Abstract
Dysregulation of hedonic processing, in which seeking of drug reward becomes more desirable than seeking natural rewards, like food, sex, and novelty, is a consequence of chronic drug exposure and potentially leads to escalating drug usage and addiction. Here, we investigated the effects of chronic cocaine treatment (10 days of escalating doses of cocaine, 10-30 mg/kg) and multiple forced abstinence periods (2, 3 or 5 weeks) on the acute rewarding properties of either cocaine (10 mg/kg) or novel-objects using the conditioned place preference procedure. Following all cocaine withdrawal periods, cocaine preference was significantly elevated while novel object preference was abolished compared to saline-treated rats. At the earliest withdrawal period, these behavioral changes were accompanied by elevations in FosB-like immunoreactive staining in the basolateral amygdala (BLA) and nucleus accumbens shell (NAc-Sh) and core (NAc-C). FosB staining in all three brain areas correlated positively with cocaine preference, but negatively with novelty preference. After 5 weeks of withdrawal, FosB staining was only elevated in the NAc-Sh and again correlated positively with elevated cocaine preference but negatively with decreased novelty preference. These data indicate that alterations in the expression of FosB-like transcription factors in the NAc can predict the dysregulation of hedonic processing that occurs during protracted withdrawal from cocaine.
Keywords: novelty, place conditioning, delta FosB, withdrawal, basolateral amygdala, anhedonia
The chronic intake of psychostimulants has devastating consequences on the lives of those individuals who become addicted. Abstinence from chronic cocaine causes anxiety, dysphoria and intense drug craving that can persist for long periods of time (Louie et al., 1989, Gawin, 1991). These symptoms are thought to result from a dysregulation in reward function caused by chronic excessive stimulation of the brain reward circuitry during periods of elevated drug intake (Di Chiara, 1998, Koob and Le Moal, 2001, Koob et al., 2004). The down regulation of brain reward function during psychostimulant abstinence causes anhedonia or a loss of satisfaction with normally rewarding life experiences. The persistence of these symptoms is a primary obstacle to successful treatment of psychostimulant addiction and one of the primary causes of relapse (Ehrman et al., 1992, Robbins and Ehrman, 1998).
Over the last ten years, accumulating evidence has shown that the alterations in reward function following chronic drug exposure may be due to long-term changes in gene regulation occurring within specific areas of the reward circuitry (Nestler, 2004). For example the nucleus accumbens (NAc) is a brain region that functions as a critical link between cortical and hypothalamic/brainstem circuits controlling the intake of primary rewards like food (Kelley, 2004) as well as the learning and execution of adaptive motor responses involved in the acquisition of such rewards (Cardinal, 2002, Robbins and Everitt, 2002). These two functions correspond to the two major subdivisions of the NAc, the shell (NAc-Sh) that is involved in reward motivation and the core (NAc-C) that is involved in the learning and execution of motor responses. Chronic exposure to virtually all drugs of abuse have been found to induce the accumulation of high levels of a stable truncated splice variant of the fosB gene, ΔFosB in the NAc (Hope et al., 1994b, Nye et al., 1995, Moratalla et al., 1996, Nye and Nestler, 1996, Hiroi et al., 1997, Pich et al., 1997). Unlike FosB, which is rapidly induced and degraded, ΔFosB is induced after repeated stimulation, is highly stable and can persist for long periods of time (weeks) even after cessation of drug intake (Nestler, 2001, McClung et al., 2004). ΔFosB then is a potential candidate for inducing the persistent changes in reward function that occur during drug abstinence. ΔFosB accumulation causes a persistent increase in transcriptionally active dimers of Fos and related Jun-family proteins known as activator protein 1 (AP-1) complexes (Hope et al., 1992) and can act as both a transcriptional activator and repressor (McClung and Nestler, 2003). Thus ΔFosB can modify gene expression in the NAc for an extended period of time.
Chronic cocaine treatment increases ΔFosB staining in the medial and lateral caudate putamen (Andersson et al., 2003), NAc-Sh and NAc-C, amygdala, as well as some areas of the frontal cortex (McClung et al., 2004). The increased expression of ΔFosB in the NAc after cocaine exposure can have functional consequences as overexpression of ΔFosB protein in the NAc has been shown to increase cocaine-seeking behaviors (Kelz, 1999, Olausson et al., 2002, Colby et al., 2003, Kumar et al., 2005, Zachariou et al., 2006).
In the current series of experiments we looked for changes in preference behavior for both drug (cocaine) and natural reward (novelty) following abstinence from a two-week exposure to escalating doses of cocaine. Rats show robust preferences for environments paired with novel objects (Besheer et al., 1999, Bevins and Bardo, 1999, Bevins et al., 2002) and recently it has been shown that this reward can be used as a sensitive measure of the anhedonia associated with nicotine abstinence (Besheer and Bevins, 2003, Bevins and Besheer, 2005). We selected two time points during forced abstinence (two and five weeks), which were previously found to be important for anhedonic responses after withdrawal from chronic drug exposure (Harris et al., 2001, Harris and Aston-Jones, 2003b, Harris and Aston-Jones, 2003a), to measure changes in reward function. At the end of each experiment, we examined FosB/ΔFosB staining using immunohistochemistry in the NAc-Sh, NAc-C and basolateral amygdala (BLA) and correlated the amount of staining in each area with preference scores to determine if changes in preference behavior were related to FosB/ΔFosB staining.
EXPERIMENTAL PROCEDURES
Subjects
Male Sprague-Dawley rats (250-300 g; Harlan, Indianapolis, IN, USA; n = 72) were used in all experiments. Rats were group-housed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and all efforts were made to minimize the number of rats used and their suffering. Rats were maintained on a 12-h light/dark cycle with food and water available ad libitum. All animal procedures were also approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Drugs
Cocaine was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in sterile saline (10-30 mg/ml) and administered via i.p. injection.
Cocaine Treatment and Forced Abstinence
Rats were given daily escalating doses of cocaine (10, 15, 20, 25, and 30 mg/kg) for 10 days with each dose given twice on consecutive days. Previously we have found this treatment procedure to produce measurable long-term changes in behavior reflective of a cocaine abstinence syndrome (Harris et al., 2001). Rats were returned to their home cage after each injection. Behavioral testing began after either two or five weeks of abstinence from cocaine treatment. Saline treated rats were given similar volumes of saline injections at the same time and were handled identically to the cocaine treated subjects.
Place Conditioning Procedure
Conditioning and testing occurred in a Plexiglas apparatus that consisted of two distinct compartments separated by a Plexiglas divider. Each compartment was equipped with photocells to record time and activity automatically (MED Associates). One compartment had a grid floor with black walls, and the second compartment had a mesh floor with black and white stripes on the walls. On the first day, rats (n = 30 for the two week withdrawn group; n = 16 for the five week withdrawn group) were allowed to freely explore the apparatus for 15 min, and the amount of time spent in each compartment was recorded. None of the rats had an initial bias for either compartment, and each was randomly assigned to a compartment for novel-object conditioning in a balanced design. Conditioning began one day after this preconditioning day. For novel-object conditioning, we used parameters reported in previous work (Bevins and Bardo, 1999, Bevins et al., 2002). For these experiments, rats require a minimum of 8 pairings with 8 novel objects to produce a preference that is similar in magnitude to three pairings with cocaine. Rats received 8 consecutive days of pairings in which they were confined to a chamber with a novel object for 10 minutes followed 1 hour later by confinement in the other chamber with no object for 10 minutes. Exposure to the novel object chamber was alternated each day so that if they were exposed to the novel object chamber first on one day they were exposed to the novel object chamber during the second session on the consecutive day. All rats received one exposure to each of the 8 different objects in the same order: white sock, newspaper, cotton ball, paper bow, sponge, egg carton, plastic ball and wood chip. One day following the last conditioning session, all rats were given a preference test with free access to both compartments. The amount of time spent in each chamber was recorded for 15 min. Novel object conditioning was performed at both two and five week withdrawal periods.
In addition, we determined whether cocaine-withdrawn rats showed differences in interaction time with the novel objects. A portion of rats from each group (n=8) were video taped during conditioning sessions. The tapes were scored later by a blind observer and the amount of time each animal spent physically interacting with the objects during each of the eight sessions was noted. Indirect contact, such as accidental contact with the object, was excluded from the count.
To establish whether cocaine-withdrawn rats had greater preferences for cocaine-associated cues than novelty-paired cues, a portion of the two-week withdrawn cocaine and saline rats (n = 14) underwent extinction after novelty conditioning. During extinction rats were tested for preference until preference for the novel object-paired chamber was below 100 seconds (2-3 days). After extinction they received cocaine place preference conditioning. As the novelty conditioning occurred at two weeks withdrawal, the cocaine conditioning began during the third week of cocaine withdrawal. For these experiments, rats received 3 consecutive days of pairings in which they were given injections of either saline paired with the former novel object-paired environment or cocaine (10 mg/kg) paired with the former non-object environment. Two 30-minute conditioning sessions occurred each day with a four-hour interval between sessions. The timing of the cocaine injections was alternated so that rats given cocaine while in one chamber in the morning were given saline in the opposite chamber in the afternoon, and on subsequent days received saline in the morning and cocaine in the afternoon. To determine if prior novel object place conditioning affected subsequent cocaine conditioning two other cocaine-conditioned groups were tested. Both of these additional groups were withdrawn from cocaine treatment for two (n = 14) and five weeks (n = 12). No novel object conditioning occurred prior to the cocaine conditioning for these groups.
FosB Immunoreactivity
Two hours after the preference tests, novelty- or cocaine-conditioned rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with ice-cold 0.9% saline followed by ice-cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4. The brains were removed and stored overnight in 4% paraformaldehyde. They were then transferred to a 20% sucrose solution and stored at 4°C for 3 days. Coronal sections (40 um-thick) were cut using a freezing microtome. Sections from saline and cocaine-withdrawn rats were processed together to equalize staining between groups. Sections were placed in a solution of 0.01 M PBS with 0.3% Triton-X added (PBS-Tx, pH 7.4) containing 2% normal donkey serum for 3 hours. Sections were incubated overnight at room temperature in this same solution of 0.01 M PBS with 0.3% Triton-X added (PBS-Tx, pH 7.4) containing 2% normal donkey serum with the addition of primary antibody that was raised against an internal region of FosB and recognizes both FosB and ΔFosB (sc-48; 1:500 Santa Cruz Biotechnology, Santa Cruz, CA, USA). Sections were rinsed three times in PBS-Tx and then incubated for 2 hours with the secondary antibody (biotinylated goat anti-rabbit 1:500, Jackson ImmunoResearch Laboratories, West Grove, PA USA). After three rinses in PBS-Tx, sections were transferred to an avidin-biotin complex (1:1000, Jackson ImmunoResearch) for 1.5 h. Sections were again rinsed two times with PBS-Tx and once with 0.05 M Tris buffer. FosB-like staining was visualized by placing the tissue in 3,3′-diaminobenzidine (DAB, 0.02%, Sigma, St Louis, MO) with 0.0002% H2O2 and 0.6% nickel ammonium sulfate in 0.01 M Tris buffer for 2.5 min. This reaction was arrested by immediate transfer into 0.05 M Tris buffer. The sections were mounted on gelatin-coated slides, stained with neutral red to identify specific structures, dehydrated through graded alcohols, cleared in xylene, and coverslipped with Permount.
Quantification of FosB-positive cells was done using Openlab image processing software (Improvision, Ltd.; Coventry England) on a Macintosh computer that was linked to a microscope and digital camera. Color images of the areas of interest were taken and saved to a disk. The numbers of FosB-positive nuclei in regions of interest were counted with a point counter tool on the saved image. This tool simultaneously marked and counted each cell so that no cells could be counted twice and the total of the number of cells counted was available. One section at each level was randomly selected from each animal. Fos-positive neurons in the structures of interest from both the right and left hemispheres were counted by a blind observer and averaged into a single score. The levels chosen corresponded to the following distances from bregma NAc-Sh and NAc-C = +1.70 mm and BLA = −2.80 mm (Paxinos and Watson, 1998).
Data analysis
Place conditioning data were analyzed by calculating the time spent in the cocaine- or object-paired chamber minus the time spent in the saline- or non-object chamber to determine chamber preference. The resulting difference (preference score) was compared between cocaine-withdrawn and saline groups using a two-way analysis of variance (ANOVA) (drug treatment and length of forced abstinence). Fos data were analyzed using a t-test comparing saline with cocaine treated rats at each time point after withdrawal. Follow-up comparisons were done using Fisher's PLSD for the two way ANOVA's. Correlations between preferences scores and Fos levels in the various brain areas in each group were performed using the Pearson's product-moment correlation coefficient (r). For correlations, data from both cocaine and saline treated rats were combined for each experimental treatment.
RESULTS
Place conditioning
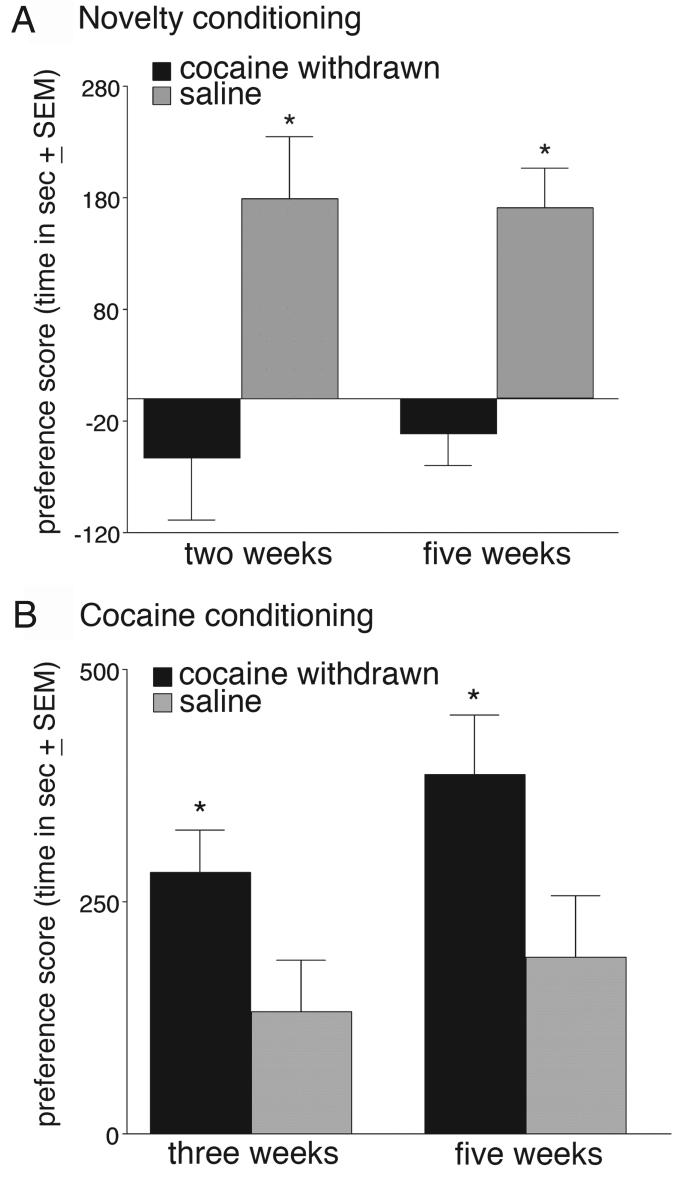
Figure 1a shows the results of the novelty place conditioning at both two and five weeks after forced cocaine abstinence. Cocaine treated rats at both two and five weeks post-withdrawal failed to develop preferences for the novel object-paired environment. A two-way ANOVA (drug treatment versus time post drug treatment) revealed a significant main effect for drug treatment (F(1,42)=19, p<.01), reflecting the fact that at both two and five weeks of withdrawal the behavior in the cocaine groups was similar. Follow-up tests revealed that saline treated rats had significantly higher preference scores for novel objects than cocaine-withdrawn rats. A comparison between pre- versus postconditioning preference scores in each group revealed that both two and five week saline groups showed significantly greater preferences for the novel object-paired environment after conditioning than pre-conditioning (two weeks: t(14)=−2.5, P>05; five weeks t(7)=3.3, P<.01), whereas cocaine-withdrawn rats did not (two weeks, t(14)=−1.0, P=.22; five weeks t(7)=−1.0, P=.32). No significant differences in locomotor activity were seen between the groups on the test day (F(1,42)=0.3, P=.90. In terms of object interaction, although cocaine-withdrawn rats spent overall less time (∼60 sec) than saline treated subjects interacting with the objects at both two and five weeks of withdrawal, they were not significantly different (two week: t(14)=0.8, P=.12; five week: t(14)=1.1, P=.23).
Fig. 1.
Conditioned place preference scores for the novel object-paired (A) or cocaine-paired (B) environments. Data are expressed as the mean time (in seconds) spent in the paired side minus the mean time spent on the non-paired side on the test day. *p<.01. Cocaine groups indicate rats that were given 10 days of cocaine injections followed by various withdrawal periods (two, three and five weeks). Saline groups indicate rats that were given a similar series of saline injections. Rats in the three-week cocaine group are a subgroup (n=14) of the two-week novelty conditioned group (n=30).
Figure 1b shows the results of cocaine conditioning. Cocaine-withdrawn rats showed larger preferences for cocaine-associated cues compared to saline rats at both the three and five week time periods. The two-way ANOVA revealed a significant main effect for drug treatment (F(1,22)=9.0, P<.01) and follow-up tests revealed that cocaine treated rats had greater preferences for the cocaine-paired environment than saline treated rats (P<.01). A comparison between pre- versus post-conditioning preference scores in each group revealed that both saline and cocaine groups showed significantly greater preferences for the cocaine-paired environment after conditioning than pre-conditioning (three weeks: saline t(6)=−2.5, P<.05; cocaine t(6)=−5.5, P<.01; five weeks saline t(5)=2.9, P<.05; cocaine t(5)=7.0, P<.01). No significant group differences were seen in locomotor activity on the test day (F(1,22)=0.6, P=.75).
To determine if prior novelty conditioning affected cocaine preference in the three week-withdrawn groups or whether it was the difference in time since cocaine treatment (i.e., three weeks of abstinence versus two weeks of abstinence), we directly compared cocaine conditioning following two weeks of forced cocaine abstinence (with no prior novelty conditioning) with cocaine conditioning that occurred after three weeks of forced abstinence (the group with prior novelty conditioning). A t-test between preference scores for these groups revealed no significant difference between the groups (t(12)=.68, P=.41; group means two week = 270±30; three week = 282±45). A t-test comparing the two week withdrawn rats with their saline-injected controls revealed that the two-week cocaine-treated rats had significantly greater preferences for the cocaine-paired environment than the saline treated rats (t(12)=−2.5, P<.05). These data indicate that at two, three and five weeks post withdrawal, cocaine-treated rats maintained a significantly greater preference for cocaine-associated cues while at similar time points (i.e., two and five weeks of withdrawal) they failed to show preference for novel objects.
FosB measurements
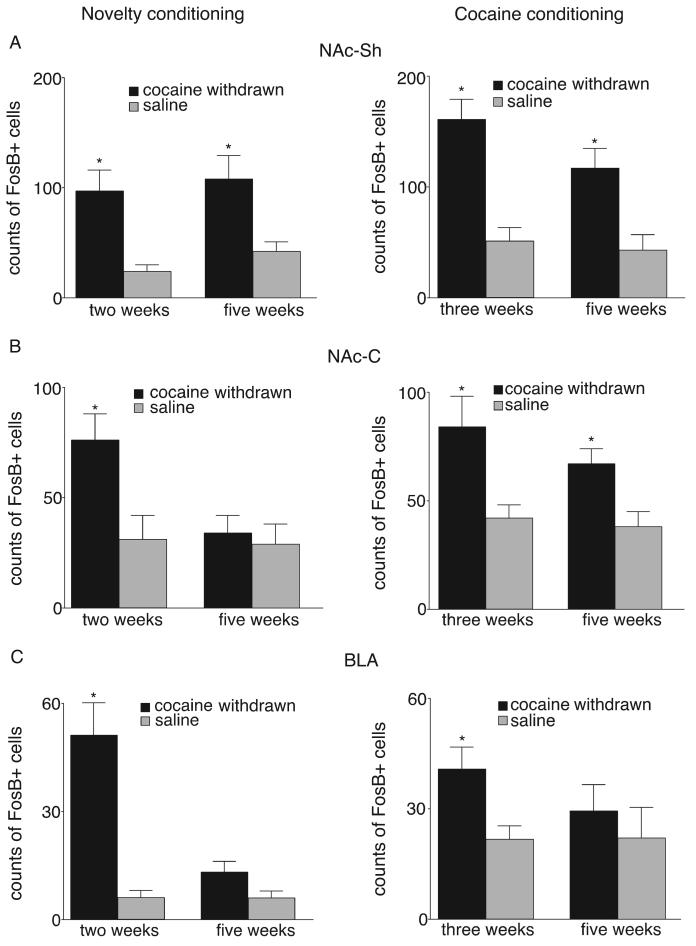
For each experiment the tissue from each separate group of cocaine-withdrawn rats was processed with their saline controls and analyzed using a t-test between the two groups. In two-week withdrawn novelty conditioned rats (n = 16, i.e., rats that were not used in the three week-withdrawn cocaine conditioning experiment), the cocaine-treated rats showed significant elevations in FosB staining in the NAc-Sh (t(14)=3.4, P<.01), NAc-C (t(14)=2.6 P<.01) and BLA (t(14)=4.8, P<.01) (Figures 2 and 3). Similar significant elevations in FosB counts were found in the three-week cocaine withdrawn rats (n = 14) in all three brain areas following cocaine conditioning (NAc-Sh t(12)=5.1, P<.01; NAc-C t(12)=2.7, P<.05; BLA t(12)=2.7, P<.05; Figure 3).
Fig. 2.
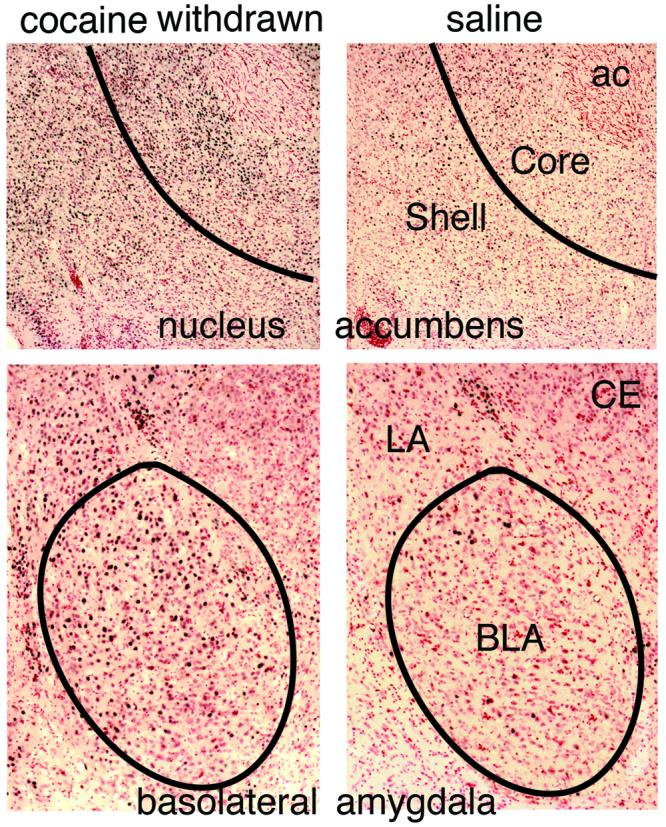
Photomicrographs of coronal brain sections from two-week-withdrawn cocaine (on left) and saline (on right) rats after novelty preference testing. Sections were stained for FosB/ΔFosB (black nuclei) and then counterstained with neutral red. ac: anterior commissure, BLA: basolateral amygdala, CE: central amygdala, LA: lateral amygdala. In all cases medial is on the right.
Fig. 3.
Counts of FosB/ΔFosB-positive neurons in the nucleus accumbens shell (NAcSh, A), core (NAc-C, B), and basolateral amygdala (BLA, C) for cocaine withdrawn and saline rats at two time points. Bar graphs on the left depict novel object conditioned rats and bar graphs on the right depict cocaine-conditioned rats. Note that FosB-like staining remains elevated for novelty conditioning at 5 weeks abstinence only in the NAc-Sh. *p<.05.
In the five-week cocaine-withdrawn group the numbers of FosB cell counts in both the BLA and NAc-C in novelty-conditioned rats dropped dramatically back to baseline levels seen in the saline controls (BLA t(14)=1.9, P=.07; NAc-C t(14)=0.4, P=.69). In contrast, FosB staining in the NAc-Sh was still significantly elevated in these 5 week-withdrawn subjects conditioned with novel objects (t(14)=2.8, P<.01). In the five-week withdrawn cocaine group given additional injections of cocaine during the three days of cocaine conditioning, the levels of FosB staining were elevated in both the NAc-Sh (t(10)=3.1, P<.01) and NAc-C (t(10)=2.9, P<.01). Additional cocaine treatments, however, did not significantly re-elevate FosB staining in the BLA (t(10)=0.8, P=.41).
Correlations
Table 1 shows the R-values for correlations between FosB levels in each brain area and preference scores for both the novel object- and cocaine-paired environments. At two-weeks of withdrawal, significant negative correlations were found between levels of FosB staining and novelty preference scores in all three brain areas. These data indicate that high FosB staining in each brain area predicted lower preferences for the novel object-paired environment. In contrast, significant positive correlations were found between preferences for cocaine-paired environments and elevated FosB staining in each brain area, indicating that higher FosB staining predicted higher cocaine preference. In the five-week withdrawn group a significant negative correlation between novelty preference scores and FosB staining was found only in the NAc-Sh. Significant positive correlations with cocaine preference in the 5 week-withdrawn group were found with FosB staining in NAc-Sh and NAc–C but not BLA. Locomotor activity, which was also measured during the preference testing, did not correlate with preference scores or FosB levels in any brain area tested (P>0.50).
Table 1.
R values for correlations between FosB staining and preference scores.
| NAc Shell | NAc Core | BLA | |
|---|---|---|---|
| Withdrawn two weeks Novelty conditioned |
−.77 P<.01 | −.87 P<.01 | −.82 P<.01 |
| Withdrawn three weeks Cocaine conditioned |
.82 P<.01 | .83 P<.01 | .71 P<.01 |
| Withdrawn five weeks Novelty conditioned |
−.86 P<.01 | −.58 NS | −.54 NS |
| Withdrawn five weeks Cocaine conditioned |
.90 P<.01 | .86 P<.01 | .58 NS |
NAc = nucleus accumbens, BLA = basolateral amygdala
DISCUSSION
The major findings of this study were that previous exposure to repeated cocaine significantly abolished the development of preference for novel object reward at both two and five weeks post-treatment. Contrary to this finding, prior repeated cocaine exposure significantly elevated preferences for a cocaine-associated environment during the same time period post-treatment. These findings suggest that prior cocaine exposure produces long-term changes in brain reward function such that preferences for natural rewards like novel objects are diminished while preferences for drug reward are augmented. Furthermore, cocaine withdrawn rats showed significant elevations in FosB-like staining in the NAc-Sh that reliably correlated with the behavioral outcomes following both withdrawal periods. Therefore, as FosB staining increased in the NAc-Sh, preference for novel objects decreased and preference for cocaine increased. In contrast, although FosB staining was also significantly elevated in the NAc-C and BLA of two-week withdrawn rats, by five weeks of cocaine withdrawal FosB levels had returned to baseline in these areas despite the fact that opposing changes in novel object and cocaine preferences had not. Thus, FosB levels in the NAc-C and BLA did not consistently predict changes in reward function across all conditions.
As there are no antibodies available to distinguish between FosB and the different isoforms of the truncated FosB protein (ΔFosB), we cannot definitely say which form of the protein we are measuring. Previous research, however, has indicated chronic cocaine exposure decreases the expression of intermediate early genes like c-Fos, JunB and FosB while alternatively increasing the accumulation of ΔFosB proteins which are highly stable and long lasting (Hope et al., 1994a, Moratalla et al., 1996, Chen et al., 1997, Hiroi et al., 1997). Acutely, cocaine transiently induces FosB expression for about six hours (Hope et al., 1992, Chen et al., 1995). After chronic exposure to cocaine, acute doses of cocaine reportedly no longer elevate FosB levels but instead lead to elevations in ΔFosB (Hope et al., 1992, Chen et al., 1995, Chen et al., 1997). Based on this evidence, it can be assumed that we are primarily measuring ΔFosB and not FosB especially in our cocaine-withdrawn rats. In addition, exposure to the testing environment is unlikely to underlie the enhanced activation of FosB in the NAc of cocaine-withdrawn rats because previous research has reported that repeated exposure to a test cage does not elevate FosB/ΔFosB expression in the NAc (Conversi et al., 2006). However, it is not known if exposure to cues conditioned to rewards cause FosB expression. As stress can also elevate FosB/ΔFosB expression in the NAc (Perrotti et al., 2004; see discussion below) we cannot rule out stress-induced FosB immunoreactivity in our conditioned groups. In addition, as mentioned above, the FosB staining we measured in the NAc-C and BLA returned to the baseline levels seen in saline treated rats after five weeks of cocaine withdrawal, even though the rats in both the two and five week groups were exposed to the same behavioral treatment during the novelty conditioning procedure. This finding indicates that the protein our antibody is measuring is not acutely induced by exposure to the novel objects or the test cage environment. The most likely explanation for the significant elevation of FosB-like staining in the NAc of withdrawn rats is the result of the accumulation of ΔFosB following the chronic cocaine exposure.
The rate of accumulation and distribution of ΔFosB vary with brain regions and treatment, and are consistent with our results (McClung et al., 2004). Withdrawal from chronic amphetamine exposure has been found to increase FosB-like expression in the NAc-Sh and BLA. This change only occurred with a procedure that incorporated escalating doses of drug similar to the procedure we used for these experiments (Murphy et al., 2003). The finding that prior exposure to cocaine increased preference for an environment paired with cocaine confirms our previous findings and those of other labs that cocaine exposure and withdrawal enhances and sensitizes cocaine place preferences (Lett, 1989, Shippenberg and Heidbreder, 1995, Harris et al., 2001). Moreover, the fact that this elevated preference was correlated with increased FosB-like immunoreactivity in the NAc is consistent with other reports indicating that overexpression of ΔFosB increases cocaine-seeking behaviors (Kelz, 1999, Olausson et al., 2002, Colby et al., 2003, Kumar et al., 2005).
Previous research has shown that acute withdrawal from nicotine suppresses the acquisition of novel object place preference without decreasing novel object interaction time (Besheer and Bevins, 2003, Bevins and Besheer, 2005). We also found that novel object interaction was not significantly decreased relative to saline treated rats at both two and five weeks of forced cocaine abstinence, despite the loss of place preference behavior. In the Besheer and Bevins paper (Besheer and Bevins, 2003) novel object reward returned to normal by day four of nicotine withdrawal whereas in our case novel object reward did not return to normal, or even show signs of recovery, up to and including five weeks after cocaine exposure. This may reflect overall differences in the drugs used, nicotine versus cocaine, or the intensity of the treatment, seven days of continuous treatment as in the case of nicotine or 10 days of escalating treatment as in the case of cocaine. A recent report has indicated that nicotine, unlike cocaine, does not produce long-lasting elevations in ΔFosB in the NAc (Marttila et al., 2006). These data support the notion that elevations of ΔFosB in the NAc may be associated with the enduring loss of novel object preference that we observed.
The cocaine conditioning results for the three-week cocaine-withdrawn rats are important because they indicated that the rats were capable of learning following cocaine withdrawal. Following two weeks of cocaine withdrawal, rats failed to demonstrate novelty preference but at three weeks of withdrawal they exhibited enhanced cocaine preference. This finding cannot be explained by differences in the time after withdrawal because when we tested a separate group of rats at two weeks post-withdrawal they also showed a similar elevation in cocaine place preference behavior. This indicates that cocaine exposure and withdrawal does not produce a generalized learning deficit. Instead, we hypothesize that the threshold for what is perceived as rewarding, or remembered as rewarding, is elevated during cocaine abstinence so that only stimuli that strongly activate reward systems (e.g., the dopaminergic system) such as cocaine are perceived and remembered as rewarding. It is notable that not only is cocaine perceived as rewarding in our subjects, but that in cocaine withdrawn rats it is perceived as more rewarding or more preferred than in saline pretreated rats as indicated by enhanced preference for the cocaine associated environment.
It has been suggested that the loss of novel object reward during withdrawal may be due to the fact that this type of reward depends selectively on D1 dopaminergic activation (Besheer et al., 1999, Bevins et al., 2002). It is possible that drugs like cocaine over-stimulate the dopaminergic system (Vezina, 2004) and cause it to dysfunction for primary rewards like novelty. Interestingly, the expression of FosB/ΔFosB in the NAc is also dependent on the activation of D1 receptors (Nye et al., 1995, Zhang et al., 2002). Therefore, it is possible that cocaine-induced increases in ΔFosB occurring through activation of the D1 receptor alters the transcription of specific genes in D1 expressing neurons thus changing the salience of natural rewards like novelty by making them less rewarding or less memorable while simultaneously increasing the salience and reward for drugs like cocaine. Several recent publications have shown that repeated cocaine administration alters gene expression specifically in D1 receptor containing neurons in the NAc (Zhang et al., 2005, Lee et al., 2006, Zhang and Xu, 2006). Intriguingly, Lee et al. (2006) reported that although dendritic spine formation and ΔFosB accumulation occurred in both D1 and D2 receptor-containing NAc neurons after chronic cocaine treatment, this effect was transient in D2 receptor-containing neurons but extremely long lasting (thirty days) in D1 receptor-containing neurons.
The unique finding of the current study compared to previous reports is that increased FosB/ΔFosB-like immunoreactivity in the NAc-Sh correlated with decreased novelty preference. This suggests that cocaine-induced ΔFosB accumulation may decrease the preference for natural rewards. However, Olausson et. al., (2006) reported that both psychostimulant exposure and ΔFosB over-expression in the NAc enhanced instrumental responding for food reinforcement. It is possible that food reward is mediated through different mechanisms from novelty reward, although both appear to involve DA release in the NAc (Besheer et al., 1999, Bevins et al., 2002, Kelley and Berridge, 2002, Kelley et al., 2005, Rauggi et al., 2005). Alternatively, these discrepancies could be explained by differences in the amount of time following cocaine exposure, which were five days in the Olausson study (Olausson et al., 2006) and fourteen to thirty-five days in the current experiments. The administration of cocaine was also different in the two studies in that we used escalating doses of cocaine that would result in high levels of cocaine followed by periods of withdrawal, whereas Olausson et al. (Olausson et al., 2006) used a dosing regimen that would result in a more constant exposure to the drug (a single dose given twice a day).
Previously we found that withdrawal from morphine (7-14 days) delayed the development of instrumental responding for food reinforcement and decreased place preference for a sweet food reward (Harris and Aston-Jones, 2003a). Morphine exposure, like cocaine exposure, has also been associated with increased ΔFosB staining in the NAc (Nye and Nestler, 1996). Over-expression of ΔFosB in the NAc increases morphine place preference (Zachariou et al., 2006), and we have shown that extended withdrawal from morphine enhances morphine place preference (Harris and Aston-Jones, 2001, Harris and Aston-Jones, 2003b, Harris and Aston-Jones, 2003a). These data indicate that both cocaine and morphine withdrawal have similar effects in decreasing the impact of primary rewards, increasing preference for drug reward, and increasing expression of ΔFosB in the NAc. Although these findings do not prove a causal relationship between increased ΔFosB expression in the NAc and changes in reward function, they strongly suggest the possibility that the two events may be linked.
Previously we showed that cocaine withdrawn rats exhibit enhanced responses to environmental stressors (Harris and Aston-Jones, 1993, Harris et al., 2001), and recently we reported that withdrawal from morphine enhanced the activation of brain stress systems (Harris and Aston-Jones, 2007). The enhanced activation of brain stress systems in that study (Harris and Aston-Jones, 2007) correlated with decreased food place preference, indicating that this is a possible mechanism whereby natural rewards become less preferred during drug abstinence. As stress has also been found to increase ΔFosB expression in the NAc (Perrotti et al., 2004), there could be an accumulative effect of previous drug exposure and stress reactivity during abstinence that further induces ΔFosB accumulation in the NAc or at least helps to maintain it in the absence of further drug exposure. This interaction between drug-induced and stress-induced ΔFosB accumulation in the NAc could explain why both high activation in stress-related brain nuclei and FosB staining in the NAc could both correlate with decreased natural reward preference and increased drug preference. It is also possible that the elevation in FosB staining we found in the NAc-Sh in the five week-withdrawn group was enhanced by stress. In this scenario, the cocaine-withdrawn rats may have experienced more stress during the daily conditioning sessions for both rewards than saline rats (due to an elevated stress reactivity) and this subsequently could have contributed to the enhanced FosB staining we saw in the NAc-Sh.
The findings of this study indicate that the NAc may be an important location in the brain where changes in gene expression regulate both enhanced preference for drug reward and decreased preference for natural rewards. Further research will be necessary to determine the precise mechanisms whereby altered gene expression in the NAc could have such divergent effects on different rewards and how alterations in brain stress systems play into this system.
Acknowledgements
Supported by PHS grant DA017289.
Abbreviations
- (NAc)
nucleus accumbens
- (NAc-Sh)
nucleus accumbens shell
- (NAc-C)
nucleus accumbens core
- (BLA)
basolateral amygdala
Footnotes
Section Editor Behavioral Neuroscience: Joan I Morrell, Ph.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson M, Westin JE, Cenci MA. Time course of striatal DeltaFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17:661–666. doi: 10.1046/j.1460-9568.2003.02469.x. [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Impact of nicotine withdrawal on novelty reward and related behaviors. Behav Neurosci. 2003;117:327–340. doi: 10.1037/0735-7044.117.2.327. [DOI] [PubMed] [Google Scholar]
- Besheer J, Jensen HC, Bevins RA. Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Bardo MT. Conditioned increase in place preference by access to novel objects: antagonism by MK-801. Behav Brain Res. 1999;99:53–60. doi: 10.1016/s0166-4328(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–714. doi: 10.1016/j.neubiorev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129:41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conversi D, Bonito-Oliva A, Orsini C, Cabib S. Habituation to the test cage influences amphetamine-induced locomotion and Fos expression and increases FosB/DeltaFosB-like immunoreactivity in mice. Neuroscience. 2006;141:597–605. doi: 10.1016/j.neuroscience.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Harris GC, Altomare K, Aston-Jones G. Preference for a cocaine-associated environment is attenuated by augmented accumbal serotonin in cocaine withdrawn rats. Psychopharmacology. 2001;156:14–22. doi: 10.1007/s002130100693. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology. 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology. 2003a;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: Association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003b;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Kelz MB, Duman RS, Nestler EJ. Chronic electroconvulsive seizure (ECS) treatment results in expression of a long-lasting AP-1 complex in brain with altered composition and characteristics. J Neurosci. 1994a;14:4318–4328. doi: 10.1523/JNEUROSCI.14-07-04318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994b;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carelzon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Louie AK, Lannon RA, Ketter TA. Treatment of cocaine-induced panic disorder. Am J Psychiatry. 1989;146:40–44. doi: 10.1176/ajp.146.1.40. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Russig H, Pezze MA, Ferger B, Feldon J. Amphetamine withdrawal modulates FosB expression in mesolimbic dopaminergic target nuclei: effects of different schedules of administration. Neuropharmacology. 2003;44:926–939. doi: 10.1016/s0028-3908(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- Olausson P, Engel JA, Soderpalm B. Involvement of serotonin in nicotine dependence: processes relevant to positive and negative regulation of drug intake. Pharmacol Biochem Behav. 2002;71:757–771. doi: 10.1016/s0091-3057(01)00673-6. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Inc; San Diego, CA: 1998. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Rauggi R, Scheggi S, Cassanelli A, De Montis MG, Tagliamonte A, Gambarana C. The mesolimbic dopaminergic response to novel palatable food consumption increases dopamine-D1 receptor-mediated signalling with complex modifications of the DARPP-32 phosphorylation pattern. J Neurochem. 2005;92:867–877. doi: 10.1111/j.1471-4159.2004.02920.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Cocaine use is associated with increased craving in outpatient cocaine abusers. Exp Clin Psychopharmacol. 1998;6:217–224. doi: 10.1037//1064-1297.6.2.217. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, ShawLutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu M. Opposite regulation of cocaine-induced intracellular signaling and gene expression by dopamine D1 and D3 receptors. Ann N Y Acad Sci. 2006;1074:1–12. doi: 10.1196/annals.1369.001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang D, Xu M. Identification of chronic cocaine-induced gene expression through dopamine d1 receptors by using cDNA microarrays. Ann N Y Acad Sci. 2002;965:1–9. doi: 10.1111/j.1749-6632.2002.tb04146.x. [DOI] [PubMed] [Google Scholar]