1. Introduction
The periaqueductal gray (PAG) has been shown to be involved in the modulation of nociception (Fardin, et al., 1984; Liebeskind, et al., 1973; Morgan, et al., 1991; Morgan, et al., 1989), regulation of the autonomic system (Depaulis, et al., 1988; Hayward, et al., 2003), and in the expression of a variety of behaviors, including sexual (Sakuma and Pfaff, 1979), maternal (Lonstein and Stern, 1997), feeding (Van Erp, et al., 1993) and defensive behaviors (Blanchard, et al., 1981; Brandao, et al., 1999; Carrive, 1993; Depaulis, et al., 1992; Fanselow, 1991; Keay and Bandler, 2001) (for review see (Behbehani, 1995)).
Canteras (2002) proposed that a medial hypothalamic defensive system (MHZ) mediates stimulus activation of the defense response elicited by a psychological threat stimulus, a predator. The MHZ initially integrates information relayed from the septohippocampal system with projections from several amygdala sites that receive or integrate information about threat stimuli (Canteras, et al., 1995). In turn, the MHZ relays the information upstream, via the thalamus, to the cortex (Risold, et al., 1997), and downstream, through descending projections to brain stem regions, particularly the PAG (Canteras, et al., 1997; Canteras and Goto, 1999; Risold and Swanson, 1997). Thus, the PAG has been proposed as part of a pathway for the expression of defensive behaviors.
Histochemical and anatomical analyses following chemical and electrical stimulation of the PAG have led to the depiction of a rostrocaudal columnar organization for this region, which is organized into four columns: the dorsolateral (dlPAG), dorsomedial (dmPAG), lateral (lPAG) and ventrolateral (vlPAG) (Bandler and Keay, 1996). Electrical and chemical (via excitatory amino acids) stimulation of the dlPAG, the superior colliculus and the inferior colliculus of the rat induces aversive behaviors such as freezing, arousal and escape (Bandler and Carrive, 1988; Bandler, et al., 1985; Brandao, et al., 1990; Brandao, et al., 1985), while lPAG stimulation elicits defecation and flight (Bandler and Shipley, 1994; Schenberg, et al., 2005). These behaviors are accompanied by changes in autonomic measures such as heart rate, mean arterial blood pressure and respiration (Brandao, et al., 1988; Carrive, 1991; Hayward et al., 2003) and are followed by analgesia (Coimbra and Brandao, 1997; Coimbra, et al., 1992; Fanselow, 1991). These reactions further vary between rostral dPAG injections, which produce a confrontational defensive reaction typically associated with orientation toward the threat and with vocalizations, and caudal dPAG injections, which trigger an escape or flight response. Both rostral and caudal dPAG-elicited-defense-reactions are accompanied by hypertension and tachycardia (reviewed in (Keay and Bandler, 2001; Keay and Bandler, 2004)).
A variety of chemical neurotransmitters mediate the defense-related behavioral responses controlled by the PAG. These include glutamate agonists, γ-amino-butyric-acid (GABA), serotonin (5HT), cholecystokinin (CCK) and neuropeptides such as opioids and corticotropin releasing factor (CRF) (Brandao et al., 1999; Brandao, et al., 1982; Cardoso, et al., 1994; Carvalho-Netto, et al., 2007; Graeff, 1997; Jenck, et al., 1986; Melo, et al., 1992). Receptor binding studies have shown a substantial population of both CRF1 and CRF2 in the PAG (Merchenthaler, 1984; Swanson, et al., 1983). In addition, CRF immunoreactive terminals in the dPAG have been traced back to the amygdala, the hypothalamus and the bed nucleus of the stria terminalis (BNST) (Gray and Magnuson, 1992).
Experiments utilizing CRF-peptide agonists and antagonists reveal potentially distinct roles played by the two-receptor subtypes in behavioral and endocrine responses. CRF1 activate the HPA axis response (Pelleymounter, et al., 2002) and also mediate direct behavioral response to stress, including anxiety (Griebel, et al., 2002), fear (Takahashi, 2001), aggression (Farrokhi, et al., 2004) and sleep pattern disruptions (Jones, et al., 1998). These effects are potentiated by CRF compounds preferential to CRF1 (ovine CRF, rat/human CRF), but not exclusive to these receptors. Recently, a novel CRF1 highly selective agonist, cortagine, has been developed. In these studies, cortagine enhanced both anxiogenic responses in the EPM and antidepressant-like behaviors in the Forced-Swim-Test (FST) (Tezval, et al., 2004; Todorovic, et al., 2005).
Research has shown a role for CRF in PAG excitation in vitro (Bowers, et al., 2003). Application of CRF produced a dose-dependent excitation of PAG neurons associated with a depolarization of membrane potential in intracellular recordings, which was blocked by the pre-application of the CRF antagonist, α-helical CRF. CRF produced responses of two different durations; a short duration lasting no more than 100 seconds, and a long one lasting between 5 to 20 minutes.
Direct injections of CRF into the dPAG have been shown to produce an anxiogenic effect in the EPM (Martins, et al., 1997). Intra-PAG injections of the CRF antagonist α-helical-CRF blocked the anxiogenic effect of 4-hours of restraint on EPM performance (Martins, et al., 2000). Recently, intra-PAG injections of oCRF have been shown to enhance avoidance behaviors in the mouse defense test battery (MDTB) and the rat exposure test (RET) (Carvalho-Netto et al., 2007). These results support a role for CRF-receptor-mediated PAG excitation in defensive behaviors.
The purpose of the present series of experiments was to characterize the role of CRF1 in the PAG using a novel and highly selective CRF1 agonist cortagine, injected directly into the caudal region of the dPAG, in modulating defensive behaviors in the MDTB and the RET. The MDTB measures defensive responses to a predatory stimulus (an anesthetized rat) including flight, freezing, risk-assessment and defensive threat and attack. In addition, pretest activity and posttest defensiveness are recorded (Blanchard, et al., 2003). The RET evaluates risk assessment, and various forms of avoidance, including freezing (passive avoidance) and burying (active avoidance) in mice previously habituated to the RET context (Yang, et al., 2004).
2. Methods
2.1 Subjects
Male Swiss-Webster mice between the ages of 12-15 weeks, obtained from Charles River Laboratories (Wilmington, MA) were used as subjects. Subjects weighed between 30-40 g at the time of surgery. Mice were individually-housed in standard polypropylene cages in a temperature controlled room (20±2 °C) with ad libitum access to food and water and were maintained on a 12 hr light/dark cycle (lights on at 06:00 AM). Testing was conducted during the light cycle between the hours of 1 p.m. and 5 p.m.
All procedures were conducted in accordance with protocols approved by the University of Hawaii Institutional Animal Care and Use Committee.
2.2 Surgical Procedure
Unilateral cannulae were implanted into the dPAG using the following coordinates from bregma: AP −4.16mm, ML −1.32mm, DV −2.27mm and entering the skull at 26 degrees. These were based on coordinates from the Mouse Brain Atlas (Paxinos and Franklin, 2001).
Animals were anesthetized with an intraperitoneal injection of pentobarbital (90mg/kg), .04 ml glycopyrolate, and isoflurane gas as needed. Thereafter, they were mounted on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The scalp was incised and retracted, and the head was positioned to place Bregma and Lambda in the same horizontal plane. One small screw (Small Parts Inc.) was inserted anterolaterally to the site of cannulation. Animals were given 7 days of recovery prior to testing. During this recovery period, cannulae were cleaned and animals were handled daily to habituate and reduce baseline stress levels during drug infusion.
2.3 Drug
Cortagine [Glu(21),Ala(40)][Svg(1-12)]×[human/rat CRF(14-30)]×[Svg(30-40)] was obtained from the Max-Planck Institute for Experimental Medicine (Gottingen, Germany). It was diluted in an artificial cerebrospinal fluid solution (CH3COOH/1xaCSF) at pH=7.4 until the final low and high doses were obtained; 30ng/0.2μl (0.034mM) and 100ng/0.2μl (0.1133mM), respectively. 0.2 μl CH3COOH/aCSF solution was used as the vehicle.
D-Amphetamine sulfate (Research Biochemicals, MA) was dissolved in sterile physiological saline and administered i.p to rats at a single dose of 5.0 mg/kg 15 min prior to placement into the RET. This procedure was used to facilitate the maintenance of motor activity and stereotypic behavior of the rat (Antoniou and Kafetzopoulos, 1991; Antoniou, et al., 1998) throughout the course of a 10-min trial duration, so that this animal serves as a constant threatening stimulus.
2.4. Apparatus
2.4.1 MDTB
The Mouse Defense Test Battery is an oval runway, 0.40m wide, 0.30m high, and 4.4 m in total length, consisting of two 2-m straight segments joined by two 0.4-m curved segments and separated by a median wall (2.0 m°—0.30 m × 0.06m). The apparatus is elevated 0.8 m from the floor to enable the experimenter to hold the rat and move with ease, while minimizing the subjects' ability to view the experimenter. All parts of the apparatus were constructed from black Plexiglas. The floor of the apparatus was marked every 20cm with white lines to facilitate measurement of locomotion distances. Two ceiling-mounted video cameras were used to record the test and the room was illuminated with one 100-watt red light.
2.4.2 Rat Exposure Test (RET)
Testing was conducted in a 46 × 24 × 21 cm clear polycarbonate cage covered with a metal lid. The exposure cage was divided into two equal sized compartments by a wire mesh screen. The home chamber was a 7cm × 7cm × 12cm box made of black Plexiglas on three sides and clear Plexiglas on one side to facilitate videotaping. The home chamber was connected to the exposure cage by a clear Plexiglas tube tunnel (4.4 cm in diameter, 13 cm in length, elevated 1.5cm from the floor of the two chambers).
2.5 Experimental Procedure
Mice were tested in the MDTB 7 days post-surgery. One week later, they were tested in the RET. Mice received an intra-PAG injection of CSF or cortagine (30ng or 100ng) and, 8 minutes later, were run in the MDTB. The Long-Evans rats used as the predator stimulus were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p) 10 minutes before the test session began in order to minimize their discomfort. A total of two rats were used for this experiment. The MDTB consists of the following subtests:
2.5.1 Experiment 1- MDTB
Seven days after surgery, each mouse was transported to the experimental room and left undisturbed for 60 min prior to testing. The apparatus was cleaned with 20% alcohol and dried with paper towels in between trials.
3-minute Pre/Post test: Line crossings, wall rears and wall climbs during the pre-predator (familiarization) period provided data on activity effects of drug treatments. The same measures, during the post-predator period compared to the pretest period, provided an index of enhanced contextual defense following predator exposure.
Predator avoidance test
Avoidance and escape distance are measured when a predator stimulus (a hand-held rat) is brought up to the subject at a speed of approximately 0.5 m/s. Approach is terminated when contact with the subject is made or the subject runs away from the approaching rat. This is repeated five times.
Chase/flight test
The hand-held rat is brought up to the subject at a speed of approximately 2 m/s. The time required to chase the subject 2 complete laps of the runway or 2 min is recorded.
Straight alley test
The runway is then converted to a straight alley, 80 cm long, by the closing of a door at one end. The rat is placed at one end while the mouse begins the test at the other. Measures are taken for 30 seconds and include immobility time (freezing), and closest distance between the subject and the rat and the number of approaches/withdrawals (these are both measures of risk assessment).
Forced contact test
The rat is brought up to contact the subject five times. For each such contact, bites, vocalizations, upright postures and jump attacks by the subjects are recorded, and used as measures of defensive threat and attack.
2.5.2 Experiment 2- The RET
Animals were tested in the RET one week after testing in the MDTB. The bedding material from each subject's home cage was placed in the chamber and surface compartments of the RET, prior to testing. The RET was cleaned and wiped down with 70% ethanol between trials. Amphetamine-treated adult male Long Evans rats (average weight, 450 grams) from breeding colonies maintained at the University of Hawaii were used as predator stimuli. A new rat was used after every five trials or if cessation of movement or stereotypy was observed. A total of three Long-Evans rats were used for this study. The RET consists of the following phases:
Phase 1: Habituation
Each subject was allowed 3 days of habituation in the apparatus. The mouse was placed in the center of the surface and was allowed to explore freely for 10 minutes with no rat present.
Phase 2: Exposure Test
On the fourth day, an amphetamine treated male Long-Evans rat was introduced behind the wire mesh. 8 minutes after receiving a single dose of injection, the mouse subject was placed in the center of the surface area.
Behavioral parameters consisted of proximal and behavioral measures. The proximal measures assessed relative to the location of the stimulus rat were the duration spent in the home chamber, tunnel, and on the surface. Locomotor activity was evaluated by the number of transits between these locations. Total contact time included time spent in contact with the wire mesh. The behavioral measures were frequency and duration of stretch attend postures (SAP, a posture in which the body is stretched forward oriented toward the threat stimulus), freezing, (complete cessation of movement except breathing), grooming, and defensive burying (sawdust pushed from the chamber into tunnel opening). Both the MDTB and the RET procedures have been previously described in detail (Blanchard, et al., 2005).
2.5.3 Drug Infusion
Mice were randomly assigned to one of the three groups: artificial cerebrospinal fluid (aCSF, vehicle control) (n=7), 30 ng (n=7), and 100 ng (n=8) cortagine. Cortagine was infused 8 minutes prior to testing via a 10 μl micro syringe (Hamilton) mounted in an infusion pump (Harvard Apparatus) to control the rate of infusion (0.4 μl/min). The 32-gauge injector was made of stainless steel tubing (Small Parts Inc. FL) and was connected to a 10-μl Hamilton micosyringe with polyethylene-20 tubing (Plastic One, VA). Each mouse was gently restrained by hand and the stylet removed, and 0.2 μl of cortagine or aCSF was injected through the guide cannula over a time course of 30 seconds. The injector was left in place for another 60 seconds to allow for drug diffusion.
2.6 Data analysis
Behaviors were scored both live and from videotape using the ethological analysis software “Hindsight” (Developed by Dr. Scott Weiss). A trained observer blind to drug condition scored these behaviors.
One-way analysis of variance (ANOVA) was used to analyze the MDTB and RET data for drug effects. Post hoc Newman Keuls tests were conducted for significant treatment effects relative to control means. A p value <0.05 was considered significant.
2.7 Histology (See Fig. 1)
Fig. 1.
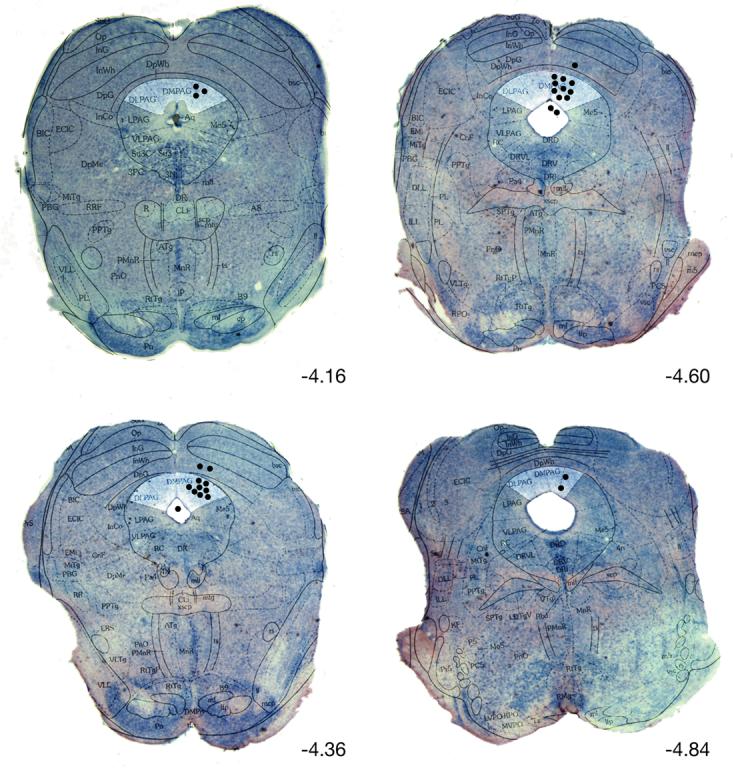
Representative photomicrographs of thionine stained coronal sections superimposed with appropriate diagrams from Paxinos and Watson's (2001) mouse brain atlas. Black dots represent infusion sites. All injections outside of the DPAG were excluded from the analysis.
Relevant abbreviations include: DLPAG dorsolateral PAG, DMPAG dorsomedial PAG, LPAG lateral PAG, VLPAG ventrolateral PAG, DpWh deep white layer of superier colliculi, DpG deep gray layer of superier colliculi, DR dorsal raphe nucleus, Aq aqueduct.
Upon completion of the experiments, the animals were deeply anaesthetized with pentobarbital (1mg/kg; Sigma, USA), infused with 0.2 μl methylene blue and transcardially perfused with 0.9% saline followed by 4% formalin. Following extraction from the skull, brains were removed, immersed (at 4 °C) in 4% formalin for 48 hours, and then transferred into 30% sucrose-formalin solution until blocking (at least 48 hours) and sectioning on a cryostat (Leica). Serial 50-μm brain sections were cut, and methylene blue staining was analyzed to locate the positions of the cannulae tip sites in the midbrain according to Paxinos and Franklin's atlas (Paxinos and Franklin, 2001). Slices between Bregma −4.16 (interaural −0.36 mm) and Bregma −4.72 (interaural −0.92) and within the dPAG region were included in the analysis. Specifically, slices that showed blue dye spread into the aqueduct, or those showing cannula tips in the superior colliculi were excluded from the analysis. The histology was done in an identical manner as in other studies from our laboratory (Carvalho-Netto, et al., 2007). Data from animals with injection sites outside the dPAG were excluded from the final analysis (n=6), leaving a total of n=7 for the control group, n=7 30ng cortagine and n=8 100ng cortagine.
3. Results
3.1 MDTB Results
Table 1 presents MDTB results. Cortagine did not produce significant differences between control and drug dose groups in any of the MDTB subtests.
Table 1.
Cortagine MDTB Table of Means
| Behaviors |
Control aCSF |
Cortagine 30 ng |
Cortagine 100 ng |
|---|---|---|---|
| Pretest activity | |||
| Line crossing | 203.14 ± 18.7 | 212.8 ± 12.8 | 208.5 ± 13.5 |
| Rears | 17.14 ± 3.08 | 19.71 ± 2.8 | 16.5 ± 4.38 |
| Predator avoidance test | |||
| Avoidance distance (cm) | 5.14 ± 4.3 | 0 ± 0 | 12.5 ± 11.78 |
| Avoidance frequency | 0.28 ± 0.23 | 0 ± 0 | 0.12 ± 0.11 |
| Escape distance (cm) | 78.2 ± 10.45 | 89 ± 11.21 | 91.5 ± 8.1 |
| Escape frequency | 4.42 ± 0.24 | 3.85 ± 0.31 | 4 ± 0.25 |
| Chase/flight test | |||
| Flight Speed (m/s) | 0.07 ± 0.015 | 0.1 ± 0.015 | 0.09 ± 0.014 |
| Stops | 0.85 ± 0.46 | 1.42 ± 0.76 | 1.66 ± 0.44 |
| Reversals | 6.14 ± 1.02 | 5.57 ± 0.7 | 5.22 ± 1.13 |
| Closed Alley Test | |||
| Approaches/withdrawals | 2.71 ± 0.35 | 2.28 ± 0.39 | 3 ± 0.37 |
| Contacts | 1.71 ± 0.47 | 2.43 ± 0.57 | 3.11 ± 0.69 |
| Jump escapes | 0.43 ± 0.24 | 0.43 ± 0.27 | 1.77 ± 0.84 |
| Freezing (s) | 0 | 0 | 0.66 ± 0.37 |
| Forced Contact test | |||
| Uprights | 12.42 ±0.6 | 12.71 ± 1.3 | 9.55 ± 1.7 |
| Vocalization | 12.71 ± 0.83 | 12.28 ± 0.97 | 11.66 ± 0.78 |
| Bites | 2.86 ± 1.02 | 2.42 ± 0.4 | 2.55 ± 0.86 |
| Jump escapes | 2.28 ± 0.53 | 2 ± 1.13 | 4.11 ± 1 |
| Post test | |||
| Line crossing | 159.42 ± 17.6 | 170.57 ± 9.34 | 194 ± 9.5 |
| Rears | 29.57 ± 4.5 | 30.71 ± 2.09 | 29 ± 2.97 |
3.2 RET Results
One-way ANOVA indicated a significant dose effect on surface duration [F(2, 19)=7.8596, p<0.01] with Neuman-Keuls post hoc revealing the 100ng cortagine group spending significantly less time on the surface when compared to the 30ng and control groups (p<0.01) (fig. 2.). Cortagine significantly increased chamber duration [F(2, 19)=8.5128, p<0.01] (fig. 2.), with the 100ng group spending more time in the chamber when compared to both controls and low dose animals (Neuman-Keuls, p<0.01). Burying duration [F(2, 19)=11.281, p<0.001] (fig. 3.) and burying frequency [F(2,19)=9.9035, p<0.05] (not shown) both showed a drug effect, with Neuman Keuls revealing a significant difference between the 100ng and other groups (burying duration, p<0.001, burying frequency, p<0.05). One-way ANOVA showed an effect of cortagine on mesh contact duration [F(2, 19)=7.0236, p<0.01] (fig. 3.) and frequency [F(2,19)=3.7559, p<0.05] (not shown) with Neuman-Keuls revealing a significant difference between the 100ng dose and the 30ng and control doses (p<0.01, p<0.05 respectively). Freezing (fig. 3.) and locomotion frequency (fig. 3.) (not shown) were not affected by cortagine.
Fig. 2.
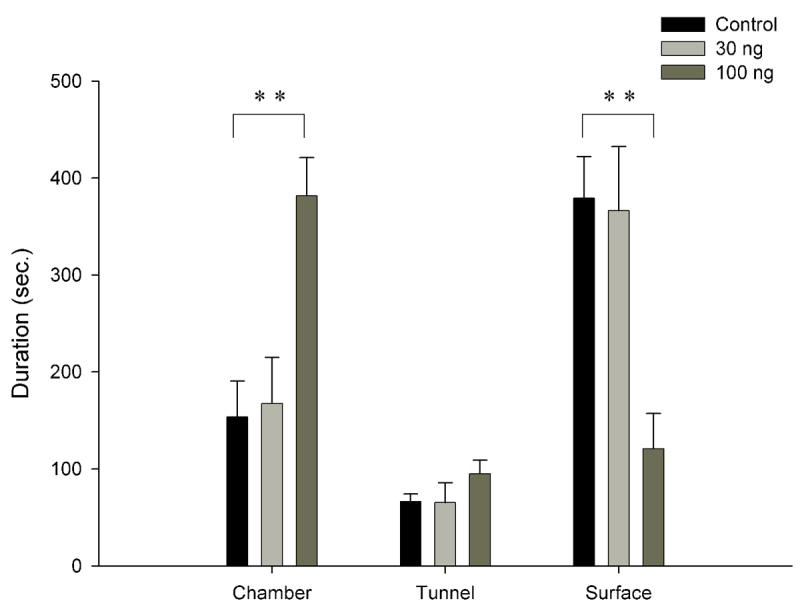
Location preference
# indicates p<0.001, ** indicates p<0.01, * indicates p<0.05
Fig. 3.
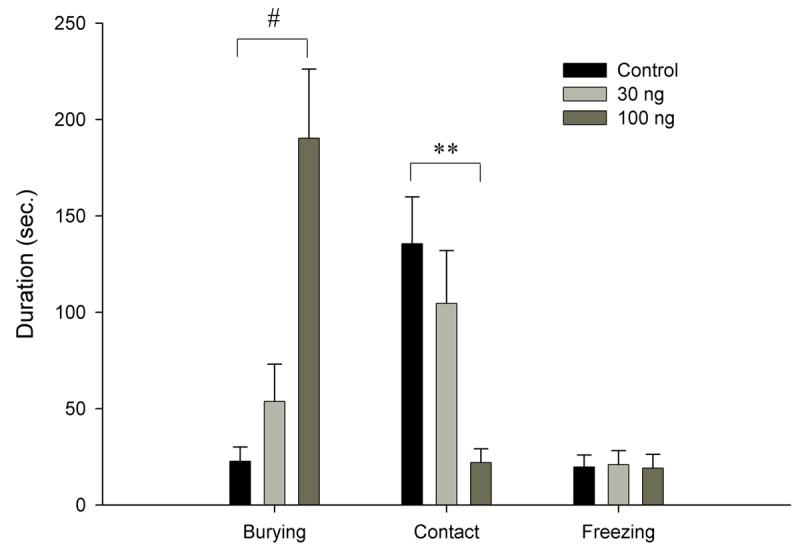
Duration of selected behaviors in the RET
# indicates p<0.001, ** indicates p<0.01, * indicates p<0.05
Table 2 presents RET data from animals showing cannulae in regions outside the dPAG, specifically the superior colliculi and the aqueduct. These data illustrate the specificity of cortagine's effect injected into the dPAG, as opposed to that at neighboring dorsal and ventral regions.
Table 2.
Selected behaviors in the RET from four subjects that received cortagine injections, yet whose cannulae were in neighboring regions to the dPAG (the superior colliculi (S.C.) and the aqueduct).
| Anatomical location |
Chamber duration (sec.) |
Tunnel duration (sec.) |
Surface duration (sec.) |
Freezing duration (sec.) |
Burying duration (sec.) |
Contact duration (sec.) |
|---|---|---|---|---|---|---|
| S. C. | 202 | 70.5 | 327.5 | 17.5 | 5.5 | 222.5 |
| S. C. | 121.5 | 47.5 | 431 | 6 | 75.5 | 85.5 |
| Aqueduct | 238.5 | 141.5 | 220 | 34 | 97.5 | 43.5 |
| Aqueduct | 174.5 | 32 | 393.5 | 22 | 25 | 131 |
4. Discussion
This study describes the effects of intra-dPAG injection of the CRF1 selective peptide, cortagine, on defensive behaviors in mice in two distinct anxiety-related models, the MDTB and the RET. While other studies have evaluated centrally administered CRF-mediated anxiety in these paradigms (Farrokhi, 2006; Yang, et al., 2006), as well as behavioral effects of a CRF agonist (Martins et al., 1997) and antagonist (Martins et al., 2000) injected directly into the dPAG in rats, this is the first in-vivo study to specifically evaluate the behavioral role of CRF1 in PAG neurons.
The MDTB and RET models differentially manipulate temporal, spatial and learned factors in order to selectively adjust both the presented threat intensity, and the prepotency of individual defensive behaviors. Consequently, the MDTB and the RET differ on several aspects. First, the MDTB tests responses to stimuli varying in intensity, ranging from a light stressor such as novelty (pretest), to an intense stressor, i.e. forced contact with a potential predator, thus providing increasing levels of anxiety/fear over the subsets of the test, whereas the RET evaluates predator avoidance, defensive coping strategies, and risk assessment to a constant threat stimulus. In particular, subjects in the RET are presented with an amphetamine treated rat behind a mesh screen, while the MDTB includes contact with a predator, a factor that may potentiate different behaviors such as defensive threat and attack. Secondly, while behaviors in the MDTB are tightly constrained by the apparatus and threat manipulations, which do not allow subjects to escape or hide from the threat, subjects in the RET may modulate their behaviors in a constant, and familiar context (habituation, see methods section) by escaping to the “home chamber” via a tunnel, thus enabling relative controllability. Thirdly, the behaviors exhibited in the MDTB consist of reactions to both a novel context and to threatening stimuli, whereas the RET isolates reactions to a predator stimulus by previous habituation to the context, thus minimizing novelty-associated anxiety. Lastly, temporal restrictions in the MDTB subtests may selectively modulate time dependent risk assessment, which may in turn influence displayed defensive behaviors. However, animals in the RET are temporally (uninterrupted 10 minutes of testing) and spatially (via the tunnel), able to assess a potential threat and act accordingly. In summary, enhanced threat together with strict temporal and spatial restrictions provide less controllability for the mouse in the MDTB context, a factor which has been known to render situations more stressful (for review see (Maier and Watkins, 2005)).
Although previous findings from our lab show a mild effect for oCRF injected into the dPAG in the MDTB (Carvalho-Netto et al., 2007), these effects were not seen for cortagine in the present series of experiments. The lack of effects may be related to a number of factors. First, although oCRF preferentially activates CRF1, CRF2 are activated as well (Tezval et al., 2004). As a result, an interaction between CRF1 and CRF2 mediated actions might contribute to a broader range of defensive behaviors, including those elicited by both high and low-level stimulus intensities, as in the case with the MDTB. In addition, although similar doses were used in the two studies, cortagine was shown to potentiate anxiety-like behavior in the EPM at a lower dose than oCRF (Tezval et al., 2004). Secondly, cortagine's enhanced potency may have created a ceiling effect at the 100ng dose, suggesting the value of examination of doses between 30ng and 100ng. Thirdly, cortagine was infused into regions significantly (t-test, p=0.0004) more rostral than our prior infusions of oCRF into the dPAG (Carvalho-Netto et al., 2007). The averages were: Carvalho-Netto et al. study= −4.67 anterior posterior axis, present study= −4.47 anterior posterior axis. Activation of rostral regions of the dPAG (implicated in confrontational defense) is characterized by decreased blood flow to skeletal muscle and viscera, with increased flow to extracranial vascular beds. On the other hand, activation of caudal regions of the dPAG (implicated in flight) results in increased blood flow to the limbs and decreased flow to viscera and to vasculature supplying muscle and skin in the extracranial regions of the oro-facial region (for review see (Keay and Bandler, 2001)). Hence, our lack of results may be attributable to a ceiling effect of CRF1 activation on rostral-dPAG controlled confrontational defense, with the relatively low levels of flight in the chase/flight test and high levels of both vocalizations and bites in the forced contact subtest strengthening this hypothesis.
The RET has been shown to produce defensive reactions toward a predator in CD-1 mice (Yang et al., 2004), and icv administration of oCRF exacerbates these defenses (Farrokhi, 2006). Farrokhi (2006) found that oCRF substantially increased avoidance of the predator stimulus, as measured by increased chamber duration, decreased surface duration and decreased mesh contact duration. Similarly, oCRF injected into the dPAG selectively increased measures of avoidance, decreasing risk assessment (as measured by stretch attend postures), although not affecting either burying or freezing (Carvalho-Netto et al., 2007).
Here, major measures in the RET were altered by injection of 100ng cortagine. Firstly, spatial measures, namely location preference and duration of contact with the mesh were affected. These animals displayed a clear preference for the chamber, while significantly reducing time spent on the surface. Furthermore, the high dose of cortagine significantly affected mesh-contact duration. These behavioral changes implicate avoidance as a preferred defensive strategy in animals injected with 100ng of cortagine. Secondly, cortagine robustly increased the duration of burying, an activity that has been shown to respond consistently to anxiolytic drugs (De Boer and Koolhaas, 2003). It is noteworthy to mention that locomotion frequency, a control for selective motor effects, did not differ between the groups, suggesting the variation in behaviors may be attributed to a change in anxiety.
In earlier studies, rats that received an intra-PAG injection of a CRF peptide and were subsequently tested in the EPM showed an increase in closed arm duration, while locomotion measures were unaffected (Martins et al., 1997). These results point to a significant effect of CRF on avoidance behaviors in the EPM. The RET and the EPM enable subjects to control the perceived intensity of the stimulus by moving toward or away from it, and the “anxiety” measures taken reflect these actions. Considering these similarities in defense across both situations the anxiogenic-like effects of intra-PAG oCRF administration in the RET (Carvalho-Netto et al., 2007) agree with prior observations in the EPM (Martins et al., 1997). The present RET results corroborate this analysis and further specify the mediating mechanisms of CRF1 in these effects.
In summary, the present results are in partial agreement with previous findings of an excitatory effect of CRF in dPAG-induced emotional responses to a stressor. They further implicate CRF1 as a primary receptor mediating these effects.
Acknowledgements
The authors gratefully acknowledge Dr. J. Spiess for his guidance and generous supply of the peptide. This work was supported by NIH grant U5 NS039406.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yoav Litvin, Department of Psychology, University of Hawaii at Manoa.
Nathan S. Pentkowski, Department of Psychology, University of Hawaii at Manoa
D. Caroline Blanchard, I. John A. Burns School of Medicine, Department of Genetics and Molecular Biology, II. Pacific Biomedical Research Center, University of Hawaii at Manoa..
Robert J. Blanchard, Department of Psychology, University of Hawaii at Manoa
REFERENCES
- Antoniou K, Kafetzopoulos E. A comparative study of the behavioral effects of d-amphetamine and apomorphine in the rat. Pharmacol Biochem Behav. 1991;39(1):61–70. doi: 10.1016/0091-3057(91)90398-l. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23(2):189–96. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439(1-2):95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav Brain Res. 1985;15(2):107–19. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res. 1996;107:285–300. doi: 10.1016/s0079-6123(08)61871-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379–89. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Griebel G. Current Protocols in Neuroscience. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Behavioral Neuroscience. Vol. 3. John Wiley & Sons, Inc.; 2005. pp. 8.19.1–8.19.20. [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463(1-3):97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Williams G, E.M.C. L, Blanchard RJ. Taming of wild Rattus norvegicus by lesions of the mesencephalic central gray. Physiological Psychology. 1981;9:157–163. [Google Scholar]
- Bowers LK, Swisher CB, Behbehani MM. Membrane and synaptic effects of corticotropin-releasing factor on periaqueductal gray neurons of the rat. Brain Res. 2003;981(1-2):52–7. doi: 10.1016/s0006-8993(03)02886-5. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Anseloni VZ, Pandossio JE, De Araujo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23(6):863–75. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Coimbra NC, Borges PC. Effects of morphine and midazolam on reactivity to peripheral noxious and central aversive stimuli. Neurosci Biobehav Rev. 1990;14(4):495–9. doi: 10.1016/s0149-7634(05)80073-9. [DOI] [PubMed] [Google Scholar]
- Brandao ML, de Aguiar JC, Graeff FG. GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol Biochem Behav. 1982;16(3):397–402. doi: 10.1016/0091-3057(82)90441-5. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Tomaz C, Borges PC, Coimbra NC, Bagri A. Defense reaction induced by microinjections of bicuculline into the inferior colliculus. Physiol Behav. 1988;44(3):361–5. doi: 10.1016/0031-9384(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Vasquez EC, Cabral AM, Schmitt P. Chlordiazepoxide and morphine reduce pressor response to brain stimulation in awake rats. Pharmacol Biochem Behav. 1985;23(6):1069–71. doi: 10.1016/0091-3057(85)90116-9. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71(3):481–91. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44(3):297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999;10(2):413–8. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360(2):213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cardoso SH, Coimbra NC, Brandao ML. Defensive reactions evoked by activation of NMDA receptors in distinct sites of the inferior colliculus. Behav Brain Res. 1994;63(1):17–24. doi: 10.1016/0166-4328(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Carrive P. Functional organization of PAG neurons controlling regional vascular beds. In: Bandler R, Depaulis A, editors. The midbrain periaqueductal grey matter: functional, anatomical and immunohistochemical organization. Plenum Press; New York: 1991. pp. 67–100. [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58(1-2):27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Litvin Y, Nunes-De-Souza RL, Blanchard DC, Blanchard RJ. Effects of Intra-PAG infusion of ovine CRF (oCRF) on defensive behaviors in Swiss-Webster Mice. Behav Brain Res. 2007;176(2):222–9. doi: 10.1016/j.bbr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra NC, Brandao ML. Effects of 5-HT2 receptors blockade on fear-induced analgesia elicited by electrical stimulation of the deep layers of the superior colliculus and dorsal periaqueductal gray. Behav Brain Res. 1997;87(1):97–103. doi: 10.1016/s0166-4328(96)02267-x. [DOI] [PubMed] [Google Scholar]
- Coimbra NC, Tomaz C, Brandao ML. Evidence for the involvement of serotonin in the antinociception induced by electrical or chemical stimulation of the mesencephalic tectum. Behav Brain Res. 1992;50(1-2):77–83. doi: 10.1016/s0166-4328(05)80289-x. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463(1-3):145–61. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90(2):307–18. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Pechnick RN, Liebeskind JC. Relationship between analgesia and cardiovascular changes induced by electrical stimulation of the mesencephalic periaqueductal gray matter in the rat. Brain Res. 1988;451(1-2):326–32. doi: 10.1016/0006-8993(88)90778-0. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Bandler R, Depaulis A, editors. The midbrain periaqueductal grey matter: functional, anatomical and immunohistochemical organization. Plenum Press; New York: 1991. pp. 151–173. [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984;306(1-2):105–23. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Farrokhi C. Comparative effects of the CRF agonist, ovine CRF, and CRF antagonist, astressin, on homecage behavior patterns and defense in the mouse. Master's, University of Hawaii at Manoa; 2006. [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77(3):465–9. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Serotonergic systems. Psychiatr Clin North Am. 1997;20(4):723–39. doi: 10.1016/s0193-953x(05)70342-7. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13(3):451–60. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4- methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301(1):333–45. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Swartz CL, Davenport PW. Respiratory response to activation or disinhibition of the dorsal periaqueductal gray in rats. J Appl Physiol. 2003;94(3):913–22. doi: 10.1152/japplphysiol.00740.2002. [DOI] [PubMed] [Google Scholar]
- Jenck F, Schmitt P, Karli P. Morphine injected into the periaqueductal gray attenuates brain stimulation-induced effects: an intensity discrimination study. Brain Res. 1986;378(2):274–84. doi: 10.1016/0006-8993(86)90930-3. [DOI] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology (Berl) 1998;138(2):124–32. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25(7-8):669–78. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Periaqueductal Gray. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- Liebeskind JC, Guilbaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50(2):441–6. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17(9):3364–78. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29(4-5):829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Martins AP, Marras RA, Guimaraes FS. Anxiogenic effect of corticotropin-releasing hormone in the dorsal periaqueductal grey. Neuroreport. 1997;8(16):3601–4. doi: 10.1097/00001756-199711100-00036. [DOI] [PubMed] [Google Scholar]
- Martins AP, Marras RA, Guimaraes FS. Anxiolytic effect of a CRH receptor antagonist in the dorsal periaqueductal gray. Depress Anxiety. 2000;12(2):99–101. doi: 10.1002/1520-6394(2000)12:2<99::AID-DA6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Melo LL, Cardoso SH, Brandao ML. Antiaversive action of benzodiazepines on escape behavior induced by electrical stimulation of the inferior colliculus. Physiol Behav. 1992;51(3):557–62. doi: 10.1016/0031-9384(92)90179-6. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Res. 1991;545(1-2):17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502(1):61–6. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Second Edition Academic Press; San Diego: 2001. [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302(1):145–52. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24(2-3):115–95. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24(2-3):197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am J Physiol. 1979;237(5):R278–84. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Povoa RM, Costa AL, Caldellas AV, Tufik S, Bittencourt AS. Functional specializations within the tectum defense systems of the rat. Neurosci Biobehav Rev. 2005;29(8):1279–98. doi: 10.1016/j.neubiorev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25(7-8):627–36. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci U S A. 2004;101(25):9468–73. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: implications of the novel CRF1 agonist cortagine. Neurosci Biobehav Rev. 2005;29(8):1323–33. doi: 10.1016/j.neubiorev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Van Erp AM, Kruk MR, Meelis W, Veening JG. Periaqueductal gray lesions do not affect grooming, induced electrically in the hypothalamic paraventricular area in the rat. Behav Brain Res. 1993;59(1-2):95–101. doi: 10.1016/0166-4328(93)90155-j. [DOI] [PubMed] [Google Scholar]
- Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav. 2004;81(3):465–73. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yang M, Farrokhi C, Vasconcellos A, Blanchard RJ, Blanchard DC. Central infusion of Ovine CRF (oCRF) potentiates defensive behaviors in CD-1 mice in the Mouse Defense Test Battery (MDTB) Behav Brain Res. 2006;171(1):1–8. doi: 10.1016/j.bbr.2006.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


