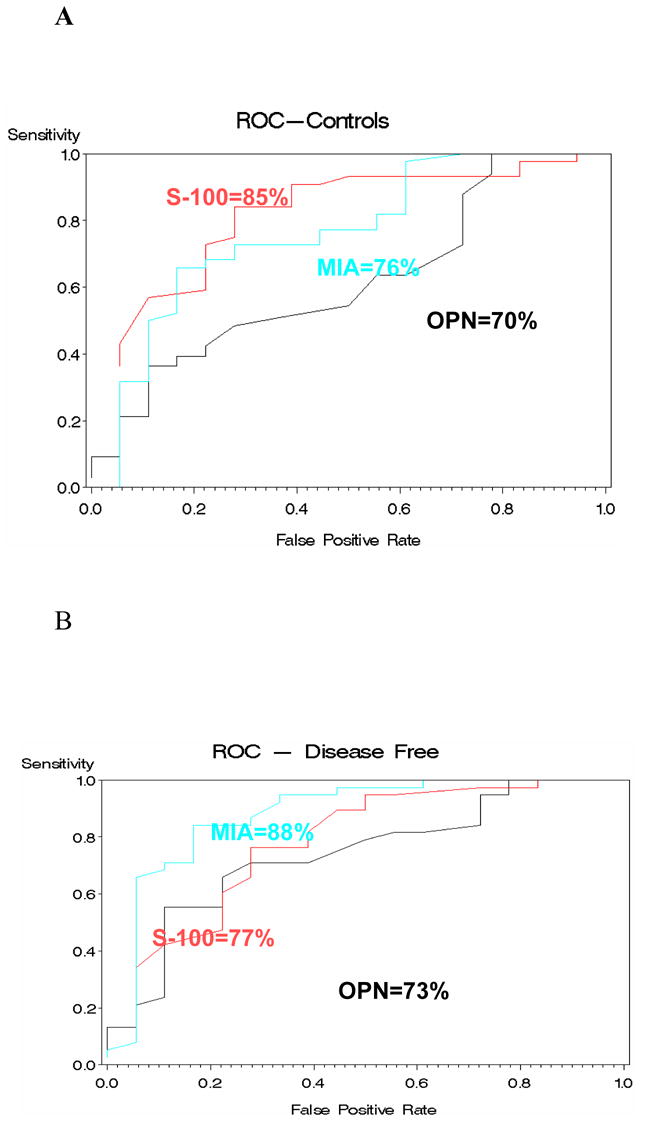
Receiver operator characteristic (ROC ) analysis performed on metastatic uveal melanoma compared to controls and to disease free patients.
Discussion
Although there are no effective treatments for metastatic UM that result in long-term “cure”, there is evidence that some modalities – such as resection of isolated hepatic metastases – can significantly prolong survival, as we have shown previously (15).
Uveal melanoma spreads preferentially to the liver as the first target of metastasis. In the past, oncologists relied upon the measurement of liver enzymes to raise the suspicion of metastases, but a significant tumor burden is usually required to elevate liver enzymes. There are reports of patients with normal liver enzymes and diffuse hepatic metastases (16).
In our recent study (17), the sensitivity and specificity of six liver function tests including bilirubin, gamma glutamyl transpeptidase (γ GT), alkaline phosphates (ALK), lactate dehydrogenase (LDH), aspartate -aminotrasferase (AST) and alanine-aminotrasferase (ALT) was much lower than the combination of the 3 serum biomarkers reported herein. For example, at the time of the diagnosis of hepatic metastasis, the sensitivity of ALT was 40%, the sensitivity for AST and γ GT was 50%, and the sensitivity of ALK and bilirubin was 60%. The sensitivity of LDH was 80% at the time of the detection of hepatic metastasis, but elevated LDH is not specific for hepatic metastasis. These data are entirely consistent with studies from other laboratories that show that the sensitivity of γ GT is 21%, the sensitivity of ALK is 25%, and the sensitivity of LDH is 67% (3). Thus, any one of the 3 serum biomarkers studied here is superior to liver enzymes and the combination of the 3 biomarkers- is far superior to liver function testing.
This report represents an evolutionary approach to the development of non-invasive tests to detect hepatic metastases from UM with a high sensitivity. Additional biomarkers are currently being screened by our group to further increase the sensitivity and specificity of these tests.
Footnotes
Supported by grant EY10457 from the National Institutes of Health (National Eye Institute).
References
- 1.Damato B. Developments in the management of uveal melanoma. Clinical and Experimental Ophthalmology. 2004;32:639–647. doi: 10.1111/j.1442-9071.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Mudhar HS, Parsons MA, Sisley K, Rundle P, Singh A, Rennie IG. A critical appraisal of the prognostic and predictive factors for uveal malignant melanoma. Histopathol. 2004;45:1–12. doi: 10.1111/j.1365-2559.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaiserman I, Amer R, Pe’er J. Liver function tests in metastatic uveal melanoma. Am J Ophthalmol. 2004;137(2):236–43. doi: 10.1016/j.ajo.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 4.Toivonen P, Makitie T, Kujala E, Kivela T. Microcirculation and tumor-infiltrating macrophages in choroidal and ciliary body melanoma and corresponding metastases. Investigative Ophthalmology and Visual Science. 2004;45:1–6. doi: 10.1167/iovs.03-0622. [DOI] [PubMed] [Google Scholar]
- 5.Hannsson L, Hauschild A, Bonfrer JMG, Duffy J, Einarsson R. Tumor markers in malignant melanoma – EGTM (European Group on Tumor Markers) Guidelines. Tumor Biol. 2006;27:35 – S1. [Google Scholar]
- 6.Bonfrer JMB, et al. The luminescence immunoassay S-100: a sensitive test to measure circulating S-100B: its prognostic value in malignant melanoma. Br J Cancer. 1998;77:2210–2214. doi: 10.1038/bjc.1998.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosserhoff AK, et al. Melanoma-inhibiting activity: MIA - a novel serum for progression of malignant melanoma. Cancer Res. 1997;57:3149–3153. [PubMed] [Google Scholar]
- 8.Hauschild A. Prospective evaluation on the utility of S-100β and technical staging procedures in the follow up of melanoma patients. Tumor Biol. 2003;24(S1):60. [Google Scholar]
- 9.Hauschild A, et al. S-100β-protein detection in serum is a significant prognostic factor in metastatic melanoma. Oncology. 1999;56:338–344. doi: 10.1159/000011989. [DOI] [PubMed] [Google Scholar]
- 10.Missotten GS, Tang NE, Korse CM, et al. Prognostic value of S-100-beta serum concentration in patients with uveal melanoma. Arch Ophthal. 2003;121:1117–1119. doi: 10.1001/archopht.121.8.1117. [DOI] [PubMed] [Google Scholar]
- 11..Rittling SR, Chambers AF. Role of osteopontin in tumor progression. British J Cancer. 2004;90:1877– 1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuck AB, O’Malley FP, Singhal H, Harris JF, Tonkin KS, Kerkvliet N, Saad Z, Doig GS, Chambers AF. Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer. 1998;79:502–508. doi: 10.1002/(sici)1097-0215(19981023)79:5<502::aid-ijc10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Kadkol S, Lyn A, Barak V, Kalickman I, Leach L, Valyi-Nagy K, Majumdar DD, Setty S, Maniotis A, Folberg R, Pe’er J. Osteopontin expression and serum levels in metastatic uveal melanoma: a pilot study. IOVS. 2006;47(3):802–806. doi: 10.1167/iovs.05-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quantitative ROC analysis, Metz CE. Basic principles of ROC analysis. Sem Nuc Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 15.Peer J, Nir I, Lotem M, Folberg R, Frenkel S. Post hepatectomy survival of metastatic Uveal Melanoma patients. IOVS. 2006;47 ARVO E-Abstract 1497. [Google Scholar]
- 16.Donoso LA, Shields JA, Augsburger JA, Orth DH, Johnson P. Metastatic uveal melanoma: diffuse hepatic metastasis in a patient with concurrent normal serum enzyme levels and liver scan. Arch Ophthalmol. 1985;103:758–758. doi: 10.1001/archopht.1985.01050060016002. [DOI] [PubMed] [Google Scholar]
- 17.Kaiserman I, Amer R, Pe’er J. Liver function tests in metastatic uveal melanoma. Am J Ophthalmol. 2004;137:236–243. doi: 10.1016/j.ajo.2003.08.045. [DOI] [PubMed] [Google Scholar]


