Abstract
Objective
Allogeneic stem cell transplantation is curative for certain cancers, but the high doses of chemotherapy and radiotherapy may lead to toxicity. This review summarizes the field of cellular immune therapy using very-low–dose conditioning for refractory cancers.
Methods
In our initial study, we treated 25 patients with refractory cancers with 100 cGy total body irradiation followed by allogeneic, nonmobilized peripheral blood cells. Eighteen patients received sibling and seven patients received unrelated cord blood stem cells.
Results
None of the 13 patients with solid tumors achieved donor chimerism or had a sustained response. Twelve patients with hematologic malignancies were treated, 1 received a cord blood transplant and 11 received sibling donor cells. Nine of these 11 patients achieved donor chimerism, ranging from 5% to 100%. Four patients had sustained complete remission of their cancers. The patients who received cord blood transplants did not respond. Development of chimerism correlated with total previous myelotoxic chemotherapy (p < 0.001). We review additional studies in this area, including data in the haploidentical and unrelated donor setting. The data presented comprises studies performed at the four institutions represented by the authors, and a review of other pertinent studies in this area.
Conclusions
Cellular immune therapy is an emerging application of transplantation therapy, which may be appropriate for refractory cancers. New studies in solid tumors, and with alternative donors, will expand the application of this new and promising treatment.
Preclinical studies were based on a mouse transplant model. Development of the cellular immune therapy approach followed from studies showing that syngeneic murine marrow could be transplanted into untreated or minimally treated (100 cGy) mice with establishment of long-term multilineage chimerism [1,2]. The level of this chimerism was based on stem cell competition. The nontoxic reduction of host stem cells secondary to 100-cGy total body irradiation resulted in very high levels of donor cell chimerism [2]. These studies led to studies of B6 SJL to BALB/C H-2 mismatched transplants using 100-cGy total body irradiation and CD-40 ligand blockade, showing stable long-term multilineage chimerism in the allogeneic setting [3]. These initial studies led to the design of the initial cellular immune therapy clinical trials.
Engraftment in nonmyeloablated mice
Studies have addressed the question of whether niches were a limiting factor for engraftment. In these studies, an accurate tally of the total murine cellularity of BALB/c mice allowed us to test our hypothesis that in the syngeneic setting the final host chimerism results from competition between donor and resident host stem cells. Total cellularity in a BALB/C mouse was a mean of 530 ±20 million total marrow cells. Considering accurate total murine cellularity numbers, which equate with total stem cell numbers, both theoretical and experimental engraftment were calculated when 40 million male BALB/c marrow cells are infused into nontreated female BALB/c hosts. Comparing these theoretical engraftment figures with those actually observed, we arrive at virtually identical values (Fig. 1). This would indicate that essentially all marrow engraftable stem cells engraft in marrow and that the final percent chimerism is determined solely by stem cell competition. Given these assumptions, the theoretical engraftment in untreated mice given 40 million cells would be 40 of 530 or 7.5%; the observed engraftment was 6.9%. This suggested that a specific stem cell homes to marrow, that the process is efficient, and that stem cell competition may be the critical determinant of sustained marrow engraftment [4].
Figure 1.
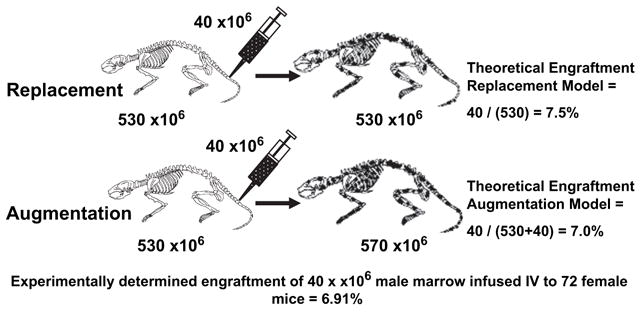
Syngeneic stem cell competition models. Theoretical and experimental syngeneic nonmyeloablative transplant models were developed based on the theory that stem cell competition between host and donor determines final chimerism. Theoretical engraftment with replacement or augmentation models compared to observed engraftment. A total of 72 unperturbed 6- to 8-week-old female BALB/c mice were injected with 40 × 106 male whole marrow cells, as controls for other experiments. Mean engraftment was found to be 7.7%. Fluorescent in situ hybridization was used to obtain engraftment results and engraftment results were confirmed in many cases by Southern blot hybridization.
Subsequently, we found that reducing host stem cells by exposure to 100 cGy resulted in marked increases in donor chimerism, also consistent with the stem cell competition model. In these more recent studies, multilineage chimerism was obtained with no irradiation, but with administration of anti-CD40 ligand when 40 million marrow cells were infused at time 0 and at 4, 5, 6, and 7 weeks, for a total of 200 million cells [5]. Interestingly, when 200 million cells were infused at time 0, there was essentially no engraftment, so scheduling was a critical component of engraftment. Most recently, we have shown that with lesser degrees of H-2 histoincompatibility there are higher levels of engraftment [4,5].
Clinical studies using matched, related donors
Over the last few years, many investigators have used non-myeloablative doses of chemotherapy or chemoradiotherapy (“mini-transplants”) to treat older patients with hematologic malignancies. This work was based on the observation, made first in chronic myelogeneous leukemia, that induction of graft-vs-host disease (GVHD) by donor lymphocyte infusions posttransplant has an antileukemia effect [6–8]. Slavin et al. [9], Khouri et al. [10], Spitzer et al. [11], and others have shown that engraftment and disease response can be achieved using reduced-intensity conditioning regimens that are well tolerated. Nonmyeloablative allogeneic transplants have been used successfully in patients who have relapsed after autologous stem cell transplantation [12,13]. This review does not encompass the entire nonmyeloablative transplant field, but focuses on those studies that use very-low–dose or no conditioning for refractory cancers. Tables 1A and 1B summarize the studies in matched related donors.
Table 1A.
Summary of studies using matched related donors–minimal or no conditioning
| Author | n | Diagnosis | Median age (y) | Conditioning | GVHD prophylaxis | Chimerism (presence of donor cells) | Outcome |
|---|---|---|---|---|---|---|---|
| Ballen [15], UMass | 18 | Refractory solid tumors and heme malignancies | 47 | 100 cGy TBI | None | 9/11 heme 0/7 solid | 4 CR |
| Porter [14], UPenn/BWH | 18 | Refractory heme malignancies | None | None | 4/4 Prior auto SCT 0/14 No prior auto SCT | 3 CR/PR |
BWH =Brigham and Women’s Hospital; CR =complete remission; GVHD =graft-vs-host disease; PR =partial remission; SCT =stem cell transplantation; TBI =total body irradiation.
Table 1B.
Summary of studies using matched related donors–reduced intensity regimens
| Author | n | Diagnosis | Median Age (y) | Conditioning | GVHD prophylaxis | Chimerism (presence of donor cells) | Outcome |
|---|---|---|---|---|---|---|---|
| DeLima [16] M.D. Anderson | 94 | MDS/AML | 57 | Flu/ARA-C/Ida or Flu/Melphalan | Tacro/MTX | 24/32
40/62 |
8/32 CR/PR
42/62 CR/PR |
| Dey [37], Mass General | 82 | Heme malignancies | 43 | Cytoxan Thymic XRT ATG or Medi 507 | Cyclosporine | 60/82 | 9/22 With lost graft CR/PR |
| Bay [19], France | 5 | Metastatic ovarian cancer | 38 | Busulfan Fludarabine ATG | Cyclosporine | 4/4 evaluable | 4/4 Evaluable PR |
| Childs [17], NIH | 23 | Metastatic renal cell | 48 | Cytoxan Fludarabine | Cyclosporine | 19/19 | 10/19 Regression |
CR =complete remission; GVHD =graft-vs-host disease; MDS/AML =myelodysplastic syndrome/acute myeloid leukemia; NIH =National Institutes of Health; PR =partial remission.
Porter et al. [14] treated 18 patients at Brigham and Women’s Hospital and at the University of Pennsylvania with donor lymphocyte infusion as primary therapy without conditioning. Four of these 18 patients had relapsed disease after a prior autologous stem cell transplant (Hodgkin’s disease, n =3; myeloma, n =1). Sustained mixed chimerism (1–5% donor chimerism) was seen in all four of these patients, but not in the less heavily pretreated patients. Three of the four patients with mixed chimerism had an antitumor response, including one patient with relapsed Hodgkin’s disease who experienced complete remission for over 30 months. Treatment-related mortality was limited to one patient who developed marrow aplasia and grade IV GVHD. Two other patients with sustained engraftment developed grade II and III acute GVHD. Therefore, this initial study demonstrated that donor lymphocyte infusion could be given as primary therapy with acceptable toxicity, and that primary donor lymphocyte infusions (DLI) without prior conditioning therapy could result in clinically significant graft-vs-tumor effect. Engraftment and tumor response were limited to the most heavily pretreated patients.
A subsequent report from this group was limited to 14 patients who received either DLI or stem cell transplantation and no conditioning regimen (n =4) or after low-dose immunosuppressive conditioning (n =10) [13]. Ten of 14 patients (71%) responded, including 6 patients who experienced a complete response (5 patients with lymphoma and 1 with myeloma). These data confirmed that sustained engraftment was frequent in heavily pretreated patients with use of no, or minimal, conditioning, and that sustained graft-vs-tumor responses, even in patients who had failed high-dose therapy, could be generated.
A Phase I cellular immune therapy trial was conducted at University of Massachusetts Memorial Health Care [15]. Twenty-five patients, median age of 47 years, with refractory cancers, were enrolled. Thirteen patients had solid tumors; the diagnoses were lung cancer (n =3), melanoma (n =2), esophageal cancer (n =2), and 1 patient each with hepatocellular carcinoma, neuroendocrine cancer, adrenocortical cancer, pancreatic cancer, renal cell carcinoma, and sarcoma. Twelve patients with hematologic malignancies were treated; they included seven patients with lymphoma, two patients with myeloma, and three patients with leukemia. Eighteen patients received a sibling graft and seven patients received a cord blood transplant. The cord blood transplants are discussed in the section on alternative donors.
Donors received no filgastrim or chemotherapy priming. The target dose was initially set at 1 × 107 CD3+ cells/kg and was then increased to 1 × 108 CD3+ cells/kg. Patients were treated with 100 cGy total body irradiation (TBI). The primary endpoint of the study was percent donor chimerism measured by polymerase chain reaction-short tandem repeats at weeks 1, 2, 3, 4, and 8 posttransplant.
Thirteen patients were treated for refractory solid tumors; six patients received a cord blood transplant and seven a sibling transplant. None of these patients showed engraftment or any sustained tumor response.
Twelve patients with hematologic malignancies were treated; 11 of these patients received a sibling graft with a cell dose of 1 × 108 CD3+ cells/kg. The chimerism values, presence of GVHD, response, and survival are outlined in Table 2. Nine of these 11 patients achieved some degree of donor chimerism. Three patients achieved 100% donor chimerism, two patients achieved 67% donor chimerism, two patients had 10% donor chimerism, and two patients 5% donor chimerism.
Table 2.
Chimerism and tumor response in patients with hematologic malignancies treated with a sibling graft–UMass data
| Patient no. | Disease | Maximal donor chimerism (%) | Response (disease state at 8 weeks) | Graft-vs-host disease | Survival (d) |
|---|---|---|---|---|---|
| 1 | Lymphoma | 5 | CR | No | 1765a |
| 2 | Lymphoma | 10 | Died of disease | Yes | 28 |
| 6 | Myeloma | 100 | CR | Yes | 116 |
| 8 | Myeloma | 10 | Stable | Yes | 229 |
| 9 | CML | 67 | No response | No | 129 |
| 11 | Lymphoma | 5 | Died of disease | No | 29 |
| 12 | Lymphoma | 100 | CR | Yes | 222 |
| 20 | Lymphoma | 67 | Died of disease | No | 37 |
| 23 | CLL | 0 | PR | Yes | 179 |
| 24 | Lymphoma | 0 | Died of disease | No | 40 |
| 25 | AML | 100 | CR | Yes | 1110 |
AML =acute myeloid leukemia; CLL =chronic lymphocytic leukemia; CML =chronic myeloid leukemia; CR =complete remission.
Patient currently alive and disease-free.
Four patients achieved a complete remission (CR) of their cancers. One patient, a 51-year-old woman with large-cell lymphoma that had relapsed after autologous transplantation, achieved CR although she only had transient 5% donor chimerism. The patient is alive and well, 58 months after transplantation.
Three other patients achieved a complete remission from their cancers. These three patients had 100% donor chimerism. A 56-year-old woman with lymphoblastic lymphoma who had relapsed after autologous transplantation was in CR by 8 weeks posttransplant. She was 100% chimeric by 4 weeks after transplant. She relapsed 5 months after infusion and died of recurrent disease. A 55-year-old woman with recurrent multiple myeloma developed complete donor chimerism by 4 weeks after transplant; CR was documented by bone marrow aspirate and biopsy. She died of liver GVHD, 3.5 months after infusion. There was no evidence of myeloma at autopsy.
The fourth patient who achieved CR was a 24-year-old woman with acute myelogeneous leukemia, who had failed three induction regimens and had refractory leukemia. She developed CR, based on bone marrow morphology and flow cytometry, and 100% donor chimerism. She died of infection, thought secondary to chronic GVHD, 3 years after transplant.
There was one treatment-related death as described above. Acute GVHD occurred in six patients. One patient survives in CR at 58 months posttherapy.
We explored associations with donor chimerism and tumor response. We developed a myelotoxicity index to evaluate the number of chemotherapy regimens. Patients who received a chemotherapy regimen with one drug were given a score of 1, two drugs a score of 2, etc. A prior bone marrow transplant was given a score of 5. The average myelotoxicity index in the patients with hematologic malignancies was 9, compared to 2 for the patients with solid tumors. This lack of prior treatment and prior immunosuppression may explain why patients with solid tumors did not have donor engraftment in this study. Patients with solid tumors also have more preserved cell-mediated immunity. Prior chemotherapy was correlated with chimerism (p < 0.001). Other variables associated with donor chimerism are outlined in Table 3. Donor chimerism was also shown to be associated with tumor response (p = 0.01). However, some patients with donor chimerism did not respond, suggesting that other factors besides donor chimerism are important for tumor response.
Table 3.
Associations with donor chimerism: UMass data
| Donor chimerism
|
Donor chimerism | |||
|---|---|---|---|---|
| Characteristic | Yes | % | No | p Value |
| Diagnosis | ||||
| hematologic malignancy | 9 | 75 | 3 | 0.0001 |
| Solid tumor | 0 | 0 | 13 | |
| Dose of T cell | ||||
| 1 × 108 | 8 | 57 | 6 | 0.03 |
| Other | 1 | 9 | 10 | |
| Graft type | ||||
| Allogeneic Cord | 0 | 0 | 7 | 0.03 |
| Allogeneic PB | 9 | 50 | 9 | |
| Acute GVHD | ||||
| Yes | 5 | 83 | 1 | 0.01 |
| No | 4 | 21 | 15 | |
| Prior myelotoxicity score | ||||
| <4 | 0 | 0 | 9 | 0.001 |
| >=4 | 9 | 55 | 7 | |
GVHD =graft-vs-host disease; PB =peripheral blood.
An interesting finding of the study was evidence of engraftment with a low CD34+ cell dose. Patients received a nonmobilized peripheral blood cell pheresis product with a target dose of 1 × 108 CD3+ cells/kg. There was no target dose of CD34+ cells; the CD34+ dose ranged from 1.5 ×104 to 2.8 × 105 cells/kg with a median dose of 5.2 × 104 CD34+ cells/kg in the infused product. This dose of CD34+ cells is about two logs lower than what is used in traditional transplantation. Thus, nonmobilized blood has low levels of CD34+ cells and can give at least short-term 100% donor chimerism.
Results of this study with low-dose TBI and donor lymphocyte infusion from matched related donors in patients with refractory cancers are as follows:
Engraftment can occur in humans with a radiation dose as low as 100 cGy.
Chimerism and tumor responses were associated with intensity of prior therapy.
Complete remission can occur with development of only transient, partial chimerism.
Engraftment can occur with a CD34+ dose as low at 104 cells/kg.
Stem cell competition, with recipient cells depleted by prior therapy, may facilitate engraftment.
The study here used very-low–dose conditioning (100 cGy TBI) for patients with refractory cancers. The M.D. Anderson group has studied the effect of the intensity of dose on disease control and survival [16]. Ninety-four patients with myelodysplastic syndrome and acute myelogeneous leukemia were treated with either a nonablative fludarabine/cytarabine/idarubicin regimen or a more myelo-suppressive regimen of fludarabine and melphalan. As shown in Table 1B, engraftment and disease control were improved in the latter group but transplant-related mortality was higher.
Several other investigators have performed studies of nonmyeloablative allogeneic transplantation for advanced solid tumors. Childs and colleagues [17] have treated renal cell carcinoma with cyclophosphamide and fludarabine, followed by allogeneic peripheral blood stem cell transplant. There was regression of metastatic disease in 53% of patients. Prior chemotherapy and a higher CD34+ dose contributed to a higher rate of engraftment [18]. Results in ovarian cancer have been inconclusive. Four of five patients with ovarian cancer had tumor regression after a nonmyeloablative transplant [19].
Results in patients without matched, related donors
Studies using umbilical cord blood
Only about 30% of patients have a matched, sibling donor [20]. Therefore, we extended this approach to patients without a 5/6 or 6/6 human leukocyte antigen (HLA) allele match in their family with use of umbilical cord blood. Banks of cryopreserved umbilical cord blood units have been established worldwide and provide an alternative stem cell source for patients without matched related or unrelated donors [21–23]. Umbilical cord blood has been shown to engraft after ablative conditioning regimens, and lead to successful transplantations, especially in pediatric patients [24–26]. However, cord blood transplantation has not been studied after very-low–dose TBI. Results in patients with alternative donors are presented in Table 4.
Table 4.
Results in patients with alternative donors
| Author | n | Diagnosis | Median age (y) | Graft source | Conditioning | Chimerism (%) | Responses |
|---|---|---|---|---|---|---|---|
| Ballen [15], Umass | 7 | 1 Lymphoma
6 Solid tumor |
47 | Cord blood | 100 cGy TBI | 0 | No response |
| Colvin [30], Roger Williams | 41 | 26 Heme mal
15 Solid tumor |
58 | Mismatched relative | 100 cGy TBI | 0 | 3/6 lymphoma 7/13 AML PR/CR |
| Loren [36], UPenn | 25 | Heme malignancy | 50 | Unrelated donor | Fludara/Cytoxan/Campath | 92 | 16 PR/CR |
| Wong, MD Anderson | 29 | Heme malignancy | 59 | Unrelated donor | Fludara/Melphalan | 86 | 18/29 CR |
AML =acute myeloid leukemia; CR =complete remission; PR =partial remission; TBI =total body irradiation.
In the initial cellular immune therapy study, we treated seven patients without sibling donors with 100 cGy TBI followed by the infusion of one cord blood unit, matched at 4/6 or more HLA loci [15]. The median number of cells infused was 3.1 × 104 CD34+ cells/kg and 1.7 × 106 CD3+ cells/kg. None of these patients achieved a tumor response or evidence of donor chimerism. The poor results seen with cord blood may be related to the low CD3+ dose infused; the dose of T cells is two logs lower than used in the sibling transplants.
Studies using mismatched related donors
Haploidentical transplants may be another option for patients without sibling donors. These transplants employ family members who are mismatched at one, two, or three HLA loci and have been utilized after both ablative and nonmyeloablative conditioning regimens [27–29]. The group at Roger Williams Hospital in Providence has extended the low-dose radiotherapy approach to patients with haploidentical donors [30]. Donors can include the patient’s parent, children, siblings, or more distant relatives.
In a Phase I study, patients with refractory hematologic malignancies received 100 cGy TBI, followed by infusion of escalating doses of CD3+ mobilized peripheral blood stem cells (1 × 106 × 108 cells/kg) [30,31]. Forty-one patients have been treated, 20 patients have been treated at the highest level.
There were no objective responses in the patients with solid tumors. The results in the hematologic malignancy patients are presented in Table 5. The most encouraging results were seen in patients with acute myeloid leukemia. Seven of 13 patients had a ≥50% decrease in bone marrow blasts and 3 patients achieved CR. There was one long-term survivor. All of the responses occurred in the absence of measurable donor chimerism.
Table 5.
Haploidentical transplant for patients with hematologic malignancy: Roger Williams data
| Diagnosis | Age (y) | CD3+ cells/kg | CD34+ cells/kg | Maximal chimerism | Response | Survival (d) |
|---|---|---|---|---|---|---|
| MDS-AML | 65 | 1 × l06 | 4.0 × 106 | 0% | Transient response | 66 |
| MDS-AML | 72 | 1 × l08 | 7.4 × 106 | 0% | Died in CR of unrelated cause | 180 |
| AML | 52 | 1 × l08 | 7.7 × 106 | N/A | NA | 6 |
| MDS-AML | 63 | 2 × 108 | 3.2 × 106 | 0% 2 wk | CR × 8.4 mo | 372 |
| AML | 58 | 2 × 108 | 7.6 × 106 | 0% 2 wk | CR × 31 mo. Back in CR day + 72 2nd BMT | 1167a,b |
| AML | 71 | 2 × 108 | 8.1 × 106 | 0% 2 wk | Transient response | 22 |
| AML | 72 | 2 × 108 | 4.3 × 106 | 17% 2 wk temporary | Transient response | 145 |
| AML | 80 | 2 × l08 | 7.8 × 106 | 48% 2 wk | Transient response | 25 |
| AML | 44 | 2 × 108 | 2.9 × 106 | 0% 2 wk | Remains in CR | 732a,b |
| AML | 70 | 2 × 108 | 10.3 × 106 | 0% 2 wk | Transient response | 100 |
| AML | 72 | 2 × 108 | 2.4 × 106 | 63% day + 1
0% 1 wk |
Transient response | 95 |
| AML | 82 | 2 × 108 | 2.9 × 106 | 11% day + 1
0% 1 wk |
Transient response | 67 |
| AML | 59 | 2 × l08 | 13.5 × 106 | 0% 2 wk | Transient response | 97 |
| Lymphoma | 49 | 3 × 107 | 2.9 × 106 | 0% 2 wk | Partial response
In CR after auto BMT |
1369a,b |
| Lymphoma | 54 | 1 × l08 | 4.0 × 106 | 0% 2 wk | Progression treated in 2nd CR | 442 |
| Lymphoma | 58 | 2 × l08 | 2.8 × 106 | 100% | Died of acute GVHD | 30 |
| Lymphoma | 50 | 1 × l08 | 1.0 × 106 | 0% 2 wk | Attained CR, minimal relapse marrow only | 1249a |
Patient currently alive;
Disease-free.
Most patients experienced a “haploimmunostorm,” a syndrome of fever, rash, and capillary leak within 12 hours of infusion. The symptoms resolved promptly after initiation of steroid therapy, which was routinely administered if fever persisted 48 hours after cell infusion. There was one treatment-related death due to GVHD.
These results suggest that donor chimerism is not necessary for tumor response. The biologic effect may be an altered host immune response breaking host tumor tolerance, and/or persistent nondetectable microchimerism.
This study of cellular immune therapy using haploidentical donors is now being extended to a multicenter Phase II study for patients with refractory leukemia. Patients receive low-dose TBI, followed by infusion of haploidentical CD3+ cells, with the option for later donor lymphocyte infusion.
Studies using unrelated donors
Well-matched unrelated donor (UD) stem cells can also be used to support nonmyeloablative allogeneic stem cell transplantation (NST), though the safety and efficacy of UD-NST is not yet well defined. Details of these studies are described in Table 4. Preliminary reports of UD-NST described a high incidence of severe acute GVHD, resulting in death in many patients [32,33]. In a subsequent trial, Alemtuzumab was added to the conditioning regimen and has been shown to reduce the incidence of GVHD after matched sibling NST [34]. Alemtuzumab can result in in vivo donor T cell depletion, and may also deplete host antigen presenting cells necessary for development of GVHD [35]. In studies of UD-NST that included Alemtuzumab, using fludarabine, Melphalan, and Alemtuzumab, a 6% incidence of grade III–IV acute GVHD was described with no cases of extensive chronic GVHD [36].
Anti-tumor responses in the absence of donor chimerism following nonmyeloablative hematopoietic stem cell transplantation
In patients with chemotherapy-refractory hematologic malignancies, meaningful antitumor responses usually occur in association with the development of full donor chimerism after stem cell transplantation (SCT) or following DLI [37]. Several patients in the studies described here, however, achieved sustained antitumor responses despite loss of donor graft. Investigators at the Massachusetts General Hospital have also recently described a number of patients who achieved anti-tumor responses despite loss of donor graft [38]. Based on a murine model, they conducted a series of trials of nonmyeloablative HLA-matched or mismatched related donor SCT with the intention of inducing mixed chimerism, then administering prophylactic DLI, for treatment of advanced hematologic malignancies [11,39]. Preparative therapy consisted of cyclophosphamide, equine antithymocyte globulin or MEDI-507 (an anti-CD2 monoclonal antibody) for in vivo T-cell depletion, thymic irradiation on day –1 and cyclosporine alone for GVHD prophylaxis. DLI(s) were given as early as 5 weeks post-SCT in patients with mixed chimerism without evidence of GVHD. All 82 evaluable patients achieved initial, both myeloid and T cell, donor chimerism. Macrochimerism is defined as >1% donor cells detectable by analyses of microsatellite variable number of tandem repeat (VNTR) or short tandem repeat markers in the setting of HLA-matched donor-recipient pairs or by either flow cytometry or microsatellite VNTR analyses in HLA-mismatched setting. Sixty patients maintained mixed chimerism or converted to full donor chimerism either spontaneously or following DLI and 22 patients (27% of total patients; bone marrow, n =20; peripheral blood stem cells, n =2) ultimately lost their graft (<1% donor cells) that could not be rescued by DLI(s). Thirty out of 60 patients (50%) who had durable donor chimerism-mixed or full donor chimerism-either spontaneously after the hematopoeitic stem cell transplantation or following donor lymphocyte infusion, achieved remission of their hematologic malignancies. Patients with transient chimerism had significantly increased numbers of circulating host T cells, mainly CD8 cells, and had sensitized antidonor cytotoxic T-lymphocyte (CTL) responses and high anti-donor CTL precursor frequencies as well as high antidonor T-helper cell frequencies, compared to the group with sustained donor chimerism within the first 100 days post-bone marrow transplantation.
Nine of 22 (41%) patients who lost donor chimerism achieved a response, including 6 who ultimately achieved a complete response. Seven of the nine patients who achieved a response despite loss of donor chimerism are alive from 2.5 to 5.5 years after SCT, including four in continuous CR. It is unlikely that the sustained response in these patients was achieved by treatment with nonmyeloablative dose cyclophosphamide alone, as most patients had chemotherapy-refractory disease at the time of transplantation with many previously treated with cyclophosphamide-based therapy. The authors favor a hypothesis that the host-vs-graft immune response associated with graft rejection has the potential to promote antitumor responses against host-type malignancies. Supporting this hypothesis are murine models, which showed that host antidonor immune responses causing spontaneous graft rejection lead to significant antitumor responses against recipient-type tumors [40]. Two patients in that series who achieved partial remission following transplantation showed clinical and radiographic evidence of further response following therapeutic DLIs. Their chimerism studies continued to show no measurable presence of donor hematopoiesis. As nearly all (20 of 22) patients, including “responders” and “nonresponders,” received DLI(s), it is not possible to attribute the antitumor responses solely to DLI. Nonetheless, the timing of tumor regression in these two patients suggests a possible DLI-mediated antitumor effect, the exact mechanisms of which are not known.
Loss of donor chimerism was not intended in these clinical protocols. However, observation of sustained tumor regression despite loss of the donor graft in conjunction with the results of the murine experiments [41] has led to development of novel transplantation strategies for achieving antitumor effects without risk of GVHD. In addition, it is possible that DLI may have a therapeutic impact in patients who have lost their grafts following nonmyeloablative allogeneic SCT.
Future directions for cellular immune therapy
A possible application of cellular immune therapy is for patients with solid tumors. These patients did not do well in the initial study [15]. Patients with tumors that respond to other types of immunotherapy-for example, cancers such as renal cell carcinoma or melanoma, may be more likely to benefit from this approach [17,42,43]. The initial early success in the studies of Childs and colleagues [17] has not been duplicated in other solid tumors. This lack of response may be due to lack of prior immunosuppressive therapy, and/or inherent biologic differences between the solid tumors and hematologic malignancies.
One approach to enhance activity of DLI is to administer ex vivo activated and expanded donor lymphocytes. The hypothesis is that ex vivo activation and expansion may reverse T cell tolerance, bypass possible in vivo T cell suppressive mechanisms, and improve the graft-vs-tumor activity [44]. This idea has been piloted as a Phase I study at the University of Pennsylvania [45,46]. Donor T cells are exposed to beads linked to anti-CD28 and anti-CD3 antibodies, which result in an approximately 130-fold expansion of CD3+ T cells. The initial trial included patients with hematologic malignancies who relapsed after allogeneic SCT. Patients received unstimulated DLI with or without standard induction chemotherapy (induction chemotherapy was given to patients with acute leukemia for cytoreduction), followed by infusion of activated donor lymphocytes on approximately day + 12 after standard donor lymphocyte infusion [47]. Eighteen patients have been treated; 8 achieved a complete remission lasting 3 to 41+ months (median 9 months) [48]. GVHD has been limited to the skin, except in two patients with stage 3 liver and gut GVHD. No patient died of GVHD-related complications. These data demonstrate that ex vivo co-stimulation results in effective expansion of donor T cells and that activated donor (aDLI) can be given with acceptable toxicity. Response rates to aDLI were impressive in diseases that have disappointing responses to conventional DLI. Subsequent trials and longer follow-up will determine if ex vivo activated DLIs will improve outcomes for relapsed patients when compared to conventional donor lymphocytes alone.
Effectiveness of donor natural killer cell alloreactivity has been demonstrated in mismatched transplants [49]. In some studies, natural killer cell alloreactive infusions have been associated with a graft-vs-leukemia effect without GVHD.
Dendritic cell therapy is a novel treatment strategy to generate tumor-specific immunity in patients with advanced cancer. Autologous dendritic cells and tumor cells are co-cultured to generate fusions, and patients are vaccinated at 3-week intervals [50]. Twenty-three patients with meta-static breast and renal cell cancer were treated with this approach. Two patients with breast cancer had a response and five patients with breast cancer and one with renal cell cancer had disease stabilization. A similar strategy is being tested in patients with multiple myeloma [51]. As patients with solid tumors did not fare well with the low-dose radiation approach, a clinical trial employing dendritic cell therapy might be considered for these patients.
A future application of cellular immune therapy may be to nonmalignant diseases. Quesenberry and colleagues have shown the “plasticity” of the stem cell, by demonstrating production of bone and muscle cells from marrow cells in a murine model [52,53]. Krause and colleagues [54] have shown that a single cell can give rise to cells in multiple tissues. These preliminary studies raise the possibility that cellular immune therapy may be beneficial in nonhematologic diseases.
Transplantation has been shown to be most effective for those patients with hematologic malignancy and chemotherapy-sensitive disease. For patients with refractory disease, traditional treatment options have been limited. The studies reviewed here suggest that low-dose conditioning followed by allogeneic stem cell therapy may be effective for patients with refractory hematologic malignancies. Patients who were heavily pretreated appeared to do better, presumably related to the higher degree of immunosuppression. For patients without a matched sibling donor, the haploidentical strategy, inducing a tumor response through graft rejection, may be an option. Cord blood transplantation is a less promising alternative, most likely related to the lower T-cell dose in cord blood. Patients with refractory solid tumors should consider enrolling in trials of dendritic cell therapy. These ideas and others form the basis for future work in this exciting field.
References
- 1.Stewart FM, Crittenden RB, Lowry PA, Pearson-White S, Quesenberry PJ. Long-term engraftment of normal and post-5-fluoro-uracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566–2571. [PubMed] [Google Scholar]
- 2.Stewart FM, Zhong S, Wuu J, Hsieh CC, Nilsson SK, Quesenberry PJ. Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood. 1998;91:3681–3687. [PubMed] [Google Scholar]
- 3.Quesenberry PJ, Zhong S, Wang H, Stewart FM. Allogeneic chimerism with low dose irradiation, antigen presensitization, and costimu-lator blockade in H-2 mismatched mice. Blood. 2001;97:557–564. doi: 10.1182/blood.v97.2.557. [DOI] [PubMed] [Google Scholar]
- 4.Colvin GA, Lambert JF, Abedi M, et al. Murine marrow cellularity and the concept of stem cell competition: geographic and quantitative determinants in stem cell biology. Leukemia. 2004;18:575–583. doi: 10.1038/sj.leu.2403268. [DOI] [PubMed] [Google Scholar]
- 5.Lambert JF, Colvin GA, Zhong S, et al. H-2 mismatched transplantation with repetitive cell infusions and CD40 ligand antibody infusions without myeloablation. Br J Hematol. 2002;119:155–163. doi: 10.1046/j.1365-2141.2002.03801.x. [DOI] [PubMed] [Google Scholar]
- 6.Kolb H, Mittermuller J, Clemm C. Donor leukocyte transfusions for treatment of recurrent chronic myelogeneous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 7.Porter D, Collins R, Shilberg O, et al. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant. 1999;5:253–261. doi: 10.1053/bbmt.1999.v5.pm10465105. [DOI] [PubMed] [Google Scholar]
- 8.Porter DL, Roth MS, McGarigle C, et al. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994;330:100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 9.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 10.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer TR, McAfee S, Dey BR, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–1752. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 12.Dey BR, McAfee S, Sackstein R, et al. Successful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed hematologic malignancy following autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:604–612. doi: 10.1053/bbmt.2001.v7.pm11760148. [DOI] [PubMed] [Google Scholar]
- 13.Porter DL, Luger SM, Duffy KM, et al. Allogeneic cell therapy for patients who relapse after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:230–238. doi: 10.1053/bbmt.2001.v7.pm11349810. [DOI] [PubMed] [Google Scholar]
- 14.Porter DL, Connors JM, Van Deerlin VM, et al. Graft-versus-tumor induction with donor leukocyte infusions as primary therapy for patients with malignancy. J Clin Oncol. 1999;17:1234–1237. doi: 10.1200/JCO.1999.17.4.1234. [DOI] [PubMed] [Google Scholar]
- 15.Ballen KK, Becker PS, Emmons RVB, et al. Low-dose total body irradiation followed by allogeneic lymphocyte infusion may induce remission in patients with refractory hematologic malignancy. Blood. 2002;100:442–450. doi: 10.1182/blood.v100.2.442. [DOI] [PubMed] [Google Scholar]
- 16.De Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 17.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral blood stem cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 18.Carballo C, Geller N, Kurlander R, et al. Prior chemotherapy and allograft CD34+ dose impact donor engraftment following nonmyeloablative allogeneic stem cell transplantation in patients with solid tumors. Blood. 2004;103:1560–1563. doi: 10.1182/blood-2003-04-1170. [DOI] [PubMed] [Google Scholar]
- 19.Bay JO, Fleury J, Choufi B, et al. Allogeneic hematopoietic stem cell transplantation in ovarian carcinoma: results of five patients. Bone Marrow Transplant. 2000;30:95–102. doi: 10.1038/sj.bmt.1703609. [DOI] [PubMed] [Google Scholar]
- 20.Schipper RF, D’amaro J, Oudshoorn M. The probability of finding a suitable related donor for bone marrow transplantation in extended families. Blood. 1996;87:800–804. [PubMed] [Google Scholar]
- 21.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryo-preservation of placental-umbilical cord blood for unrelated marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballen KK, Wilson M, Valinski H, et al. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplantat. 2000;27:7–14. doi: 10.1038/sj.bmt.1702729. [DOI] [PubMed] [Google Scholar]
- 23.Ballen KK, Kurtzberg J, Lane TA, et al. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–275. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 26.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensitiy conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 27.Daly A, McAfee S, Dey B, et al. Nonmyeloablative bone marrow transplantation: infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9:373–382. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 28.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 29.Sykes M, Preffer F, McAfee S, et al. Mixed lymphohematopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 30.Colvin GA, Abedi M, Rathore R, et al. Mini-haploidentical transplantation for refractory acute myeloid leukemia. Blood. 2004;140:2150a. abstract. [Google Scholar]
- 31.Colvin GA, Rathore R, Lambert JF, et al. HLA-haploidentical stem cell infusions for refractory hematologic malignancies: a universal outpatient immunotherapeutic approach in 100 cGy treated patients. In: Dicke K, Keating A, editors. Autologous Blood and Marrow Transplantation. Charlottesville, VA: Carden Jennings Publishing Co; 2003. pp. 267–277. [Google Scholar]
- 32.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 33.Sacks N, Orloff G, Duffy K, et al. High incidence of graft-versus-host disease (GVHD) in nonmyeloablative allogeneic (NMA) stem cell transplants (SCT) with unrelated donors (UD) Proc Am Soc Clin Oncol. 2002;21:282b. [Google Scholar]
- 34.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 35.Shlomchik W, Couzens M, Tang C, et al. Prevention of graft-versus-host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 36.Loren AW, Luger SM, Stadtmauer EA, et al. Intensive graft-versus-host disease prophylaxis is required after unrelated-donor nonmyeloa-blative stem cell transplantation. Bone Marrow Transplant. 2005;35:921–926. doi: 10.1038/sj.bmt.1704887. [DOI] [PubMed] [Google Scholar]
- 37.Dey BR, McAfee S, Colby C, et al. Impact of prophylactic donor leukocyte infusion on mixed chimerism, graft-versus-host disease, and antitumor responses in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 38.Dey BR, McAfee S, Colby C, et al. Anti-tumor response despite loss of donor chimerism in patients treated with nonmyeloablative conditioning and allogeneic stem cell transplantation. Br J Hematol. 2005;128:351–359. doi: 10.1111/j.1365-2141.2004.05328.x. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer TR, McAfee S, Sackstein R, et al. The intentional induction of mixed chimerism and achievement of anti-tumor responses following non-myeloablative conditioning therapy and HLA-matched donor bone marrow transplant for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6:309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 40.Kraus AB, Shaffer J, Toh HC, et al. Early host CD8 T-cell recovery and sensitized anti-donor interleukin-2-producing and cytotoxic T-cell responses associated with marrow graft rejection following nonmyeloablative allogeneic bone marrow transplantation. Exp Hematol. 2003;31:609–621. doi: 10.1016/s0301-472x(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 41.Rubio MT, Kim YM, Sachs T, Mapara M, Zhao G, Sykes M. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: critical role for recipient-derived interferon gamma. Blood. 2003;102:2300–2307. doi: 10.1182/blood-2002-12-3949. [DOI] [PubMed] [Google Scholar]
- 42.Bregni M, Dodero A, Peccatori J, et al. Nonmyeloablative conditioning followed by hematopoietic cell allografting and donor lymphocyte infusions for patients with metastatic renal and breast cancer. Blood. 2002;99:4234–4236. doi: 10.1182/blood.v99.11.4234. [DOI] [PubMed] [Google Scholar]
- 43.Bay JO, Choufi B, Pomel C, et al. Potential allogeneic graft-versus-tumor effect in a patient with ovarian cancer. Bone Marrow Transplant. 2000;25:681–682. doi: 10.1038/sj.bmt.1702206. [DOI] [PubMed] [Google Scholar]
- 44.Liebowitz L, Lee KP, June CH. Costimulatory approaches to adoptive immunotherapy. Curr Opin Oncol. 1998;10:533–541. doi: 10.1097/00001622-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Porter D, Bunin N, Laport G, et al. Graft-vs-tumor induction with donor leukocyte infusions expanded ex-vivo by activation with CD3/CD28 stimulation. Blood. 2001;98:404a. abstract. [Google Scholar]
- 46.Porter DL. Donor leukocyte infusions in acute myelogeneous leukemia. Leukemia. 2003;17:1035–1037. doi: 10.1038/sj.leu.2402950. [DOI] [PubMed] [Google Scholar]
- 47.Levine J, Braun T, Penza S, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 48.Porter D, Levine B, Bunin N, et al. Enhancing graft-vs-tumor (GVT) activity of donor leukocyte infusions by ex-vivo expansion and activation with CD3/CD28 co-stimulation. Blood. 2004;104:794a. abstract. [Google Scholar]
- 49.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 50.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 51.Raje N, Hideshima T, Davies FE, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Hematol. 2004;125:343–352. doi: 10.1111/j.1365-2141.2004.04929.x. [DOI] [PubMed] [Google Scholar]
- 52.Abedi M, Greer D, Demers D, et al. Bone marrow cells participate in regeneration of skeletal muscle by transdifferentiation to muscle cells. Exp Hematol. 2003;31(suppl 1):134. [Google Scholar]
- 53.Nilsson SK, Dooner MS, Weier HU, et al. Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J Exp Med. 1999;189:729–734. doi: 10.1084/jem.189.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]


