Abstract
Background and Objectives
Following total knee arthroplasty (TKA), hospitalization may be shortened by allowing patients to return home with a continuous femoral nerve block (CFNB). This study quantified the hospitalization costs for 10 TKA patients receiving ambulatory CFNB versus a matched cohort of 10 patients who received CFNB only during hospitalization.
Methods
We examined the medical records (n=125) of patients who underwent a unilateral, primary, tricompartment TKA with a postoperative CFNB by one surgeon at one institution in an 18-month period beginning January 2004. Each of the ten patients discharged home with an ambulatory CFNB (cases) was matched with a patient with a hospital-only CFNB (controls) for age, gender, body mass index, and health status. Financial data were extracted from the hospital micro-costing database.
Results
Nine patients with ambulatory CFNB (cases) were discharged home on postoperative day (POD) 1, and one on POD 4. Of the controls, 3 were discharged home on POD 3, 6 on POD 4, and 1 on POD 5. The median (range) costs of hospitalization (excluding implant and professional fees) was $5,292 (4,326 – 7,929) for ambulatory cases, compared with $7,974 (6,931 – 9,979) for inpatient controls (difference=$2,682, 34% decrease, P<0.001). The total charges for hospitalization, the implant, and professional fees was $33,646 (31,816 – 38,468) for cases, compared with $39,100 (36,096 – 44,098) for controls (difference=$5,454, 14% decrease, P<0.001).
Conclusions
This study provides evidence that ambulatory CFNB for selected patients undergoing TKA has the potential to reduce hospital length-of-stay and associated costs and charges. However, the current study has significant inherent limitations based on the study design. Additional research is required to replicate these results in a prospective, randomized, controlled trial and to determine whether any savings exceed additional CFNB costs such as from complications, having caregivers provide care at home, and additional hospital/health-care provider visits.
Keywords: ambulatory continuous peripheral nerve block, ambulatory continuous femoral nerve block, continuous peripheral nerve block, continuous femoral nerve block, ambulatory perineural local anesthetic infusion, health economics
INTRODUCTION
Total knee arthroplasty (TKA) is one of the most common elective surgical procedures, with over 450,000 cases performed annually in the United States alone.1 The number of TKA procedures has doubled in each of the past 2 decades, and is expected to continue to increase as the population ages.1–3 These surgeries are among the most expensive procedures, with hospital, implant, and physician charges averaging nearly $40,000 per case, and totaling $15–18 billion annually in the United States.2;3 Despite these high costs, TKA is one of the most cost-effective interventions relative to other medical treatments as TKA provides substantial improvements in quality of life due to reduced pain and increased mobility.4;5 How society will underwrite the associated costs of high-value interventions like TKA is unknown.3;6;7
Consequently, health care providers have attempted to contain costs through various interventions such as competitive bidding for implants,7;8 decreasing medication costs,8–10 clinical pathways,11 and shortening the period of hospitalization.12 Using a hospital-based epidural or continuous femoral nerve block (CFNB) for 48–72 h, anesthesiologists have reported shortened institutionalization from 50 to 40 days (France), 21 to 17 days (Belgium), and 5 to 4 days (United States) compared with using only IV opioids.13–15 However, hospitalization length-of-stay in the United States has changed little over the past decade,1 persistently averaging 4 days, at least in part because of the need for potent analgesia following TKA.16
Unlike IV opioids or epidural infusion of local anesthetic with or without opioids, perineural local anesthetic infusion does not require hospitalization.17 In a recent prospective longitudinal cohort study, conventional TKA (non-minimally invasive) was performed as an overnight-stay procedure in 9 of 10 selected patients using small, portable infusion pumps that allowed discharge directly home with the CFNB in situ for over 4 postoperative days (POD).18 All of these patients reported analgesia equal or superior to patients with hospital-based CFNB.13–15;19 without any associated complications. Providing ambulatory CFNB was primarily an attempt to enable earlier discharge in motivated patients who desired convalescence in the comfort of their own homes. However, this study did not include a control group and any reduction in length-of-stay remains speculative. Moreover, an additional possible indirect benefit of this intervention may have been a decrease in the associated costs of hospitalization.
Therefore, the goal of this retrospective, case-control study was to examine the relationship between providing a CFNB on an ambulatory versus hospital-only basis, and hospitalization costs following conventional TKA.
METHODS
Following Institutional Review Board (University of Florida, Gainesville, FL) approval, we examined the medical records for patients who underwent TKA at one institution (Shands Hospital at the University of Florida, Gainesville, FL) with a single orthopedic surgeon during an 18-month period beginning January 2004 (Fig. 1). Patients who had participated in a pilot study or a randomized clinical trial (RCT) were excluded. Subjects were retained for further evaluation if they had undergone unilateral, primary, tricompartment, cemented TKA for osteoarthritis under a general anesthetic. Furthermore, the postoperative analgesic regimen required for study retention included a CFNB with ropivacaine, 0.2% (basal infusion ≥ 5 mL/h), and 10 mg of controlled-release oxycodone administered twice each day.
Figure 1.
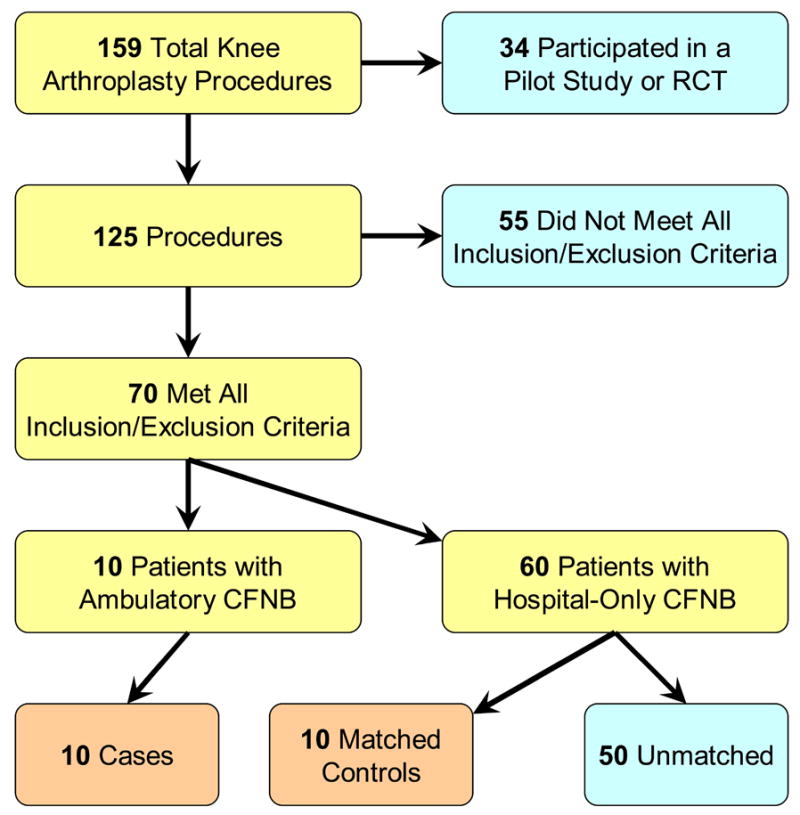
Study inclusion and matching. RCT: Randomized clinical trial. CFNB: Continuous femoral nerve block.
Study Group Assignment
“Cases” were defined as patients who were discharged home with their CFNB, all of whom had participated in a previously-published Institutional Review Board-approved prospective longitudinal cohort study.18 Of note, these subjects were a unique subset of patients in that they were all under 70 years of age, had no known cardiac disease or risk factors for deep-vein thrombosis, and were interested in returning home the day following a TKA.18 All other remaining patients who had received a hospital-based CFNB were designated as “controls.” Care and resource use for each of the treatment groups was similar except for the earlier discharge in the CFNB group (Table 1). For example, physical therapy for CFNB patients was delivered at home by the same department as the inpatients, so costs for physical therapy were captured under hospital costs/charges for both groups.
Table 1.
Comparison of protocol and clinical pathway used for the treatment groups.
| Cases with an Ambulatory CFNB | Controls with a Hospitalized CFNB | Costs/Charges Included? | |
|---|---|---|---|
| Pre- and Intraoperative | |||
| Preoperative clinic visit | Shands Hospital | Same | Yes |
| Surgical procedure | Primary, unilateral, tricompartment, cemented TKA via a 12–18 cm midline incision and parapatellar approach | Same | Yes |
| ASA Physical Status | 2 | Same | N/A |
| Femoral catheter placed | Regional anesthesia induction area | Same | Yes |
| Perineural infusion local anesthetic | Ropivacaine 0.2% initiated in the operating room | Same | Yes |
| Perineural infusion basal infusion rate | 8 mL/h until day of discharge, then 5 mL/h | 5–10 mL/h | N/A |
| Perineural infusion bolus dose and lockout | 4 mL bolus; 30 min lockout until POD 1, then 60 min | 2–5 mL bolus; 20–30 min lockout | N/A |
| Primary anesthetic | Sevoflurane, nitrous oxide | Inhalation agent, not specified | Yes |
| IV fluids | Hetastarch 15 mL/kg | Not specified | Yes |
| Recovery room supplemental analgesic | IV morphine | Same | Yes |
| Cases with an Ambulatory CFNB (n=10) | Controls with a Hospitalized CFNB (n=10) | Cost/Charges Included? | |
| Postoperative/Hospitalized | |||
| Hospital unit | General Clinical Research Center | General orthopedic unit | Yes |
| Scheduled analgesics | Controlled-release oxycodone 10 mg every 12 h and acetaminophen 975 mg every 6 h | Same | Yes |
| DVT prophylaxis | Enteric-coated aspirin 650 mg daily | Same | Yes |
| Supplemental analgesics | Oxycodone 5–10 mg (NRS ≤ 7) IV morphine (NRS > 7) | Oxycodone 5–10 mg and IV morphine (NRS ranges not specified) | Yes |
| Transfusion | 2 units packed red blood cells for hematocrit < 30 | Same | Yes |
| Duration of CFNB | Catheter removed at home on postoperative day 4 | Catheter removed in the hospital at surgeon’s discretion | Yes |
| Pain management | Anesthesiologist | Anesthesiologist during CFNB, then orthopedic surgeon | Yes |
| Physical therapy | Twice daily until discharge | Same | Yes |
| Primary discharge criteria | NRS consistently ≤ 4
IV morphine < 5 mg previous 24 h Ambulate > 30 m |
NRS: same
IV morphine: same Ambulate > 24–30 m |
N/A |
| Home management | |||
| Scheduled analgesics | Controlled-release oxycodone 10 mg every 12 h and acetaminophen 975 mg every 6 h | Same | No |
| DVT prophylaxis | Enteric-coated aspirin 650 mg daily | Same | No |
| Supplemental analgesics | Oxycodone 5–10 mg | Same | No |
| Home nursing visit | Day following discharge | Same | Yes |
| Home physical therapy | Daily through postoperative day 5 | Same | Yes |
| Pain management | Anesthesiologist (available by pager and daily phone calls) | Orthopedic surgeon (available by pager) | Yes |
ASA: American Society of Anesthesiologists
N/A: Not applicable
TKA: total knee arthroplasty
CFNB: continuous femoral nerve block
DVT: deep vein thrombosis
NRS: numeric rating pain scale
Data was transferred by keypunch entry into a computerized database (Office Excel 2003, Microsoft Corporation, Bellevue, WA). Each case was matched with one control for the following variables: age (±5 yr), gender, body mass index (±5 kg/m2), and ASA Physical Status. Investigators were blinded to the duration of both hospitalization and CFNB for the control group during the matching process.
Outcome Measures
The primary end point was the hospitalization cost. Included in this value were costs for the preoperative clinic visit, day-of-surgery, postoperative hospital course including room and board, home nursing visit, and physical and occupational therapy during the first postoperative week (for both in- and out-patient sessions). The cost of the knee implant itself and physician professional fees were excluded for the primary end point. Secondary end points included hospitalization charges (including room and board); room/board costs and charges (separate from other costs and charges); implant charges; and physician professional fees. Additional secondary endpoints included the surgical, CFNB, and hospitalization durations. Financial data was extracted using the hospital’s internally-developed micro-costing database. Due to the short time span of charges that were reviewed (18 months), no adjustments were made for inflation.
Statistical Analysis
Sample size calculations were derived based on the primary hypothesis that providing ambulatory CFNB is associated with a decrease in hospitalization costs compared with providing CFNB solely during hospitalization. Therefore, the costs of hospitalization (excluding physician professional fees and implant cost) were used to estimate a probable sample size. Since the cases were comprised of only 10 patients who were provided ambulatory CFNB, the only variable that could be manipulated to power the study was the number of control subjects assigned to each ambulatory CFNB subject. Based on published data,1;3;12;20 mean costs of hospitalization for patients with hospital-only CFNB (controls) was anticipated to be $10,000 in U.S. dollars. A $1,500 (15%) difference in costs between groups was considered to be economically relevant. Assuming a SD in both groups of $1,000, a two-sided type I error protection of 0.05, and a power of 0.80, only one control subject per case subject was required to demonstrate an economically-significant difference between study groups for the primary end point (SigmaStat 3.1, SPSS, Inc., Chicago, IL).
Normality of distribution was determined using the Kolmogorov-Smirnov test (NCSS Statistical Software, Kaysville, UT). Possible differences between groups were analyzed using a t-test or Wilcoxon Rank-Sum test for parametric and nonparametric data, respectively. Categorical data were analyzed using the Chi square test with Yates continuity correction. P<0.05 was considered significant.
RESULTS
Ten cases were successfully matched with an equal number of controls (Fig. 1, Table 2).
Table 2.
Demographic and surgical information.
| Parameter | Cases with an Ambulatory CFNB (n=10) | Controls with a Hospitalized CFNB (n=10) |
|---|---|---|
| Age (yr) | 61 ±5 | 62 ±7 |
| Sex (F/M) | 4/6 | 4/6 |
| Height (cm) | 172 ± 11 | 174 ± 11 |
| Weight (kg) | 95 ± 13 | 96 ± 20 |
| Body mass index (kg / m2) | 32 ± 6 | 31 ± 6 |
| ASA Physical Status | 2 ± 0 | 2 ± 0 |
| Surgical duration (min) | 113 ± 16 | 125 ± 18 |
Values are reported as mean ± SD
There were no statistically significant differences between treatment groups
CFNB: Continuous femoral nerve block
ASA: American Society of Anesthesiologists
Primary Endpoint
The total median (range) hospitalization costs for ambulatory CFNB cases was $5292 (4,326 – 7,929), compared with $7,974 (6,931 – 9,979) for control patients with a CFNB only during hospitalization (difference=$2,682, 34% lower than the cost of controls), P<0.001, Fig. 2).
Figure 2.
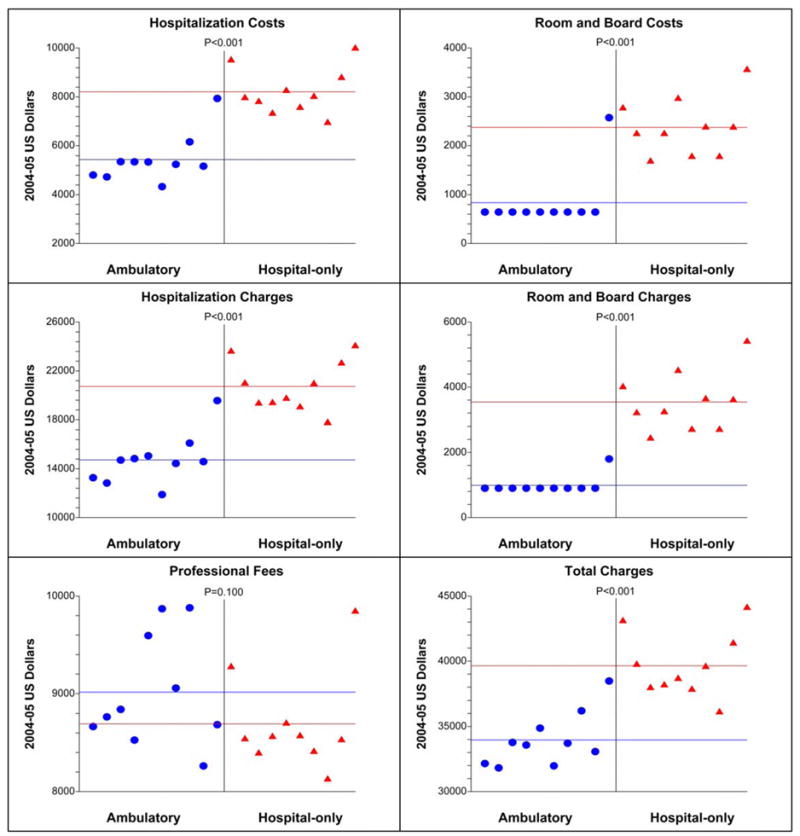
The costs and charges related to total knee replacement surgery for patients with an ambulatory continuous femoral nerve block (CFNB, “cases”) and hospital-only CFNB (“controls”). The horizontal lines demarcate the group means. Of the patients in the ambulatory CFNB group, the highest data point in every panel (except Professional Fees) denotes the one subject who required four days of hospitalization as a result of a bowel obstruction.
Secondary Endpoints
Of the 10 cases with ambulatory CFNB, 9 patients were discharged home on POD 1, and one was discharged home in the morning of POD 4 because of a bowel obstruction that resolved on POD 3.18 All cases retained their CFNB using portable infusion pumps upon discharge that enabled perineural ropivacaine infusion at home until POD 4. In contrast, control subjects had their CFNB discontinued prior to discharge, with an infusion duration of 2 days for 7 patients, and 3 days for 3 patients. Of these 10 control subjects, discharge occurred on POD 3 for 3 patients, POD 4 for 6 patients, and POD 5 for 1 patient. The total charges for hospitalization, the implant, and professional fees were $33,646 (31,816 – 38,468) for cases, compared with $39,100 (36,096 – 44,098) for controls (difference=$5,454, 14% lower than the cost of controls, P<0.001, Fig. 2). Additional secondary endpoints are presented in Figure 2 and Table 3.
Table 3.
Financial endpoints, presented in 2004–2005 U.S. dollars.
| Cases with an Ambulatory CFNB (n=10) | Controls with a Hospitalized CFNB (n=10) | P- value | Difference between Cases and Controls | Difference as Percentage of Controls | |
|---|---|---|---|---|---|
| Costs | |||||
| Hospitalization (including room & board) | 5,292 (4,326 – 7,929)
5,438 ± 1,000 |
7,974 (6,931 – 9,979)
8,203 ± 959 |
<0.001 | 2,682 | 34% |
| Room & board alone | 643 (643 – 2,572)
836 ± 610 |
2,304 (1,776 – 3,552)
2,372 ± 588 |
<0.001 | 1,661 | 72% |
|
| |||||
| Charges | |||||
| Hospitalization (including room & board) | 14,643 (11,875 – 19,556)
14,720 ± 2,090 |
20,726 (17,745 – 24,029)
20,726 ± 2,092 |
<0.001 | 6,083 | 29% |
| Room & board alone | 900 (900 – 1,800)
990 ± 285 |
3,415 (2,430 – 5,400)
3,539 ± 910 |
<0.001 | 2,515 | 74% |
| Implant | 10,227 (10,227 – 10,227)
10,227 ± 0 |
10,227 (10,227 – 10,227)
10,227 ± 0 |
1.000 | 0 | 0% |
| Professional fees | 8,804 (8,262 – 9,880)
9,015 ± 572 |
8,549 (8,391 – 9,842)
8,693 ± 499 |
0.100 | (−) 255 | (−) 3% |
| Total charges* | 33,646 (31,816 – 38,468)
33,962 ± 2,083 |
39,100 (36,096 – 44,098)
39,646 ± 2,511 |
<0.001 | 5,454 | 14% |
Values are reported as median (range) and mean ± SD.
CFNB: Continuous femoral nerve block
Includes hospitalization and implant charges plus professional fees
DISCUSSION
This retrospective, case-control study provides evidence that following conventional (non-minimally invasive) TKA, allowing hospital discharge with an ambulatory CFNB is associated with a 34% ($2,682 per patient) decrease in hospitalization costs compared to inpatient-only CFNB. Nearly two-thirds of the savings resulted from lower room and board costs, as might be expected with all but one of the case subjects hospitalized just one night, compared with 3–5 nights for control subjects. However, this leaves over 38% of the cost savings resulting from additional sources.
When charges—as opposed to costs—are compared, the per-patient savings increases to $6,083 (29%). This finding may be relevant to insurers with contracts to pay discounted fee-for-service rates. When the total TKA-related charges are calculated with the addition of the implant and physician professional fees, the per-patient savings was $5,454 (14%). Considering over 450,000 TKA procedures are performed annually in the United States alone, the potential savings to society may be significant, as long as increased complication or caretaker costs do not appreciably increase total costs. This study helps quantify the potential for anesthesiologists to contribute to TKA cost-containment by providing ambulatory CFNB. A reliable projection of possible annual savings requires information not currently available from either this study or the published literature.
Cost-minimization analysis
Convened by the United States Public Health Service, the Panel of Cost Effectiveness in Health and Medicine published a set of standards to facilitate the comparability of cost-benefit analyses.21 The current study follows these methodological guidelines.21 In cost-effectiveness analysis, as defined by the Panel, the “added costs and health outcomes associated with a program are used to calculate the incremental cost-effectiveness ratio relative to some comparator.”21 However, the retrospective nature of the current study did not allow for comparison of similarly-collected health-outcome data. Therefore, the present study is a type of cost-effectiveness analysis labeled cost-minimization analysis, in which the “effectiveness of the intervention and the comparator are presumed to be equal.”21 Although preliminary evidence suggests that providing CFNB at home on POD 1–4 results in equal or superior outcomes compared with hospital-only CFNB,13;14;18;19;22 substantiation of this assertion requires a large-sample, multiyear RCT. While there were no complications related to early discharge in the small cohort on which the current study is based, a hospital readmission rate of only 10% would probably negate any cost savings.
Related to these issues, the Panel proposed that, “although the effectiveness of alternative programs is rarely exactly equal, this assumption may be a reasonable approximation in some cases [of cost-minimization analysis].” And while the patients of the current study did not receive low molecular weight heparin, given the recent report of clinically-relevant hematoma following femoral catheter placement for TKA associated with low molecular weight heparin administration, great caution in is warranted by all practitioners discharging patients home with a catheter in situ.23
As recommended by the Panel, the primary outcome measure for this analysis involved costs, as opposed to charges.21 Analyzing costs instead of charges is preferred because market distortion or imperfection may significantly influence the relationship between prices (charges) and resource requirements (costs).21;24 Typically, hospitals increase charges by a certain percentage each year. Hospitals tend not to individually adjust the pricing for each charge code to bring the charge value in line with the item’s current cost. Thus, the relationship between charge values and items' actual costs can be inconsistent. However, secondary outcome measures are provided to allow comparisons from additional perspectives, which may provide useful information to various specific interests and audiences.
A distinctive feature of cost data in health care is its asymmetrical distribution—skewed to the right—and large variance. Causes of skewed (e.g., right-sided tail) data include that the severe cases require substantially more resources. The three measures of central tendency or “average” value of the distribution are mode, median, and mean. The high-cost patients will influence some measures of central tendency (the mean cost will be “increased” due to the right tail cases), but not others (medians). For these reasons, we have presented data as median (ranges). However, when information about the costs of alternative treatments is to be used to guide healthcare policy decision making, it is the total budget needed to treat patients with the disease that is relevant. An estimate of this total cost is obtained from data in a trial by multiplying the arithmetic mean (average) cost in a particular treatment group by the total number of patients to be treated. It is therefore the arithmetic mean that is the informative measure for cost data in pragmatic clinical trials, and we have therefore provided the mean (±SD) data in Table 3.
Cost shifting
Similarly, some of the costs of care on POD 2–5 may be transferred from hospitals and orthopedic surgeons to anesthesiologists and family members. In the current study, there was minimal change in anesthesiologists’ time commitment (costs) by providing ambulatory CFNB since they provide analgesia regardless of the patients’ location, or reimbursement (charges) since CFNB is billed as a global fee regardless of infusion duration. However, patients returning home with an ambulatory CFNB required a caretaker for the duration of the infusion.18 For the cases, patient caretakers were comprised exclusively of retired spouses, and therefore calculations did not include forgone wages. But information regarding potential caretakers for control subjects was unavailable due to the retrospective nature of the study design, and it is therefore impossible to calculate any opportunity cost for these individuals. Regardless of these missing data, the Panel noted that, “if an intervention requires… time spent away from other activities (including leisure), then there has been a change in the way resources are used, even if there is no monetary transaction. In such cases, there are opportunities forgone, and a ‘cost’ results in the form of the loss of the benefits that would come from the alternative use of time.”21 Such a cost calculation is extremely complex and highly population-dependent,25 and was therefore not attempted by the authors. It is thus an acknowledged weakness of the present study. Future cost-effectiveness investigations into ambulatory CFNB should include both the costs and effects of requiring patient caretakers to remain at home for various populations.
Other factors besides caretaker opportunity costs were beyond the scope of this analysis, yet would be expected to influence the financial scenario of implementing ambulatory CFNB. For example, although the median per-patient total charges were $5,454 (14%) less in the ambulatory CFNB group and therefore a fall in the per-patient average hospital revenue might be expected, total hospital revenue might actually be increased if the resulting empty hospital beds allowed performance of additional TKA procedures. Given this scenario, ambulatory CFNB may not reduce financial costs from a societal perspective, but rather enable an increased number of procedures, and thereby increase quality-of-life by decreasing the often long wait for joint replacement.26;27
In addition, charges must not be confused with collections: although ambulatory CFNB was associated with a median per-patient decrease in total charges of $5,454 (14%), actual collections remained unchanged. This may be explained by the hospital having pre-negotiated TKA reimbursement with various insurers, and payment is independent of hospitalization duration. Therefore, since hospital costs decreased by a median of $2,682 (34%) per patient, the hospital may have actually increased its per-patient profit margin, and at the same time increased its ward capacity having staffed beds now available for other patients.
Study limitations
This investigation involved only one orthopedic surgeon operating from a single university hospital. Evidence for internal validity may be found in the one subject who was provided ambulatory CFNB but remained hospitalized until POD 4 because of a bowel obstruction: the costs and charges of her care were similar to those of control subjects (Fig. 2). However, the external validity remains unknown, and undoubtedly would have been improved by a larger sample of institutions and surgeons. In addition, this study had a limited number of patients. We are currently designing larger studies now that investigation feasibility is established.18 Of note, the average per-patient total charge for the control group is similar to the national mean, suggesting that the hospital of this study is a relatively good representation of other institutions in the United States.2;3
A significant limitation of the present study is its retrospective, non-randomized design, which introduces inherent biases shared by all case-control studies. We controlled for eleven confounding variables, but certainly do not imply definitive conclusions with these data. One potential source of bias is the study protocol used for the ten patients provided ambulatory CFNB.18 However, this protocol was designed specifically to reflect the one surgeon’s standard clinical pathway that was also utilized for the control patients (including specific discharge criteria). Differences between the protocol used for the cases and controls were minimal, but may have had an effect on the outcome measures (Table 1). Of note, we could not account for the post-discharge oral analgesic and thromboprophylactic prescription costs/charges as these were financially covered by patients’ insurance companies and not included in the hospital micro-costing database. Yet, prescriptions for patients of both groups were identical, so this omission should have no effect on between-group comparisons. In addition, we cannot exclude a possible “healthy volunteer effect” in which patients who choose to participate in prospective research have better outcomes than other patients.28
Analysis model
The time-horizon for this study began with the preoperative visit, and included the day of surgery and first postoperative week. However, if ambulatory CFNB increased or decreased subsequent care requirements, those costs and charges should be included in any future cost-consequence analysis. Follow-up analysis of the ambulatory CFNB group does not suggest supplemental care was necessary, but a large RCT is required for confirmation.
In summary, the results of this retrospective, case-control investigation suggest that providing ambulatory CFNB following TKA is associated with a decrease in hospitalization costs and charges. However, the current study has significant inherent limitations based on the study design. Additional research is required to confirm these findings with a prospective RCT and measure the long-term costs and benefits of this relatively new intervention. While these results help identify and quantify the potential for anesthesiologists to contribute to TKA cost-containment, it is not the authors’ intention to suggest that financial considerations dictate the use of ambulatory CFNB to enable earlier discharge following TKA. Rather, ambulatory CFNB may be provided for multiple other reasons—all of which require additional confirmatory study—with a decrease in related costs being an indirect benefit.18 It is well established that, “changing technology in medicine results in increased spending and accounts for one half to two thirds of the increase in health care spending in excess of general inflation [in the United States].”6 It is therefore notable that ambulatory CFNB may be a rare instance of a new technology holding the promise of both improving outcomes, and reducing societal costs.
Acknowledgments
The authors gratefully acknowledge the valuable assistance of Nikolaus Gravenstein, MD, Department Chair, Department of Anesthesiology, University of Florida, Gainesville, FL, for his assistance in preparing this manuscript.
Funding for this project provided by the University of Florida, Department of Anesthesiology. Dr. Ilfeld is supported by NIH grant GM077026 (Bethesda, MD), and a Mentored Research Training Grant from the Foundation of Anesthesia Education and Research (Rochester, MN). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities. Dr. Ilfeld has received research funds and product from companies producing perineural catheters (Arrow International, Reading, PA; B. Braun Medical Inc., Bethlehem, PA) and portable infusion pumps (Smiths Medical, St. Paul, MN; Sorenson Medical, West Jordan, UT; Stryker Instruments, Kalamazoo, MI). Dr. Williams has received honoraria, unrestricted research funds, and/or product from companies producing perineural catheters (Arrow International, Reading, PA; B. Braun Medical Inc., Bethlehem, PA) and portable infusion pumps (I-Flow Corporation, Lake Forest, CA). These companies had no input into any aspect of study conceptualization, initiation, and design; data collection, analysis and interpretation; or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.American Academy of Orthopaedic Surgeons website. [4–22-2006.]; http://www.aaos.org.
- 2.Weinstein J. The Dartmouth Atlas of Musculoskeletal Health Care. Chicago, IL: AHA Press; 2000. pp. 72–78. [PubMed] [Google Scholar]
- 3.Mushinski M. Average charges for a total knee replacement: United States, 1994. StatBullMetropInsurCo. 1996;77:24–30. [PubMed] [Google Scholar]
- 4.Liang MH, Cullen KE, Larson MG, Thompson MS, Schwartz JA, Fossel AH, Roberts WN, Sledge CB. Cost-effectiveness of total joint arthroplasty in osteoarthritis. Arthritis Rheum. 1986;29:937–43. doi: 10.1002/art.1780290801. [DOI] [PubMed] [Google Scholar]
- 5.Rissanen P, Aro S, Sintonen H, Asikainen K, Slatis P, Paavolainen P. Costs and cost-effectiveness in hip and knee replacements. A prospective study. Int J Technol Assess Health Care. 1997;13:575–88. doi: 10.1017/s0266462300010059. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg PB. Controlling health care costs. NEnglJ Med. 2004;351:1591–3. doi: 10.1056/NEJMp048159. [DOI] [PubMed] [Google Scholar]
- 7.Clark CR. Cost containment: total joint implants. J Bone Joint Surg Am. 1994;76:799–800. doi: 10.2106/00004623-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman JD, Kummer FJ, Frankel VH. The effectiveness of a hospital-based strategy to reduce the cost of total joint implants. J Bone Joint Surg Am. 1994;76:807–811. doi: 10.2106/00004623-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone RE, Jozefczyk KG. Costs of anesthetic drugs: experiences with a cost education trial. Anesth Analg. 1994;78:766–771. doi: 10.1213/00000539-199404000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Becker KE, Jr, Carrithers J. Practical methods of cost containment in anesthesia and surgery. J Clin Anesth. 1994;6:388–399. doi: 10.1016/s0952-8180(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 11.Macario A, Horne M, Goodman S, Vitez T, Dexter F, Heinen R, Brown B. The effect of a perioperative clinical pathway for knee replacement surgery on hospital costs. Anesth Analg. 1998;86:978–984. doi: 10.1097/00000539-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279:847–852. doi: 10.1001/jama.279.11.847. [DOI] [PubMed] [Google Scholar]
- 13.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, Criswell A. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16:436–445. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 16.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94:130–137. doi: 10.1097/00000539-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100:1822–1833. doi: 10.1213/01.ANE.0000151719.26785.86. [DOI] [PubMed] [Google Scholar]
- 18.Ilfeld BM, Gearen PF, Enneking FK, Berry LF, Spadoni EH, George SZ, Vandenborne K. Total knee arthroplasty as an overnight-stay procedure using continuous femoral nerve blocks at home: a prospective feasibility study. Anesth Analg. 2006;102:87–90. doi: 10.1213/01.ane.0000189562.86969.9f. [DOI] [PubMed] [Google Scholar]
- 19.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 20.Healy WL, Finn D. The hospital cost and the cost of the implant for total knee arthroplasty. A comparison between 1983 and 1991 for one hospital. J Bone Joint Surg Am. 1994;76:801–806. doi: 10.2106/00004623-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. 1. New York: Oxford University Press; 1996. [Google Scholar]
- 22.Ilfeld BM, Gearen PF, Enneking FK, George SZ, Vandenborne K. The effects of ambulatory continuous femoral nerve blocks on readiness-for-discharge following total knee arthroplasty: a randomized, double-blind, placebo-controlled pilot study, abstracted. Anesthesiology. 2005;103:A1013. [Google Scholar]
- 23.Bickler P, Brandes J, Lee M, Bozic K, Chesbro B, Claassen J. Bleeding complications from femoral and sciatic nerve catheters in patients receiving low molecular weight heparin. Anesth Analg. 2006;103:1036–1037. doi: 10.1213/01.ane.0000237230.40246.44. [DOI] [PubMed] [Google Scholar]
- 24.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96:102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 25.Gronau R. Leisure, home production, and work--the theory of the allocation of time revisited. J Political Economy. 1977;85:1099–1123. [Google Scholar]
- 26.Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34:1499–1518. [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton BH, Hamilton VH, Mayo NE. What are the costs of queuing for hip fracture surgery in Canada? J Health Econ. 1996;15:161–185. doi: 10.1016/0167-6296(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 28.Hunter CP, Frelick RW, Feldman AR, Bavier AR, Dunlap WH, Ford L, Henson D, Macfarlane D, Smart CR, Yancik R. Selection factors in clinical trials: results from the Community Clinical Oncology Program Physician's Patient Log. Cancer Treat Rep. 1987;71:559–565. [PubMed] [Google Scholar]


