Abstract
The present study investigated urinary scent marking behavior in male C57BL/6J (C57) mice as olfactory social signaling. In Experiment 1, when compared scent marking toward adult males, C57 males showed substantial scent marking toward CD-1 males and even toward the odor alone of CD-1 males, but not toward C57 males. Experiment 2 explored scent marking in C57 males of different ages to males and females, and juveniles and adults of the same strain. C57 males deposited more marks than control conditions only toward an adult C57 female when tested at 100 days of age, but not at 60 days of age. Development of urine marking behavior was investigated in C57 males at the ages of 30, 60, 90, and 120 days in Experiment 3. When tested alone (control) or confronted with a C57 male, C57 males showed diminished scent marks throughout development. Compared to controls, marking toward a CD-1 male increased after the age of 60 days, while marks toward an adult female showed significant increases after the age of 90 days. This difference in scent marking depending on the sex of the stimulus animal is likely to be associated with development of sexual behavior, in which males need to set up territories against other males prior to advertising to females. Although highly inbred strains have similar odor components, C57 males are able to detect and deposit urine marks after puberty as social communication depending on age, sex, and genetic differences in the opponents.
Keywords: C57BL/6J, urine marking, sexual behavior, aggressive behavior, odor recognition, urinary odor
1. Introduction
Mice show urinary scent marking behavior, which functions in social communication [25-26], including individual recognition [10], assessment of dominance [28,32-33], and determination of reproductive condition of potential mates [5,43]. Mice are territorial [1,15], and urine marking serves to indicate territorial boundaries. Dominant male mice make more urine marks, whereas subordinate males urinate in fewer locations of a novel environment [17,27]. Scent marking may also be used by males to signal females to advertise males' quality [43] and a primer for reproduction [12].
Environmental factors, such as food type, bacterial gut flora, and social status induce changes in volatile odors emitted by animals [54,61]. The major histocompatibility complex (MHC) peptides influences the volatile and nonvolatile scents produced by mice [60-61]. Another source of odor complexity in rodents resides in a class of proteins, termed major urinary proteins (MUPs) [8,29], that bind and release small nonvolatile pheromones [4]. MHC peptides and MUPs remain fixed for life [8,61], which provide each individual with an identity signature. Urinary MUPs are expressed at high concentrations by adult mice of both sexes [8,51], although males invest more than females in both urine marks [27] and MUP production [8]. Individuals of highly inbred strains have the identical MHC peptides and MUP patterns, which are likely to be essential in allowing mice to distinguish another mouse's urine mark from their own [29,47], and thus are unable to discriminate between each others' urinary odors when kept under identical conditions [36,46,59]. Based on the genetic differences in odor from urine or body, dominant male mice increase their scent marking in the vicinity of scent marks from other males to countermark the scent, but show no such response to their own scent marks or to these from adult males genetically identical to themselves [27,46-47]. As olfaction is the primary sensory modality of mice and mice use scent marks to communication with conspecifics [13], scent marking provides a specific behavioral test for the social communication in mice [20,24,46].
Mouse models involving with gene-manipulations may provide a useful research tool to advance the investigation of genes associated with specific neurodevelopmental communication disorders such as autism [3,19,31,62] and attention deficit hyperactivity disorder [57]. As specific behavioral or physiological parameters of a background strain may influence the expression or the salience of genetic manipulation effects [e.g. 16,21,37,42], an understanding of these parameters is important in interpreting such manipulations. C57BL/6J (C57) inbred mice were used for sequencing the mouse genome [21,58], and have served as background for a number of genetic manipulations [e.g. 14,16,23,55]. C57 mice can detect scent marks from opposite-sex conspecifics and then deposit marks as countermarks [20]. However, as inbred male mice cannot either discriminate between same-sex individuals of same inbred strain or assess familiarity with other same strain conspecifics [6,46], it is important to understand how social/cognitive aspects including sex, development, and genetic differences may influence on urinary scent marking behavior in inbred C57 mice for genome study on human behavioral/communication disorder.
In Experiment 1, urine marking behavior in male C57 mice toward adult males was explored. Frequencies of urine marks toward an adult male of same strain, or a different outbred strain and their odors alone were compared. In Experiment 2, sex and developmental effects of exposure to C57 males and females on urine marking to same-strain stimuli were investigated: Male C57 mice aged 60 and 100 days old were confronted by male and female, juvenile and adult mice of the same strain. These developmental features were expanded in experiment 3, which evaluated urine marking toward same-age males or adult females of the same strain, as well as adult males of an outbred strain, for male C57 mice aged 30, 60, 90, and 120 days old.
2. Materials and methods
2.1. Animals
Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals [NIH, 1996]. All protocols and animal handling and treatment were approved by the Institutional Anial Care and Use Committee at the University of Hawaii.
C57BL/6J mice were bred from stock obtained from the Jackson Laboratory (Bar Harbor, ME). All subjects were weaned at 23-26 days of age, and then housed, in groups of 2-3 same sex animals, in standard polypropylene cages, 26.5 × 17 × 11.5 (H) cm, under 12L:12D cycle (lights on 06:00) in a temperature- (22±2 °C) and humidity- (60 %) controlled room at the University of Hawaii Laboratory Animal Services. They were housed in pairs of same sex animals for 1 week prior to the test. CD-1 males were obtained from Charles River Laboratories (Wilmington, MA). They were housed individually in polypropylene cages at least for 1 week prior to the test. All animals were allowed free access to food and water in their home cages.
2.1.1. Experiment 1
Twelve male C57 mice (25-30g) 14-18 weeks of age were used as the subjects. Six male outbred CD-1 mice 12 weeks of age were used as stimulus animals.
2.1.2. Experiment 2
Twenty four male C57 mice were used as the subjects, half of which was assigned to each group tested at 60 or 100 days of age. Twelve female C57 mice 12-14 weeks of age (20-24 g), and twenty four C57 mice 30 days of age (male 12, female 12) were used as the stimulus animals.
2.1.3. Experiment 3
Forty eight male C57 mice were used as the subjects. They were divided into four groups (N=12 in each group), aged 30, 60, 90, and 120 days on the first test day. Twelve female C57 mice 12-13 weeks of age and twelve CD-1 outbred male mice 13 weeks of age were used as the stimulus animals.
2.2. Apparatus
Testing of urine marking was conducted in a bottomless Polycarbonate cage (46 × 24 × 21 cm), placed upside-down on a rough paper (457 × 365 mm, Rough Newsprint paper, Bienfang) substrate. The cage was divided into two equal-sized compartments by a wire mesh screen that prevented direct physical contact between subject and stimulus, but allowed olfactory, visual, and auditory cues to be received.
2.3. Procedure
All test trials were conducted during the light phase of the light/dark cycle under dim light conditions. Twenty min before the beginning of the test, subjects were moved from the holding room to the experiment room in their homecages. Subjects were placed in one compartment of the test chamber cage for 20 min. Behaviors were recorded using an over-head video camera. Between trials, the entire apparatus was cleaned with 15% alcohol and dried with paper towels, and the paper substrate was changed.
Mouse urine was fixed by Ninhydrin spray (LC-NIN-16, Criminal Research Products, LLC). After 24 hours drying, the number of urine marks was measured by placing a transparent grid sheet over the substrate paper and counting the number of grids (each 10 × 10 mm) containing urine marks (maximum: 552 squares). Pools of urine larger than four square grids that formed a larger quadrant were not included in this count. Four squares in a row, however, were included. Total number of squares with urine marks was counted. The number of urine marks was also separately counted by each near and far side of the marked area from the cage of stimulus animals (each maximum: 226 squares). The total size (# of squares) of pools and the number of feces were recorded.
The order of the trials with different stimuli in each Experiment was counterbalanced in a Latin Square design. Inter-trial-interval was 24 hrs in all experiments. In experiment 1, animals were tested with four stimuli: Single (S: no stimulus animal); C57 (novel adult C57 male stimulus); CD-1 (novel adult CD1 male); and CD-1 odor (fresh urine marks of an adult CD1 male on the stimulus side of the cage). In experiment 2, each male C57 mouse aged 60 or 100 days old was exposed singly (S); or with an adult male (AM), an adult female (AF), a juvenile male (JM), or juvenile female (JF) of the C57 strain. In experiment 3, subjects at 30, 60, 90, or 120 days old were tested singly (S), or with a same-aged male (C57m), an adult C57 female (C57f), or an adult CD-1 male (CD1m).
2.4. Statistical analysis
For experiment 1, data were analyzed by a one-way analysis of variance (ANOVA) with a within-subject stimulus factor (S, C57, CD1, or CD1 odor). For experiment 2, two-way ANOVAs with a between-subject factor of age (60 or 100 days of age) and within-subject stimulus factor (S, AM, AF, JM, or JF) were conducted. For experiment 3, two-way ANOVAs with the between-subjects factor of age (30, 60, 90, or 120 days of age) and the within-subject stimulus factor (S, C57m, C57f, or CD1m) were used. Post hoc comparisons used the Bonferroni test for within-subject factors and the Tukey's HSD test for between-subject factors. A probability level of p<.05 was adopted as the level of statistical significance for all analyses.
3. Results
3.1. Experiment 1
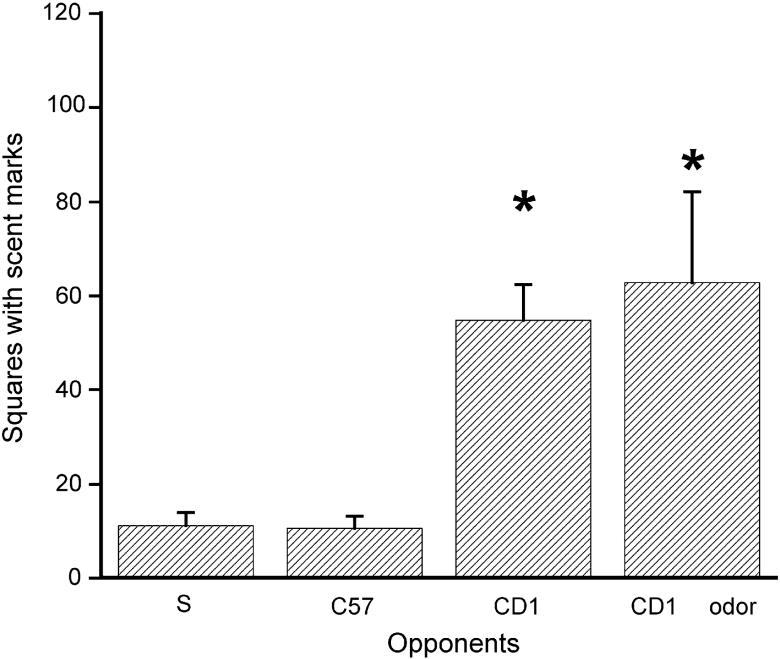
The total number of squares with urine marks for each stimulus group is presented in Fig. 1. A one-way ANOVA conducted on these scores showed a significant difference between groups [F(3,44)= 7.125, p<.001]. When exposed to CD-1 males or their odor alone, C57 males displayed higher number of urine marks than when exposed singly or to C57 males. There were no significant differences between tests without stimuli, and those involving C57 male stimuli.
Fig. 1.
Total number of squares with urine marks for C57 males (N=12) during 20 min exposed singly (S), or with a C57 male (C57), a CD-1 male (CD1), or odor of CD-1 male (OD1 odor). Data are expressed as mean (± S.E.M.). * indicates significant differences between groups compared to Group S, p<.05.
The number of squares with urine marks in the near and far sides of floor of marked cage from stimulus animals for each stimulus group is shown in Table 1. A one-way ANOVA conducted on these scores of each side showed a significant difference between groups for near and far sides [F(3,44)= 8.211, p<.001; 5.820, p<.01, respectively]. These results had a similar tendency to the scores of whole marked area; in the near side, C57 males showed higher number of marking when confronted with CD-1 males and CD-1 odor alone, than when exposed singly or to C57 males. In the far side, C57 males exposed to CD-1 males displayed higher urine marks than that exposed singly or to C57 males.
Table 1.
The numbers of squares with scent marks in each side of marked cage and squares with urine pool and feces toward each opponent for male C57 mice in Experiment 1.
| Scent marks |
Pool size | Fecal boli | ||
|---|---|---|---|---|
| Near side | Far side | |||
| S | 6.50 (1.68) | 5.17 (1.33) | 23.25 (7.08) | 3.58 (0.56) |
| C57 | 8.33 (2.00) | 3.17 (0.81) | 18.83 (5.47) | 2.58 (0.65) |
| CD1 | 32.25 (4.61)* | 22.67 (3.40)* | 11.00 (4.43) | 4.92 (0.79) |
| CD1 odor | 40.58 (11.22)* | 23.92 (8.11) | 15.83 (4.25) | 4.58 (0.75) |
Data are means (S.E.M.). Post hoc significant differences between condition of opponents compared to S
p < .05.
A one-way ANOVA conducted on the total squares that contained pools of urine did not find significant differences between groups (Table 1). The total number of feces for each group (Table 1), did not differ significantly between groups.
3.2. Experiment 2
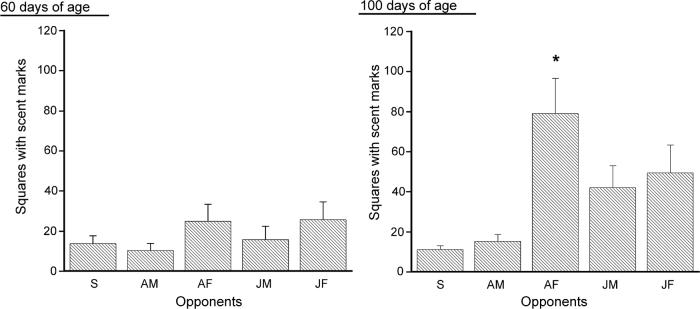
Fig. 2 depicts the total number of squares with urine marks for each group in Experiment 2. A two-way ANOVA conducted on age (2) x stimulus (5) scores found significant main effects of age [F(1,22) =6.559, p<.05] and stimulus [F(4,88)= 7.240, p<.01]. The interaction between age and stimulus was also significant [F(4,88)= 3.110, p<.05]. Subsequent analysis of age effects indicated only one significant difference; that 100 day-old males made more urine marks to females than did 60 day-old males. At 100 days of age, male mice exposed to an adult female made more urine marks than those exposed to no stimulus or to an adult male.
Fig. 2.
Total number of squares with urine marks for C57 males aged 60 days (left panel) and 100 days (right panel) (each N=12) during 20 min exposure singly (S), or with an adult C57 male (AM), an adult C57 female (AF), a juvenile C57 male (JM), or a juvenile C57 female (JF). Data are expressed as mean (± S.E.M.). * indicates significant differences between groups compared to Group S, p<.05.
The number of squares with urine marks in each near and far side of the cage for each group is presented in Table 2. A two-way ANOVA conducted on the scores in the near side of marked cage found significant main effects of age [F(1,22)= 9.206, p<.01] and stimulus [F(4,88)= 6.940, p<.001]. The interaction between age and stimulus for the near side was also significant [F(4,88)= 3.656, p<.01]. Subsequent analysis indicated a similar tendency to the scores of total urine marks; in the near side, while C57 mice aged 60 days did not show any differences depending on the opponents, C57 mice aged 100 days displayed higher level of marking toward adult C57 females compared to these exposed singly or to adult C57 males. In the far side, a two-way ANOVA showed significant main effects of stimulus [F(4,88)= 6.04, p<.001], but not of age [F(1,22)= 2.992, n.s.]. The interaction between age and stimulus for the scores of far side area was not significant [F(4,88)= 1.717, n.s.]. Thus C57 males showed higher number of urine marks when confronted with adult C57 females compared with those when exposed singly or to adult C57 males, regardless of their age.
Table 2.
The numbers of squares with scent marks in each side of marked cage and squares with urine pool and feces toward each opponent for male C57 mice aged 60 or 100 days in Experiment 2
| Scent marks |
Pool size | Fecal boli | ||
|---|---|---|---|---|
| Near side | Far side | |||
| 60 days of age | ||||
| S | 9.50 (2.90) | 4.17 (1.36) | 15.75 (4.06) | 3.33 (0.50) |
| AM | 8.00 (2.68) | 2.42 (1.02) | 9.25 (3.21) | 4.42 (0.59) |
| AF | 14.92 (5.05) | 9.92 (4.04)* | 20.00 (4.51) | 6.17 (0.44) |
| JM | 10.25 (4.40) | 5.58 (2.32) | 12.00 (5.62) | 3.42 (0.84) |
| JF | 16.67 (6.06) | 9.08 (3.13) | 14.67 (4.03) | 3.17 (0.56) |
| 100 days of age | ||||
| S | 7.92 (1.60) | 3.25 (0.71) | 14.42 (4.05) | 4.91 (0.66) |
| AM | 11.58 (2.50) | 3.75 (0.98) | 24.75 (6.08) | 4.25 (0.54) |
| AF | 53.17 (10.01)* | 26.00 (7.64)* | 13.00 (4.00) | 4.75 (0.86) |
| JM | 28.92 (7.74) | 13.09 (3.52) | 16.08 (4.57) | 4.75 (0.58) |
| JF | 34.42 (8.75) | 15.08 (5.80) | 18.75 (6.66) | 4.67 (0.78) |
Data are means (S.E.M.). Post hoc significant differences between condition of opponents compared to S
p < .05.
Table 2 presents the total squares that contained pools of urine and the total number of feces for each group. A two-way ANOVA conducted on these scores of each index did not show any significant main effects or interaction between age and stimulus.
3.3. Experiment 3
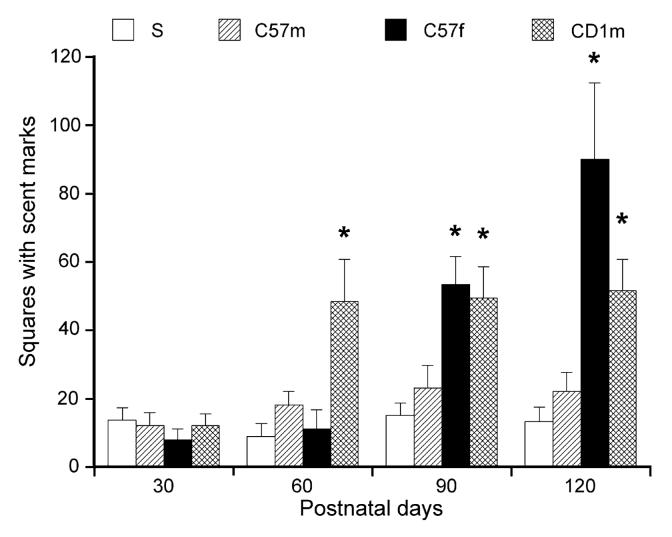
Fig. 3 presents the total number of squares with urine marks for each group of male C57BL/6Js aged 30, 60, 90, and 120 days. A two-way ANOVA conducted on age (4) x stimulus (4) scores showed significant main effects of age [F(3,44) =6.341, p<.01] and stimulus [F(3,132)= 12.884, p<.01]. The interaction between age and stimulus was also significant [F(9,132)= 5.397, p<.01]. At 60, 90, and 120 days of age, males deposited more urine marks to the CD-1 male than either those placed singly or exposed to a C57 male. At 90 and 120 days of age, males made more urine marks to an adult female than those without a stimulus, or those exposed to a C57 male. At 120 days of age, they showed tendency (but not significant, p=.061) for more urine marks to the female than to the CD-1 male. Urine marking to the female was higher at 120 days than at either 30 or 60 days of age. Marking to the CD-1 male stimulus was higher at 90 or 120 days than at 30 days.
Fig. 3.
Total number of squares with urine marks for C57 males aged 30, 60, 90, and 120 days (each N=12) during 20 min exposure singly (S), or with a C57 male (C57m), with an adult C57 female (C57f), or an adult CD-1 male (CD1m). Data are expressed as mean (± S.E.M.). * indicates significant differences between groups compared to Group S, p<.05.
The number of squares that C57 males deposited urine marking in each side of marked area for each group is presented in Table 3. A two-way ANOVA conducted on these scores in the near side area found significant main effects of age [F(3,44)= 6.314, p<.001] and stimulus [F(3,132)= 14.819, p<.001]. The interaction between age and stimulus was also significant [F(9,132)= 6.291, p<.001]. Subsequent post-hoc analysis for the scores in the near side area found a similar tendency to these of total scent marks; In the near side of marked area, at 60 days of age, urine marking toward the CD-1 male was higher than all other conditions, while at 90 and 120 days of age, those toward a CD-1 male and an adult female were higher than either those without a stimulus or those exposed to a C57 male. Additionally, at 120 days of age, marking to an adult female was higher than those to a CD-1 male. Urine marking toward a CD-1 male was higher at 60, 90, or 120 days than at 30 days. Marking to an adult female stimulus was higher at 90 or 120 days than at either 30 or 60 days. A two-way ANOVA conducted on the scores in the far side of marked area found significant main effects of age [F(3,44)= 5.467, p<.01] and stimulus [F(3,132)= 7.786, p<.001]. The interaction between them was also significant [F(9,132)= 3.260, p<.01]. Subsequent post hoc analysis for the scores in far side area indicated similar tendency to these for the total number of urine marks; at 60 and 90 days of age, urine marking toward a CD-1 male was higher than those without a stimulus. At 120 days of age, urine marking toward either a C57 male or an adult female was higher than those without stimulus animals, and additionally marking to an adult female was higher than those exposed to a C57 male. When exposed to a CD-1 male, urine marking was higher at 60, 90 or 120 days than at 30 days. When exposed to an adult female, marking was higher at 120 days than at 30 days.
Table 3.
The numbers of squares with scent marks in each side of marked cage and squares with urine pool and feces toward each opponents for male C57 mice in Experiment 3.
| Scent marks |
Pool size | Fecal boli | ||
|---|---|---|---|---|
| Near side | Far side | |||
| 30 days of Age | ||||
| S | 9.67 (2.65) | 3.83 (1.09) | 21.92 (4.10) | 6.50 (0.85) |
| C57m | 9.33 (3.32) | 2.83 (0.67) | 11.75 (4.19) | 5.67 (0.97) |
| C57f | 6.58 (2.61) | 1.33 (0.70) | 5.25 (2.89) | 5.50 (0.76) |
| CD1m | 9.08 (2.39) | 3.17 (1.16) | 4.67 (2.53) | 5.00 (0.49) |
| 60 days of age | ||||
| S | 5.67 (2.70) | 3.25 (1.08) | 2.75 (1.93) | 3.50 (0.67) |
| C57m | 12.50 (3.30) | 5.58 (1.40) | 15.25 (4.92) | 3.83 (0.63) |
| C57f | 6.50 (3.94) | 4.67 (1.82) | 1.17 (2.55) | 5.00 (0.66) |
| CD1m | 30.42 (6.60)*# | 18.08 (6.83)*# | 7.25 (2.98) | 2.25 (0.47) |
| 90 days of age | ||||
| S | 9.00 (2.76) | 5.17 (1.41) | 7.67 (2.32) | 4.83 (0.63) |
| C57m | 14.17 (3.95) | 8.92 (3.32) | 22.08 (5.81) | 4.67 (0.89) |
| C57f | 37.83 (6.71)*# | 15.67 (2.67) | 7.5 (2.55) | 4.92 (0.88) |
| CD1m | 31.00 (5.36)*# | 18.50 (4.69)*# | 9.08 (3.27) | 4.17 (0.47) |
| 120 days of age | ||||
| S | 10.83 (2.63) | 5.42 (1.61) | 14.42 (4.66) | 5.17 (0.72) |
| C57m | 14.83 (3.41) | 7.25 (1.94) | 13.67 (4.36) | 1.92 (0.68) |
| C57f | 59.75 (14.05)*# | 32.75 (9.46)*# | 10.17 (5.02) | 4.25 (0.66) |
| CD1m | 33.50 (5.77)*# | 18.17 (4.33)*# | 7.58 (4.15) | 2.50 (0.50) |
Data are means (S.E.M.). Significant post hoc differences between conditions of opponents compared to S
p < .05, and between ages compared to 30 days
p < .05.
For pool size and fecal boli, there no significant differences between conditions of opponents or age of testing.
Table 3 shows the total squares that contained pools of urine and the number of feces for each group in Experiment 3. A two-way ANOVA conducted on these scores did not show any significant main effects and interaction between age and stimulus.
4. Discussion
Urinary scent marking in a novel environment and to other males are important components of dominance advertisement among male mice and have a strong influence on their aggressive interactions [28,44]. Males can indicate their domination of an area by scent marking and ensuring that their scent marks are the freshest in that area as countermarks [53]. In the present study, findings of substantial marking by C57 males exposed to adult CD-1 males (experiments 1 and 3) or CD-1 male urine odors alone (experiment 1), or to C57 females (experiment 2 and 3), in a unmarked situation provide validation for the protocol used to elicit scent marking. The same findings indicate that, although C57 males have been shown to make reduced scent marks in comparison to the C3H/He strain [22], and learn simple odor discrimination less readily than DBA/2J mice [41] they are clearly capable of responding to such stimuli in a typical mouse pattern, i.e. by marking as a response to sexual and genetic differences. C57 males made no more scent marks when confronted with a male mouse of the same, inbred, C57 strain, than to the identical situation without any mouse present (experiments 1, 2, and 3). This lack of marking failed to change over development (30 to 120 days of age), and regardless of the age (juvenile or adult) of the conspecific male stimulus of same strain. This absent of urine marks toward juvenile or adult males of same strain may reflect that mice of highly inbred strains have the same urine odor, ascribable to the same MHC and MUP patterns through the life [29,47,61].
In the present experiments, C57 males were housed with same-sex littermates from weaning, a factor that might be involved in their lack of responsivity to other male conspecifics. As the number of scent marks can be predictors of both aggression scores and social dominance status in mice [18], it is possible that subordinate members of these male groups might have shown marked suppression of scent marks, as do subordinate males generally [17]. However, if group-housing of these animals had produced strong dominant relationships, with dominant males showing normal scent marking, while subordinates did not under situation including confronted with a CD-1 male, a pattern of intermediate levels of marking and high variability should have been obtained. Results from experiments 1, 2 and 3 are consistent in showing no evidence whatever of such a pattern in response to the C57 adult male stimulus groups. An additional consideration is that, when housed with same-sex littermates from weaning, male mice do not show either serious aggressive fighting or apparent dominant-subordinate relationships among cage mates [2,7,38]. This also argues against a view that subordination-induced suppression of scent marking is responsible for the present findings of no significant increase in C57 male marking to conspecific males. Thus, during testing the strange male conspecific stimulus, with an odor that is similar to or even indistinguishable from, that of the familiar cage/litter mate, should elicit less urine marking [47].
Findings that wild male mice aged 55-60 days [39] and adult CF-1 male mice [40] deposited similar amounts of scent marks toward male and female conspecifics, suggesting that the magnitude of marking may not be determined by the sex of the opponent. Indeed, the present finding (experiment 2) of no difference in scent marking toward any of the stimuli presented for 60 day old C57 males is partly congruent with this report. However, C57 mice can discriminate between volatile chemosignals in urine from male and female conspecifics when tested in a Y-maze [10], and male C57BL/6 mice can discriminate between urinary odors from estrous versus ovariectomized females [50] or non-estrous females [35] of the same strain. These findings indicate that even inbred strains can discriminate sex and estrous condition from urinary odor, consonant with the present findings of a clear spike in scent marking toward conspecific females in C57 males at 100 (experiment 2) or 90 and 120 (experiment 3) days of age. A previous research has shown that male mice deposit scent marks at high levels to females at all points in the estrous cycle [48], suggesting that the estrous cycle of female stimuli should not affect the amount of scent marking in males. Therefore, in the present experiments, the status of the estrous cycle of the female stimuli was not monitored, and as the mouse estrous cycle is about 4 days in length, these females were undoubtedly used as stimuli while in both estrous and non-estrous, as they were used in tests that extended over 5 successive days in experiment 2 and 4 successive days in experiment 3. These procedures should offset the estrous effect.
A particularly intriguing finding (experiment 3) is that of clear developmental differences for scent marks to adult CD-1 males vs adult C57 females. Compared to marks to male C57 stimuli, scent marks to adult CD-1 males increased sharply between 30 and 60 days, remaining stable thereafter, while marks to adult females remained low at 60 days, but increased at 90 days, increasing further (i.e. significantly higher than to either controls or conspecific, and higher tendency than to CD-1 males) at 120 days. This developmental difference may be related to possible differences in the chemical signatures to adult male and female mice. Mouse urinary odor may contain specific information concerning the species, sex, and individual identity of the owner [11,30,61]. For example, female odor induced increased approaches by dominant males but not subordinates, while odors of dominant males were avoided by both dominant and subordinate males [33]. This sex difference in response to urinary odors occurs after puberty, a time when urine excretion increases in males, with staged activation of androgen-dependent endocrine system [30,51], which modulates male-specific marking [34,56]. Thus male-specific marking should emerge after puberty, as was found here for responses to the CD-1 male (experiment 3).
However, male marking to the presence of an adult female was delayed in onset compared to marking to an adult CD-1 male. A possible functional explanation for this difference may be related to findings that the presence of an adult female strongly elicits aggression in male mice, even those that have lived amicably together prior to the appearance of the female [49]. Thus, even though 60 days is well above the period when copulation begins in male mice [45], this suggests that scent marking of an unknown area containing a female may be a particularly dangerous undertaking for a young and small, albeit sexually mature, male. Scent is functional signaling to males to avoid territory [25] and to females to advertise males' quality [53]. This indicates that the developmental timing of scent marking to males and females odors may reflect the necessary of males to establish and defend territories against other males prior to advertising to females.
Odor discrimination and subsequent deposition of urine marks have an ethologically important role in social communication among conspecifics [29,47,52]. C57 mice are a highly inbred strain, indicating they possess the same chemosignals in their urine associated with MUP and MHC peptides [29,47]. This should act to prevent C57 males from discriminating odors of male mice of the same inbred strain, regardless of their age. However, C57 males did display discriminative deposition of urine marks toward outbred CD-1 males and toward adult C57 females. This ability is important because C57 mice have been a background strain for many genetic manipulations in mice [9,16,23,37]; especially for animal models of social communication disorders such as autism [14,21,31]. The present findings suggest that urine marking in C57 males may be sufficiently responsive to subject age and stimulus gender and genetic factors as to provide a highly sensitive paradigm of social behavior that includes both social signaling and communication factors.
Acknowledgements
This study was supported by SNRP grant 5U54NS039406 to RJB and JSPS Grant-in-Aid for Scientific Research No.16.1415 to HA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson PK, Hill JL. Mus musculus: experimental induction of territory formation. Science. 1965;148:1753–5. doi: 10.1126/science.148.3678.1753. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa H. Changes in the pattern of exploratory behavior are associated with the emergence of social dominance relationships in male rats. Dev Psychobiol. 2006;48:39–47. doi: 10.1002/dev.20114. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchini A, Gaetani E, Cavaggioni A. Pheromone binding proteins of the mouse, Mus musculus. Experientia. 1992;48:419–21. doi: 10.1007/BF01923448. [DOI] [PubMed] [Google Scholar]
- 5.Barnard CJ, Fitzsimmons J. Kin recognition and mate choice in mice: the effects of kinship familiarity and social interference on intersexual interactions. Anim Behav. 1988;36:1078–90. [Google Scholar]
- 6.Barnard CJ, Hurst JL, Aldhous P. Of mice and kin: the functional significance of kin bias in social behaviour. Biol Rev. 1991;66:379–430. doi: 10.1111/j.1469-185x.1991.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 7.Bartolomucci A, Chirieleison A, Gioiosa L, Ceresini G, Parmigiani S, Palanza P. Age at group formation alters behavior and physiology in male but not female CD-1 mice. Physiol Behav. 2004;823:425–34. doi: 10.1016/j.physbeh.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Beynon RJ, Hurst JL. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem Soc trans. 2003;2003;31:142–6. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard DC, Arakawa H, Crawley JN, Blanchard RJ. Social Behaviors in wild and laboratory mice with a special emphasis on the C57BL/6J inbred strain. In: Crusio WE, Sluyter F, Gerlai RT, editors. Handbook of Behavioral Genetics of the Mouse. Elsevier; in press. [Google Scholar]
- 10.Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–10. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- 11.Boyse EA, Beauchamp GK, Yamazaki K. The genetics of body scent. Trends Genet. 1987;3:97–102. [Google Scholar]
- 12.Bronson FH, Desjardins C. Relationships between scent marking by male mice and the pheromone-induced secretion of the gonadotropic and ovarian hormones that accompany puberty in female mice. In: Montagna W, Sadler WA, editors. Reproductive behavior. Plenum Press; New York: 1974. pp. 157–178. [DOI] [PubMed] [Google Scholar]
- 13.Brown RE, McDonald DW. Social odours in mammals. 1 & 2. Clarendon Press; Oxford: 1985. [Google Scholar]
- 14.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Payler R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacol. 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 15.Crowcroft P, Rowe FP. Social organization and territorial behaviour in the wild house mouse (Mus musculus L.) Proc Zool Soc Lond. 1963;140:517–31. [Google Scholar]
- 16.Crusio WE. Flanking gene and genetic background problems in genetically manipulated mice. Biol Psychiatry. 2004;56:381–5. doi: 10.1016/j.biopsych.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–41. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 18.Drickamer LC. Urine marking and social dominance in male house mice (Mus musculus domesticus) Behav Processes. 2001;53:113–20. doi: 10.1016/s0376-6357(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 20.Ferkin MH, Li HZ. A battery of olfactory-based screens for phenotyping the social and sexual behaviors of mice. Physiol Behav. 2005;85:489–99. doi: 10.1016/j.physbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 22.Hale RL, Randall CL, Becker HC, Middaugh LD. The effect of prenatal ethanol exposure on scentmarking in the C57BL/6J and C3H/He mouse strains. Alcohol. 1992;9:287–92. doi: 10.1016/0741-8329(92)90068-l. [DOI] [PubMed] [Google Scholar]
- 23.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Humphries RE, Robertson DHL, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mice. Anim Behav. 1999;58:1177–90. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- 25.Hurst JL. The functions of urine marking in a free-living population of house mice, Mus domesticus Rutty. Anim Behav. 1987;35:1433–42. [Google Scholar]
- 26.Hurst JL. The complex network of olfactory communication in populations of wild house mice, Mus domesticus Rutty: urine marking and investigation within family groups. Anim Behav. 1989;37:705–25. [Google Scholar]
- 27.Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. I. communication between males. Anim Behav. 1990;40:209–22. [Google Scholar]
- 28.Hurst JL. The priming effects of urine substrate marks on interacgtions between male house mice, Mus musculus domesticus Schwarz & Schwarz. Anim Behav. 1993;45:55–81. [Google Scholar]
- 29.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 30.Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signaling in mice. BioEssays. 2004;26:1288–98. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- 31.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 32.Jones RB, Nowell NW. Aversive and aggression-promoting properties of urine from dominant and subordinate male mice. Anim Behav. 1973;21:207–10. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- 33.Jones RB, Nowell NW. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Anim Learn Behav. 1974;2:141–4. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- 34.Jones RB, Nowell NW. Aversive potency of urine from dominant and subordinate male laboratory mice (Mus musculus): resolution of a conflict. Aggress Behav. 1989;15:291–6. [Google Scholar]
- 35.Kavaliers M, Kinsella DM. Male preference for the odors of estrous female mice is reduced by the neurosteroid pregnenolone sulfate. Brain Res. 1995;682:222–226. doi: 10.1016/0006-8993(95)00335-n. [DOI] [PubMed] [Google Scholar]
- 36.Lacey JC, Beynon RJ, Hurst JL. The importance of exposure to other male scents in determining competitive behaviour among inbred male mice. Appl Anim Behav Sci. 2006;104:130–42. [Google Scholar]
- 37.Le Roy I, Pothion S, Mortaud S, Chabert C, Nicolas L, Cherfouh A, Roubertoux PL. Loss of aggression, after transfer onto a C57BL/6J background, in mice carrying a targeted disruption of the neuronal nitric oxide synthase gene. Behav Genet. 2000;30:367–73. doi: 10.1023/a:1002796404278. [DOI] [PubMed] [Google Scholar]
- 38.Lore RK, Stipo-Flaherty A. Postweaning social experience and adult aggression in rats. Physiol Behav. 1984;33:571–4. doi: 10.1016/0031-9384(84)90373-1. [DOI] [PubMed] [Google Scholar]
- 39.Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav. 1974;12:1035–9. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- 40.Maruniak JA, Wysocki CJ, Taylor JA. Mediation of male mouse urine marking and aggression by the vomeronasal organ. Physiol Behav. 1986;37:655–7. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- 41.Mihalick SM, Langlois JC, Krienke JD. Strain and sex differences on olfactory discrimination learning in C57BL/6J and DBA/2J inbred mice (Mus musculus) J Comp Psychol. 2000;114:365–70. doi: 10.1037/0735-7036.114.4.365. [DOI] [PubMed] [Google Scholar]
- 42.Mogil JS, Wilson SG. Nociceptive and morphine antinociceptive sensitivity of 129 and C57BL/6 inbred mouse strains: implications for transgenic knock-out studies. Euro J Pain. 1997;1:293–7. doi: 10.1016/s1090-3801(97)90038-0. [DOI] [PubMed] [Google Scholar]
- 43.Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–8. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- 44.Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;226:967–8. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod. 1990;42:649–55. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- 46.Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. The consequences of inbreeding for recognizing competitors. Proc Biol Sci. 2000;267:687–94. doi: 10.1098/rspb.2000.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in mouse scent marks is involatile. Proc Royal Soc B. 2003;270:1957–63. doi: 10.1098/rspb.2003.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell V, Blanchard RJ, Blanchard DC. Mouse aggression increases after 24 hours of isolation or housing with females. Behav Neural Biol. 1981;32:89–103. doi: 10.1016/s0163-1047(81)90317-4. [DOI] [PubMed] [Google Scholar]
- 50.Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–7. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne CE, Malone N, Humphries RE, Bradbrook C, Veggerby C, Beynon RJ, Hurst JL. Heterogeneity of major urinary proteins in house mice: population and sex differences. In: Marchelewska-Koj A, Muller-Schwarze D, Lepri J, editors. Chemical Signals in Vertebrates. Vol. 9. Plenum; New York: 2001. pp. 233–240. [Google Scholar]
- 52.Ralls K. Mammalian scent marking. Science. 1971;171:443–9. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- 53.Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim Behav. 1999;58:1027–37. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- 54.Schellinck HM, West AM, Brown RE. Rats can discriminate between the urine odors of genetically identical mice maintained on different diets. Physiol Behav. 1992;51:1079–82. doi: 10.1016/0031-9384(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 55.Silver LM. Mouse Genetics. Oxford University Press; Oxford: 1995. [Google Scholar]
- 56.Sipos ML, Nyby JG. Intracranial androgenic activation of male-typical behaviours in house mice: concurrent stimulation of the medial preoptic area and medial nucleus of the amygdala. J Neuroendocrinol. 1998;10:577–86. doi: 10.1046/j.1365-2826.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- 57.Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr Opin Neurobiol. 1998;8:263–71. doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- 58.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci U S A. 1981;78:5817–20. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamazaki K, Yamaguchi M, Baranoski L, Bard J, Boyse EA, Thomas L. Recognition among mice: evidence from the use of a Y-maze differentially scented by congenic mice of different major histocompatibility types. J Exp Med. 1979;150:755–60. doi: 10.1084/jem.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamazaki K, Beauchamp GK, Singer A, Bard J, Boyse EA. Odortypes: their origin and composition. Proc Natl Acad Sci USA. 1999;96:1522–5. doi: 10.1073/pnas.96.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiatry. 2002;7:S38–39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]