Abstract
Stem cell homing has been studied in syngeneic models and appears to be rapid (<1 h) and dependent on cellular adhesion and migration factors. We utilized a full H2-mismatched transplantation model to determine the basics of allogeneic homing. C57BL/6J Lin-Sca-1+ cells were labeled with CFSE and injected in non-myeloablated BALB/c mice. Fluorescent cell detection was via high-speed FACS analysis. Alternatively, B6.SJL whole bone marrow cells were injected in lethally irradiated BALB/c mice (10 Gy). One, 3, 6, and 24 h after transplant, marrow was harvested and cells were either plated for high proliferative potential colony-forming cell (HPP-CFC) assay or secondarily injected into myeloablated (8 Gy) C57BL/6J mice using 10% competing C57BL/6J marrow. Chimerism was evaluated at 8 weeks. CFSE+ cells were detected in the bone marrow 1, 3, and 6 h after injection. The numbers were moderately lower when compared to syngeneic homing possibly due to strain effect. Conversely, utilizing a surrogate or secondary assay, we observed a decline of secondary engraftment of harvested cells over time, but not of HPP-CFC. Combining experiments and normalizing the 1-h time point to 100% (to allow comparison), we observed a mean relative engraftment of 87 ± 29%, 72 ± 21%, 84 ± 35% of the 1 h level at 3, 6, and 24 h respectively. HPP-CFC assay showed no significant variation as a homing surrogate over 1–6 h. These data indicate a rapid homing into allogeneic recipients with a plateau at 1 h. The decline of secondary engraftability over time may indicate a phenotype alteration of homed cells.
Hematopoetic stem cells (HSC) are defined by their capacity to fully reconstitute marrow of lethally irradiated hosts and give rise to all marrow-derived hematopoietic elements. Stem cell transplantation is a clinical approach used to restore defective hematopoiesis due either to high-dose chemoradiotherapy or to intrinsic marrow diseases. In this process, intravenously infused stem cells rapidly find their way to specific periendosteal location in the marrow-termed niches. The totality of this process defines homing (Quesenberry and Becker, 1998). Using syngeneic inbred mice, many aspects of stem cell homing have been described (Vos et al., 1980; Visser and Eliason, 1983; Bertoncello et al., 1985; Tavassoli and Aizawa, 1987; Hardy et al., 1991; Hendrikx et al., 1996; Nilsson et al., 1996; Nibley et al., 1997; Shaaban et al., 1999; Lanzkron et al., 1999a; Askenasy et al., 2003). The process involves rapid phenotypic changes in the infused stem cells and the interactions of multiple adhesion protein and their receptors (Papayannopoulou and Craddock, 1997; Frenette et al., 1998; Becker et al., 1999; Lapidot et al., 2005). Marrow stem cells enter S phase within about 12 h after infusion in vivo. The numbers of murine marrow stem cells in the marrow after infusion, plateaus after 1 h (Nilsson et al., 1997, 1998; Cerny et al., 2002; Avigdor et al., 2004; Cancelas et al., 2005).
A wide variety of adhesion proteins and chemokines are involved in mediating the movement of stem cells throughout the blood, into the marrow cavity and to the endosteal surface. HSC in both human and mouse express α4 β1 (VLA-4 or CD49d/CD29), a receptor for VCAM-1 (CD106), fibronectin, and αLβ2 (LFA-1 or CD11a/CD18), a co-receptor for ICAM-1 (CD54), L-selectin (CD62-L), a ligand for the CD34 form found on endothelial cells and the glycoprotein CD44, which binds to hyaluronate and other ECM proteins. Stromal-derived growth factor 1 and CXCR4 appear to form an especially critical axis for homing of stem cells and along with the VLA-4/VCAM-1 axis play a prominent role in marrow homing. More recently, utilizing a phage display approach, Nowakowski et al. (2004) identified CD84 as being a marrow homing protein and the calcium receptor has been proposed to be important in the intra-marrow movement of stem cells to the endosteal niche (Papayannopoulou and Nakamoto, 1993; Papayannopoulou et al., 1995; Simon et al., 1998; Zanjani et al., 1999; Adams et al., 2006; Jung et al., 2006; Qian et al., 2006).
The homing capacity of stem cells appears to modulate with cell cycle passage and this is, in turn, reflected in changes in long-term (8 week and 6 month) engraftment. The capacity to purify stem cells using lineage depletion with magnetic beads and fluorescent-activated cell sorting for specific epitopes or rhodamine and Hoechst dye retention, along with the knowledge of the kinetics of stem cell proliferation after intravenous infusion in the mouse, allowed for the structuring of a direct homing assay in normal non-irradiated syngeneic mice. Thus lineage negative Sca-1 positive (Lin-Sca-1+) stem cells labeled with CFSE are infused, and the mice sacrifice after the plateau of homing, but before cell division (3 h). Linearity was established between 50,000 and one million cells. Accordingly, our standard syngeneic homing assay involves infusing from 1–3 × 105 CFSE labeled Lin-Sca-1+ cells into syngeneic host mice with harvest of the marrow 3 h after cell infusion and evaluation of the percent fluorescent events by flow analysis. Studies using this approach in syngeneic non-treated host mice indicate that homing plateaued by 1 h and there were 35–70 homed events per million host marrow cells representing 8.5–10% of the infused marrow cells. Homing in the allogeneic situation imposes additional immunological variables on the process. The following study was undertaken to access marrow stem cell homing in the fully H2-mismatched allogeneic situation and to compare this to our previous result in a syngeneic model.
Materials and Methods
BALB/c (H2d) mice were purchased from Taconic Farms, Inc., (Georgetown, NY). Congenic male B6.SJL-PtprcaPep3b/Boy.J (Ly5.1) (CD45.1) (H2k) and male C57BL/6J-Thy 1.2, Ly5.2-Pep (CD45.2) (H2k) were obtained from The Jackson Lab (Bar Harbor, ME). All animals were 6–8 weeks of age and housed in a conventional clean facility for at least a week before experimental use, housed in micro-isolator cages, given ad libitum access to autoclaved mouse chow and acidified water. The animals were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Roger Williams Medical Center.
Preparation of lineage-negative Sca-1-positive HSC from bone marrow mononuclear cells
Isolation of lineage negative, stem cell antigen-1 positive (Lin-Sca-1+) purified stem cells was obtained using bone marrow isolated from the iliac bones, femurs, and tibiae of C57BL/6J mice 6 to 8 weeks of age. A low-density fraction (1.077 g/cm3) was isolated on Nycoprep (Accurate Chemical and Scientific Corporation; Westbury, NY). The cells were lineage depleted as previously described (to obtain Lin− cells) by incubating with the following monoclonal rat-anti-mouse antibodies: Ter119, B220, Mac-1, GR-1, Lyt-2, L3T4, YW25.12.7 (Bertoncello et al., 1985). Antibody-coated cells were removed with a magnet by mixing with Dynabeads M450 anti-rat IgG (Dynal; Lake Success, NY) in order to recover the lineage negative cells only. To obtain Lin− Sca-1+ cells, the lineage depleted cells were then labeled with FITC-anti-Sca-1 (Pharmingen, San Diego, CA). Aliquots of the bead-free cells were also stained with isotype-matched immunoglobulin. The cells were then washed with ice-cold PBS buffer and resuspended in PBS containing 1 μg/ml propidium iodide (PI; Sigma Chemical Co., St. Louis, MO). The samples were filtered through a 35 μm small blue cap filter (Falcon Cat # 2235). Fluorescence-activated cell sorting was performed on a multi-laser MoFlo® cell sorter (Cytomation, Inc., Fort Collins, CO). Cells were sequentially selected for sorting as PI-negative, FITC-negative, and Sca-1-positive.
Direct homing assay
C57BL/6J Lin− Sca-1+ cells were prepared from whole bone marrow (see above) and incubated with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE), Sigma-Aldrich Corp., St. Louis, MO) at a final concentration 2 μM for 15 min at 37°C. The reaction was stopped by washing with ice cold Dulbecco’s Phosphate-buffered saline, without calcium or magnesium chloride (PBS) (Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal calf serum. Using this method, more than 97% of the cells show an intense green fluorescence between 2 and 3 logs over background. One million CFSE-labeled cells were transplanted by tail vein injection into non-myeloablated BALB/c mice (full H2d mismatch). At different times after transplant, bone marrow from hind limb bones was flushed with PBS and immediately fixed in paraformaldehyde (1% final concentration). Highly fluorescent cells were detected by high-speed FACS analysis. In order to obtain a statistical reproducibility, at least 10 × 106 events were analyzed for each sample. A negative control was also analyzed with high number of events in order to subtract the background from experimental samples.
HPP-CFC in vitro progenitor assay
Bone marrow cells were harvested from 1 to 6 h after infusion. One million cells per dish were plated in soft agar using 35-mm plastic tissue culture dishes. The agar-based underlay consisted of α-minimal essential medium with 20% heat inactivated fetal calf serum (Hyclone, Logan, UT) with 1% penicillin/streptomycin (Invitrogen Corp., Carlsbad, CA) and 1% L-glutamine (Invitrogen Corp.). Seven growth factors (CSF-1, 104 U/dish; GM-CSF, 5 ng/dish, G-CSF, 10 ng/dish; IL-1α, 500 U/dish; IL-3, 200 U/dish; SCF, 200 ng/dish; and bFGF, 10 ng/dish) were included in a 1 ml underlayer with a final agar concentration of 0.5%. Cells were included in a 0.5 ml overlay of 0.3% agar in α-MEM with 20% HI-FCS. Culture dishes were incubated at 37°C for 14 days in presence of 5% O2, 10% CO2, and 85% N2. Five plates were set up for each recipient mouse. Plates were scored on a dissecting microscope for high-proliferative potential colony-forming cells (HPP-CFC, highly dense colonies >0.5 mm in diameter) (Bradley and Hodgson, 1979).
Secondary or surrogate engraftment and progenitor assay
Frenette et al. (1998) used a surrogate assay to evaluate the progenitor potential of homed whole bone marrow cells. In our experiments, B6.SJL (Ly5.1) whole bone marrow cells (100 × 106) were injected into lethally irradiated BALB/c mice (10 Gy). One, 3, 6, and 24 h after transplant, bone marrow was harvested. Cells were either plated for HPP-CFC (7 factor-responsive) or secondarily injected into myeloablated (8 Gy) B6.SJL mice using 10% competing B6.SJL marrow cells and short-term engraftment evaluation at 8 weeks (CD45.1/(CD45.1 + CD45.2) ratio). This second approach allowed comparing direct homing of purified stem cells with homing of whole bone marrow and subsequent engraftability of the homed cells. A schematic representation of the experiment is shown in Figure 1.
Fig. 1.
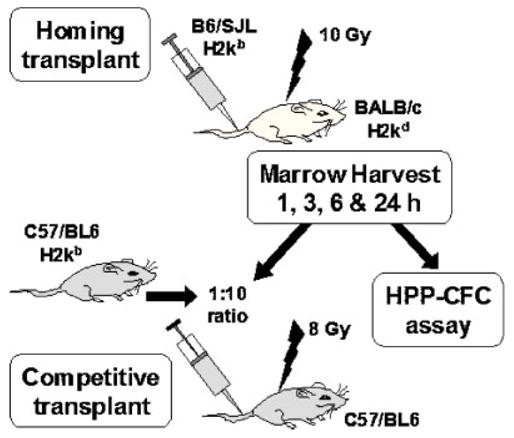
Surrogate transplant assay evaluation of allogeneic marrow homing. This shows the schema used for the surrogate assay.
Statistical analysis
Transplant and HPP progenitor assay data was stored using an Excel 2000 database and analyzed using Intercooled Stata 6.0 (Stata Corporation, College Station, TX) for Windows. We used the non-parametric Wilcoxon rank-sum test for comparison, and the trend test developed by Cuzick for testing trend. The non-parametric Spearman’s rank-correlation coefficient was used to assess the correlation between groups. The level of statistical significance is set at 0.05. Data are presented as mean ±one standard error of the mean. All P-values are two sided (Lang and Secic, 1997; Armitage et al., 2001).
Results
Direct homing assay of CFSE-labeled HSC-injected into allogeneic recipients
Purified lineage negative, Sca-1+ B6/SJL marrow cells were isolated, stained with fluorescent CFSE dye and 105 cells were injected into the tail vein of allogeneic BALB/c mice. Peripheral blood was collected at 1, 3, and 6 h after infusion and bone marrow was harvested from hind limb bones at 1, 3, and 6 h after injection. Positive fluorescent events were evaluated by high-speed FACS analysis. Figure 2 shows rapid disappearance of fluorescent Lin− Sca-1+ cells from peripheral blood (2A) by 1 h and homing to bone marrow (2B) completed by 1 h after infusion.
Fig. 2.
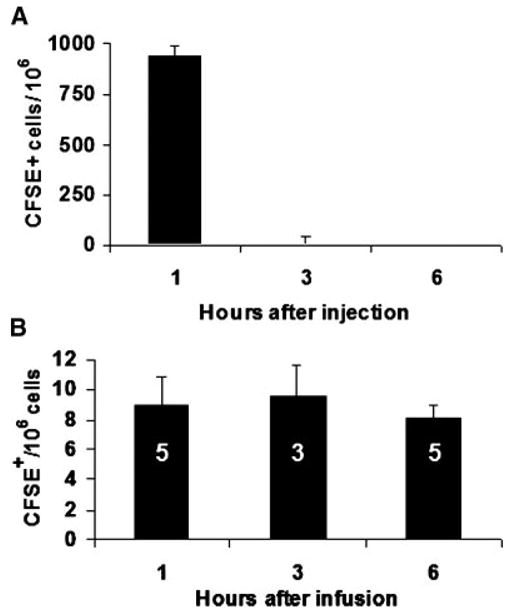
Detection of CFSE fluorescent cells in blood (A) and whole bone marrow (B) harvested serially after transplant. One hundred five CFSE-stained Lin− Sca-1+ cells (C57BL/6J) were injected into unconditioned BALB/c. A MoFlo® high-speed sorter was used to analyze a minimum of 107 events per marrow sample. Fluorescent events were detected in bone marrow at 1, 3, and 6 h after infusion. The number of CFSE+ cells is shown per thousand blood cells or per million marrow cells analyzed. Bars show standard error of the mean (SEM). Number of animals per group is shown in white for bone marrow analysis.
Evaluation of total marrow cellularity decay over time after 10 Gy total body irradiation
After total body irradiation (10 Gy), we observed a rapid decrease of total bone marrow cellularity as assessed by manual counts of cells harvested from femurs and tibias. In order to be able to quantitatively compare time-points, we created an enrichment ratio that corrected for the cellular decay induced by irradiation. Figure 3 shows the actual numbers and postulated relative enrichment of allogeneic homed cells that should not be affected by irradiation. Based on that enrichment ratio, we were able to correct the secondary in vitro and in vivo studies in order to compare homing values at different time points.
Fig. 3.
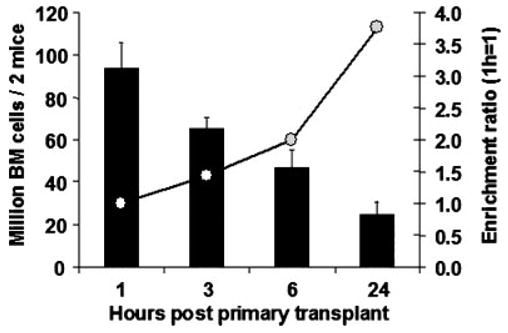
Cellular decay assay of bone marrow cellularity after 10-Gy irradiation. Bone marrow was harvested from femurs and tibias 1 to 24 h after infusion of allogeneic marrow cells. The recipient mice were irradiated with 10-Gy 2 h prior to transplant, thus times indicated reflect the cellularity decay 3, 5, 8, and 26 h after irradiation. Based on the cellularity, an enrichment ratio was calculated; this ratio allows calculating the relative increase of non-irradiated allogeneic homed cells compared to the host bone marrow cells. Using this data, an enrichment ratio was used to correct progenitor assays and secondary engraftment data to allow quantization and comparison between groups (infravide). There were six groups of two mice pooled together; standard error of the mean is shown.
In vitro evaluation of homed allogeneic bone marrow by HPP-CFC assay
In order to confirm the observation obtained with direct measurement of labeled stem cells, we used an indirect method of assessment of hematopoietic cell homing looking at the in vitro progenitor capacity by HPP-CFC assay. As described in Figure 1, one hundred million marrow cells from B6/SJL males (CD45.1) were injected IV in 10 Gy-irradiated BALB/c recipients. After different homing times (1 to 24 h), bone marrow was harvested and 2 million cells were plated in double layer soft agar with a 7-factor cytokine cocktail, and then incubated for 14 days in low O2. The number of dense colonies (HPP) is shown in Figure 4. The progenitors obtained from recovered marrow were similar at 1, 3, and 6 h after infusion, indicating that most of the progenitors had homed by 1 h after transplant and corrected with the enrichment ratio.
Fig. 4.
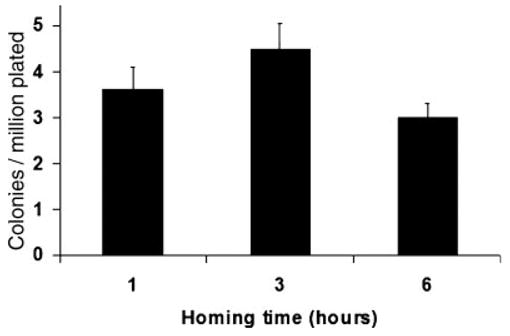
Surrogate evaluation of homing by high proliferative potential colony-forming cell (HPP-CFC) assay. At different times after transplantation of bone marrow cells into allogeneic radio-ablated recipients, marrow was collected and plated into two layer soft agar with seven cytokines in the lower layer. Colonies were counted according to size and density (see methods). The numbers of colonies per million cells plated are shown. Mean values (five plates per group of two recipients, three groups per time-point) and SEM are shown. The Kruskal–Wallis test was performed to compare all groups.
Secondary or surrogate engraftment assay of homed allogeneic bone marrow with competitive transplants
The true nature of stem cells is its capacity to home to bone marrow and to give rise to multilineage hematopoietic cells. In order to further verify the direct homing assay data, we used a secondary transplant to confirm that purified fluorescent stem cells found early in the bone marrow were capable of repopulating a secondary mouse. In this surrogate assay, 108 B6/SJL whole bone marrow cells were injected in lethally irradiated BALB/c mice (10 Gy) (five per time point). One three, and six hours after transplant, bone marrow was harvested and cells were secondarily injected into myeloablated (8 Gy) C57/BL6 mice (12 per time point). A ratio of competing C57/BL6 bone marrow cells equal to 1:1 or 1:10 was used to support early recovery of transplanted mice. We evaluated final chimerism by FACS for CD45.1 and CD45.2 in the bone marrow, spleen, and thymus at 8 weeks post secondary transplant (see Fig. 1). When a ratio of competitor cells of 1:1 was used, we observed a final engraftment in the bone marrow of 0.77 ± 0.18, 0.86 ± 0.41, and 0.85 ± 0.70 % (N=13, 12, and 7), for 1, 3, and 6 h, respectively. CD45.1 chimerism was similarly detected in spleen and thymus (data not shown). In further experiments, we conducted the secondary transplants with a 10:1 harvested to competitor ratio to enhance secondary engraftment and thus accuracy of final reading.
These experiments show that secondary engrafted B6/JL Ly5.1 cells were detected in bone marrow, spleen, and thymus of myeloablated C57/BL6 recipient. Percentages of Ly5.1 cells over total Ly5.1 + Ly5.2 cells are shown in Figure 5.
Fig. 5.
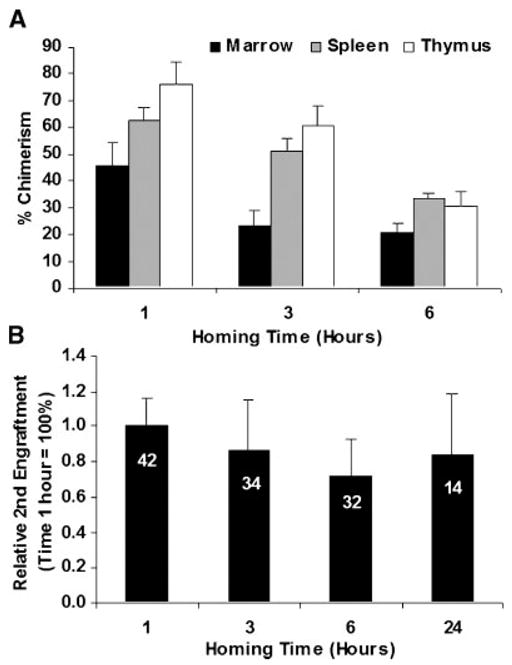
Secondary engraftment of bone marrow at different times after initial allogeneic homing. A: The percentage of chimerism at 8-weeks in bone marrow, spleen, and thymus by FACS staining for CD45.1 and CD45.2 is presented. The data was corrected according to the relative enrichment ratio observed after 10-Gy lethal irradiation. Mean and SEM is shown, there were respectively 13, 10, and 13 mice for each time point. The difference between 1 and 6 h were significantin all organs (P = 0.043, <0.001, and 0.001 for bone marrow, spleen, and thymus, respectively). A test for trend from 1 to 6 h was significant for spleen and thymus (P < 0.005). B: Serial analysis of the percent of relative secondary engraftment in three pooled experiments compared with 1 h homing values. Data were corrected for cellularity decay and normalized based on the average marrow chimerism of each experiment. This data shows that homing and the subsequent engraftment chimerism percent plateaus within 1 h. There was decreased homing and engraftment with marrow collected 6 h after primary homing before retrieval and secondary homing and engraftment. The difference between 1 and 6 h was significant (P = 0.017). A test for trend from 1 to 6 h was significant (P = 0.01). The number of animals in each time-point is seen in white on each data bar. The Kruskal–Wallis test was performed to compare all groups.
Discussion
The aim of this study was to evaluate homing in an allogeneic setting. We showed a rapid homing of purified lineage depleted Sca-1-positive marrow cells with a plateau reached by 1 h after intravenous infusion. These observations were confirmed by surrogate assay evaluation of homing using an in vitro early progenitor assay (HPP-CFC) and via a secondary competitive transplant. Other surrogate progenitor assays could have included the LTC-IC, but, in our hands, the HPP-CFC has been a reasonable surrogate assay (Bertoncello et al., 1985, 1988, 1992; McNiece et al., 1987, 1990; Lowry et al., 1991). For the competitive transplant assay, fluorescent labeling of hematopoietic progenitors with the cytoplasmic fluorescent dye, CFSE to label Lin− Sca-1+ marrow progenitors was used. This dye allows strong fluorescent labeling to follow labeled cells up to seven mitotic divisions. It does not interfere with long-term engraftment capacity. Previous studies from our group using a syngeneic direct homing model showed that 8.5–10.7% of CFSE stained Lin−Sca-1+ injected cells had homed to the bone marrow by 3 h (Cerny et al., 2002). We have also shown a dose–effect relationship with a linear range of homing efficiency between 5 × 104 and 106 total stem cells injected. Cell doses over 106 resulted in a decreasing homing efficiency, thus indicating that saturation of homing niches was achieved in non-conditioned recipients. Szilvassy et al. (1999) who used a PKH26 label/retrieval surrogate assay, estimated a plateau of homing in bone marrow of ‘‘6% to 8% of input after 3 h.’’ In the present study, taking an allogeneic homing frequency of 9.5 fluorescent events/106 cells analyzed at 3 h, and a total bone marrow cellularity of 526 × 106 cells, we calculated that 5.0% of the injected CFSE-labeled cells (1 × 106) had homed to the bone marrow (Colvin et al., 2004). This result is highly comparable to the published data on syngeneic homing. For the surrogate evaluation, the homing efficiency was calculated as follow: If 100% of the engraftable cell had homed to bone marrow, the 1:1 ratio would give a 50% final engraftment; similarly, the 10:1 ratio would give 50% engraftment if 10% of engraftable cell homed to bone marrow. The experiments showed 0.86% and 23.35% chimerism, respectively, therefore, the homing frequencies are 100 × 0.86/50 = 1.7% and 10 × 23.35/50 = 4.7%.
Table 1 summarizes the calculated homing frequency for direct labeling and surrogate in vivo evaluation of allogeneic homing from our experiments. Indirect measurement gave a similar homing frequency.
TABLE 1.
HSC homing to allogeneic bone marrow after 3 hours
| Experiment | Homing frequency (%) |
|---|---|
| Direct fluorescent homing | 5.0 |
| Surrogate transplant 1:1 ratio | 1.7 |
| Surrogate transplant 10:1 ratio | 4.7 |
In the surrogate transplant, we observed a trend towards decrease of secondary engraftment between 1 and 6 h (Fig. 5). This was not observed in vitro (Fig. 4) and we believe that delayed immunologic destruction likely had a significant role in the final engraftment results. There was little evidence that there was rapid elimination of allogeneic homed cell by the recipient’s immune system because the direct homing data showed constant homing efficiency between 1 and 6 h does not support this possibility. We could speculate that the decrease of secondary engraftment capacity of homed cells was exacerbated by the stem cells being provoked to rapidly enter into cell cycle and hence change their stem cell phenotype/function (e.g., toward a more differentiated progenitor) which could affect the stem cells ability to find its true bone marrow niche and later undergo apoptosis or replicative extinction by differentiation. Evidence for this has been shown by Nilsson et al. (1997) finding that homed stem cells enter into cell cycle and encounter their first division only 12 h after seeding. Additionally, there are other cell cycle-related changes that could have altered the final engraftment efficiency with variation of surface antigen expression (Becker et al., 1999), homing (Cerny et al., 2002), engraftment (Habibian et al., 1998), and gene expression profile (Lambert et al., 2003) through cell cycle position of the stem cells prior to initial cell division. Askenasy and Farkas (2003) have looked at allogeneic and syngeneic homing with whole marrow or lineage-depleted marrow labeled with PKH. The homing efficiency was evaluated, using an in vivo observation method with a surgically implanted glass slide over the distal femoral epiphysis. Using lineage negative bone marrow cells, they observed a homing efficiency of 1.52% in allogeneic recipients, with a slight decrease of homing over time. Our study, using more efficient stem cell purification by adding a stem cell marker positive selection (Sca-1), showed similar, although higher homing efficiencies by direct or surrogate (secondary) evaluation. Direct homing evaluation using vital stains has been criticized for overestimating homing by including cell that are passing though the bone marrow, but unable to home to the final niche. Nilsson et al. (2001) have shown that injected purified stem cells rapidly leave the central vein area after infusion and reach the endosteal niches within a few hours. Frimberger et al. (2001) have also shown in an in vitro Dexter model that bone marrow stem cells are capable of extremely rapid adherence to the stroma within 20 min after deposition on the flasks with secondary engraftability used as a read-out. These observations indicate that measurement of bone homing at 3 h after transplant is an adequate time to infer that the cell in the marrow space are indeed marrow stem/progenitor cells. In addition, knowing that stem cells divide within 12 h after seeding, a read-out at 24 h would skew the homing results by including fluorescent cells that had already divided. Furthermore, our data indicating a clearance of marked stem cells from peripheral blood after 1 h argues against the possibility that remaining intravenous cells passing through the bone capillaries would be miscounted as homed cells. The use of whole bone marrow is another source of bias, because differentiated or stromal cells may also contaminate the bone marrow sample. Using a surrogate assay, we were able to evaluate true homing of secondary engraftable stem cells. Despite some variability in secondary transplant efficiency, our results confirm the direct homing data. The homing efficiency was smaller, thus suggesting a saturation of homing niches in the surrogate assay while using a large amount of unpurified bone marrow cells (100 × 106 cells).
In our study, we used lethal irradiation conditioning (10 Gy) for the surrogate assay. Hendrikx et al. (1996) have shown a deleterious effect of irradiation on homing; although this effect was maximized by 24 h post irradiation. Our data showed smaller homing efficiency in the surrogate assay compared to the direct fluorescent assay, which may indicate a negative effect of irradiation, despite the short time between irradiation and stem cell infusion (2 h). Another potential pitfall of this analysis is the possibility that stem cell engraftment and homing could happen in two steps with a first group of stem cells seeding directly into the bone marrow and a second group first seeding in spleen and secondary migrating to bone marrow (Shaaban et al., 1999). However, long-term engraftment is not necessary predicted by the CFU-spleen assay, indicating that cells homing to spleen cannot be used as a surrogate for engraftment (Vos et al., 1980; Lanzkron et al., 1999b). Homing is a critical step for autologous and allogeneic peripheral blood stem cell or bone marrow transplantation. In this study, we showed that allogeneic homing is comparable to syngeneic homing in term of speed and efficiency. A better understanding of homing and immediate engraftment should help defining new approaches to decrease time to engraftment and to enhance engraftment of in vitro expanded HSC. New approaches may also overcome the engraftment/homing defect observed after in vitro culture of HSC.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Contract grant sponsors: National Institutes of Health (NIH), National Center for Research Resources (NCRR); Contract grant numbers: COBRE P20 RR 018757–02, NIDDK K08DK6498–01, P01-HL-56920, P01-DK-5022, R01-DK-49650, R01-DK2742.
Literature Cited
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4. Oxford, Great Britain: Blackwell Science Ltd; 2001. pp. 1–85. [Google Scholar]
- Askenasy N, Farkas DL. In vivo imaging studies of the effect of recipient conditioning, donor cell phenotype and antigen disparity on homing of haematopoietic cells to the bone marrow. Br J Haematol. 2003b;120:505–515. doi: 10.1046/j.1365-2141.2003.04114.x. [DOI] [PubMed] [Google Scholar]
- Askenasy N, Stein J, Yaniv I, Farkas DL. The topologic and chronologic patterns of hematopoietic cell seeding in host femoral bone marrow after transplantation. Biol Blood Marrow Transplant. 2003a;9:496–504. doi: 10.1016/s1083-8791(03)00150-2. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, Hsieh CC, Quesenberry PJ. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: Modulation by cytokines and cell cycle status. Exp Hematol. 1999;27:533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Bertoncello I. Status of high proliferative potential colony-forming cells in the hematopoietic stem cell hierarchy. Curr Top Microbiol Immunol. 1992;177:83–94. doi: 10.1007/978-3-642-76912-2_7. [DOI] [PubMed] [Google Scholar]
- Bertoncello I, Hodgson GS, Bradley TR. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985;13:999–1006. [PubMed] [Google Scholar]
- Bertoncello I, Hodgson GS, Bradley TR. Multiparameter analysis of transplantable hemopoietic stem cells. II. Stem cells of long-term bone marrow-reconstituted recipients. Exp Hematol. 1988;16:245–249. [PubMed] [Google Scholar]
- Bradley TR, Hodgson GS. Blood. 1979;54:1446–1450. [PubMed] [Google Scholar]
- Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- Cerny J, Dooner M, McAuliffe C, Habibian H, Stencil K, Berrios V, Reilly J, Carlson J, Cerny AM, d’Hondt L, Benoit B, Lambert JF, Colvin G, Nilsson S, Becker P, Quesenberry P. Homing of purified murine lymphohematopoietic stem cells: A cytokine-induced defect. J Hematother Stem Cell Res. 2002;11:913–922. doi: 10.1089/152581602321080574. [DOI] [PubMed] [Google Scholar]
- Colvin GA, Lambert JF, Abedi M, Hsieh CC, Carlson JE, Stewart FM, Quesenberry PJ. Murine marrow cellularity and the concept of stem cell competition: Geographic and quantitative determinants in stem cell biology. Leukemia. 2004;18:575–583. doi: 10.1038/sj.leu.2403268. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimberger AE, Stering AI, Quesenberry PJ. An in vitro model of hematopoietic stem cell homing demonstrates rapid homing and maintenance of engraftable stem cells. Blood. 2001;98:1012–1018. doi: 10.1182/blood.v98.4.1012. [DOI] [PubMed] [Google Scholar]
- Habibian HK, Peters SO, Hsieh CC, Wuu J, Vergilis K, Grimaldi CI, Reilly J, Carlson JE, Frimberger AE, Stewart FM, Quesenberry PJ. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188:393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CL, Matsuoka T, Tavassoli M. Distribution of homing protein on hemopoietic stromal and progenitor cells. Exp Hematol. 1991;19:968–972. [PubMed] [Google Scholar]
- Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. [PubMed] [Google Scholar]
- Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, McCauley LK, Taichman RS. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lambert JF, Liu M, Colvin GA, Dooner M, McAuliffe CI, Becker PS, Forget BG, Weissman SM, Quesenberry PJ. Marrow stem cells shift gene expression and engraftment phenotype with cell cycle transit. J Exp Med. 2003;197:1563–1572. doi: 10.1084/jem.20030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang TA, Secic M. How to Report Statistics in Medicine. Philadelphia, PA: American College of Physicians; 1997. pp. 39–146. [Google Scholar]
- Lanzkron SM, Collector MI, Sharkis SJ. Homing of long-term and short-term engrafting cells in vivo. Ann N Y Acad Sci. 1999a;872:48–54. doi: 10.1111/j.1749-6632.1999.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: A comparison of short-term and long-term repopulating cells. Blood. 1999b;93:1916–1921. [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lowry PA, Zsebo KM, Deacon DH, Eichman CE, Quesenberry PJ. Effects of rrSCF on multiple cytokine responsive HPP-CFC generated from SCA + Lin− murine hematopoietic progenitors. Exp Hematol. 1991;19:994–996. [PubMed] [Google Scholar]
- McNiece IK, Williams NT, Johnson GR, Kriegler AB, Bradley TR, Hodgson GS. Generation of murine hematopoietic precursor cells from macrophage high-proliferative-potential colony-forming cells. Exp Hematol. 1987;15:972–977. [PubMed] [Google Scholar]
- McNiece IK, Bertoncello I, Kriegler AB, Quesenberry PJ. Colony-forming cells with high proliferative potential (HPP-CFC) Int J Cell Cloning. 1990;8:146–160. doi: 10.1002/stem.5530080302. [DOI] [PubMed] [Google Scholar]
- Nibley WE, Pohlmann SJ, Spangrude GJ. Patterns of organ-specific engraftment by stem cell subsets and committed progenitors. Stem Cells. 1997;15:31–38. doi: 10.1002/stem.5530150806. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Hulspas R, Weier HU, Quesenberry PJ. In situ detection of individual transplanted bone marrow cells using FISH on sections of paraffin-embedded whole murine femurs. J Histochem Cytochem. 1996;44:1069–1074. doi: 10.1177/44.9.8773573. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Quesenberry PJ. Synchronized cell-cycle induction of engrafting long-term repopulating stem cells. Blood. 1997;90:4646–4650. [PubMed] [Google Scholar]
- Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: Inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Nowakowski GS, Dooner MS, Valinski HM, Mihaliak AM, Quesenberry PJ, Becker PS. A specific heptapeptide from a phage display peptide library homes to bone marrow and binds to primitive hematopoietic stem cells. Stem Cells. 2004;22:1030–1038. doi: 10.1634/stemcells.22-6-1030. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- Quesenberry PJ, Becker PS. Stem cell homing: Rolling, crawling, and nesting. Proc Natl Acad Sci USA. 1998;95:15155–15157. doi: 10.1073/pnas.95.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban AF, Kim HB, Milner R, Flake AW. A kinetic model for the homing and migration of prenatally transplanted marrow. Blood. 1999;94:3251–3257. [PubMed] [Google Scholar]
- Simon AR, Warrens AN, Yazzie NP, Seebach JD, Sachs DH, Sykes M. Cross-species interaction of porcine and human integrins with their respective ligands: Implications for xenogeneic tolerance induction. Transplantation. 1998;66:385–394. doi: 10.1097/00007890-199808150-00017. [DOI] [PubMed] [Google Scholar]
- Szilvassy SJ, Bass MJ, Van Zant G, Grimes B. Organ-selective homing defines engraftment kinetics of murine hematopoietic stem cells and is compromised by ex vivo expansion. Blood. 1999;93:1557–1566. [PubMed] [Google Scholar]
- Tavassoli M, Aizawa S. Homing receptors for hemopoietic stem cells are lectins with galactosyl and mannosyl specificities. Trans Assoc Am Physicians. 1987;100:294–299. [PubMed] [Google Scholar]
- Visser JW, Eliason JF. In vivo studies on the regeneration kinetics of enriched populations of haemopoietic spleen colony-forming cells from normal bone marrow. Cell Tissue Kinet. 1983;16:385–392. [PubMed] [Google Scholar]
- Vos O, Luiten F, Ploemacher RE. Lodging of CFU(S) under various circumstances in bone marrow, spleen and liver. Exp Hematol. 1980;8:860–866. [PubMed] [Google Scholar]
- Zanjani ED, Flake AW, Almeida-Porada G, Tran N, Papayannopoulou T. Homing of human cells in the fetal sheep model: Modulation by antibodies activating or inhibiting very late activation antigen-4-dependent function. Blood. 1999;94:2515–2522. [PubMed] [Google Scholar]


